Abstract
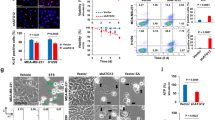
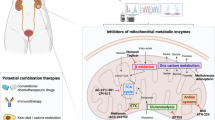
Citrin, encoded by SLC25A13 gene, is an inner mitochondrial transporter that is part of the malate–aspartate shuttle, which regulates the NAD+/NADH ratio between the cytosol and mitochondria. Citrullinemia type II (CTLN-II) is an inherited disorder caused by germline mutations in SLC25A13, manifesting clinically in growth failure that can be alleviated by dietary restriction of carbohydrates. The association of citrin with glycolysis and NAD+/NADH ratio led us to hypothesize that it may play a role in carcinogenesis. Indeed, we find that citrin is upregulated in multiple cancer types and is essential for supplementing NAD+ for glycolysis and NADH for oxidative phosphorylation. Consequently, citrin deficiency associates with autophagy, whereas its overexpression in cancer cells increases energy production and cancer invasion. Furthermore, based on the human deleterious mutations in citrin, we found a potential inhibitor of citrin that restricts cancerous phenotypes in cells. Collectively, our findings suggest that targeting citrin may be of benefit for cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Amoedo ND, Punzi G, Obre E, Lacombe D, De Grassi A, Pierri CL, et al. AGC1/2, the mitochondrial aspartate-glutamate carriers. Biochim Biophys Acta. 2016;1863:2394–412.
Rabinovich S, Adler L, Yizhak K, Sarver A, Silberman A, Agron S, et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature. 2015;527:379–83.
Satrustegui J, Pardo B, Del Arco A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev. 2007;87:29–67.
Song YZ, Deng M, Chen FP, Wen F, Guo L, Cao SL, et al. Genotypic and phenotypic features of citrin deficiency: five-year experience in a Chinese pediatric center. Int J Mol Med. 2011;28:33–40.
Takeuchi S, Yazaki M, Yamada S, Fukuyama T, Inui A, Iwasaki Y, et al. An adolescent case of citrin deficiency with severe anorexia mimicking anorexia nervosa. Pediatrics 2015;136:e530–4.
Saheki T, Inoue K, Tushima A, Mutoh K, Kobayashi K. Citrin deficiency and current treatment concepts. Mol Genet Metab 2010;100(Suppl 1):S59–64.
Kimura N, Kubo N, Narumi S, Toyoki Y, Ishido K, Kudo D, et al. Liver transplantation versus conservative treatment for adult-onset type II citrullinemia: our experience and a review of the literature. Transplant Proc 2013;45:3432–7.
Chang KW, Chen HL, Chien YH, Chen TC, Yeh CT. SLC25A13 gene mutations in Taiwanese patients with non-viral hepatocellular carcinoma. Mol Genet Metab 2011;103:293–6.
Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 2008;28:264–78.
Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 2010;584:1374–8.
Shinohara M, Adachi Y, Mitsushita J, Kuwabara M, Nagasawa A, Harada S, et al. Reactive oxygen generated by NADPH oxidase 1 (Nox1) contributes to cell invasion by regulating matrix metalloprotease-9 production and cell migration. J Biol Chem 2010;285:4481–8.
Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol 2007;23:164–70.
Shaul YD, Yuan B, Thiru P, Nutter-Upham A, McCallum S, Lanzkron C, et al. MERAV: a tool for comparing gene expression across human tissues and cell types. Nucleic Acids Res. 2015;44(D1):D560–6.
Fiermonte G, Parisi G, Martinelli D, De Leonardis F, Torre G, Pierri CL, et al. A new Caucasian case of neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD): a clinical, molecular, and functional study. Mol Genet Metab 2011;104:501–6.
Corbet C, Bastien E, Draoui N, Doix B, Mignion L, Jordan BF, et al. Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nat Commun. 2018;9:1208.
van Niekerk G, Engelbrecht AM. Role of PKM2 in directing the metabolic fate of glucose in cancer: a potential therapeutic target. Cell Oncol (Dordrecht). 2018;14:343–51.
Porporato PE, Payen VL, Perez-Escuredo J, De Saedeleer CJ, Danhier P, Copetti T, et al. A mitochondrial switch promotes tumor metastasis. Cell Rep 2014;8:754–66.
Kluza J, Corazao-Rozas P, Touil Y, Jendoubi M, Maire C, Guerreschi P, et al. Inactivation of the HIF-1alpha/PDK3 signaling axis drives melanoma toward mitochondrial oxidative metabolism and potentiates the therapeutic activity of pro-oxidants. Cancer Res 2012;72:5035–47.
Sullivan LB, Luengo A, Danai LV, Bush LN, Diehl FF, Hosios AM, et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nat Cell Biol. 2018;20:782–8.
Garcia-Bermudez J, Baudrier L, La K, Zhu XG, Fidelin J, Sviderskiy VO, et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat Cell Biol. 2018;20:775–81.
Gaude E, Schmidt C, Gammage PA, Dugourd A, Blacker T, Chew SP, et al. NADH shuttling couples cytosolic reductive carboxylation of glutamine with glycolysis in cells with mitochondrial dysfunction. Mol Cell 2018;69:581–93.e7.
Hollinshead KE, Tennant DA. Mitochondrial metabolic remodeling in response to genetic and environmental perturbations. Wiley Inter Rev Syst Biol Med. 2016;8:272–85.
Xian ZY, Liu JM, Chen QK, Chen HZ, Ye CJ, Xue J, et al. Inhibition of LDHA suppresses tumor progression in prostate cancer. Tumour Biol 2015;36:8093–100.
Erez A, DeBerardinis RJ. Metabolic dysregulation in monogenic disorders and cancer - finding method in madness. Nat Rev Cancer. 2015;15:440–8.
Torrano V, Valcarcel-Jimenez L, Cortazar AR, Liu X, Urosevic J, Castillo-Martin M, et al. The metabolic co-regulator PGC1alpha suppresses prostate cancer metastasis. Nat Cell Biol. 2016;18:645–56.
Chung HY, Park YK. Rationale, feasibility and acceptability of ketogenic diet for cancer treatment. J Cancer Prev 2017;22:127–34.
Fass E, Shvets E, Degani I, Hirschberg K, Elazar Z. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem 2006;281:36303–16.
Hung YP, Albeck JG, Tantama M, Yellen G. Imaging cytosolic NADH-NAD(+) redox state with a genetically encoded fluorescent biosensor. Cell Metab 2011;14:545–54.
Salem M, Bernach M, Bajdzienko K, Giavalisco P. A simple fractionated extraction method for the comprehensive analysis of metabolites, lipids, and proteins from a single sample. J Vis Exp. 2017;1:124.
Malitsky S, Ziv C, Rosenwasser S, Zheng S, Schatz D, Porat Z, et al. Viral infection of the marine alga Emiliania huxleyi triggers lipidome remodeling and induces the production of highly saturated triacylglycerol. New Phytol 2016;210:88–96.
Zheng L, Cardaci S, Jerby L, MacKenzie ED, Sciacovelli M, Johnson TI, et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat Commun. 2015;6:6001.
Robinson BH, McKay N, Goodyer P, Lancaster G. Defective intramitochondrial NADH oxidation in skin fibroblasts from an infant with fatal neonatal lacticacidemia. Am J Hum Genet 1985;37:938–46.
Dimmock D, Maranda B, Dionisi-Vici C, Wang J, Kleppe S, Fiermonte G, et al. Citrin deficiency, a perplexing global disorder. Mol Genet Metab 2009;96:44–9.
Dimmock D, Kobayashi K, Iijima M, Tabata A, Wong LJ, Saheki T, et al. Citrin deficiency: a novel cause of failure to thrive that responds to a high-protein, low-carbohydrate diet. Pediatrics 2007;119:e773–7.
Dimmock D, Tang LY, Schmitt ES, Wong LJ. Quantitative evaluation of the mitochondrial DNA depletion syndrome. Clin Chem. 2010;56:1119–27.
Bartolome F, Abramov AY. Measurement of mitochondrial NADH and FAD autofluorescence in live cells. Methods Mol Biol (Clifton, NJ) 2015;1264:263–70.
Acknowledgements
We acknowledge and thank the Weizmann Institute for providing financial and infrastructural support. We thank Tomer Shlomi and his student Won Dong-Lee, Gad Asher, Zvulun Elazar and his student Adi Abada, Yoav Shaul, Irit Sagi, Ron Rotkopf, and Sivan Galai for the intellectual and technical assistance. AE is incumbent of the Leah Omenn Career Development Chair and is supported by research grants from the European research program (CIG618113, ERC614204), the Israel Science Foundation (1343/13; 1952/13), and from the Minerva grant award (711730). AE received additional support from the Adelis Foundation, the Henry S. and Anne S. Reich Research Fund, the Dukler Fund for Cancer Research, the Paul Sparr Foundation, the Saul and Theresa Esman Foundation, from Joseph Piko Baruch, and from the estate of Fannie Sherr. The authors declare applying for a patent for citrin inhibition as cancer therapy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rabinovich, S., Silberman, A., Adler, L. et al. The mitochondrial carrier Citrin plays a role in regulating cellular energy during carcinogenesis. Oncogene 39, 164–175 (2020). https://doi.org/10.1038/s41388-019-0976-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0976-2
This article is cited by
-
Citrin mediated metabolic rewiring in response to altered basal subcellular Ca2+ homeostasis
Communications Biology (2022)
-
ASS1 and ASL suppress growth in clear cell renal cell carcinoma via altered nitrogen metabolism
Cancer & Metabolism (2021)
-
Effect of hypoxia factors gene silencing on ROS production and metabolic status of A375 malignant melanoma cells
Scientific Reports (2021)