Abstract
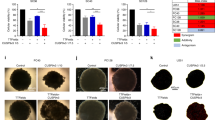
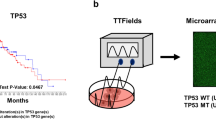
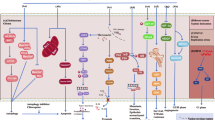
Tumor-treating fields (TTFs) — a type of electromagnetic field-based therapy using low-intensity electrical fields — has recently been characterized as a potential anticancer therapy for glioblastoma multiforme (GBM). However, the molecular mechanisms involved remain poorly understood. Our results show that the activation of autophagy contributes to the TTF-induced anti-GBM activity in vitro or in vivo and GBM patient stem cells or primary in vivo culture systems. TTF-treatment upregulated several autophagy-related genes (~2-fold) and induced cytomorphological changes. TTF-induced autophagy in GBM was associated with decreased Akt2 expression, not Akt1 or Akt3, via the mTOR/p70S6K pathway. An Affymetrix GeneChip miRNA 4.0 Array analysis revealed that TTFs altered the expression of many microRNAs (miRNAs). TTF-induced autophagy upregulated miR-29b, which subsequently suppressed the Akt signaling pathway. A luciferase reporter assay confirmed that TTFs induced miR-29b to target Akt2, negatively affecting Akt2 expression thereby triggering autophagy. TTF-induced autophagy suppressed tumor growth in GBM mouse models subjected to TTFs as determined by positron emission tomography and computed tomography (PET-CT). GBM patient stem cells and a primary in vivo culture system with high Akt2 levels also showed TTF-induced inhibition. Taken together, our results identified autophagy as a critical cell death pathway triggered by TTFs in GBM and indicate that TTF is a potential treatment option for GBM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Johnson DR, Ma DJ, Buckner JC, Hammack JE. Conditional probability of long‐term survival in glioblastoma. Cancer. 2012;118:5608–13.
Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–66.
Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64:3288–95.
NovoTTF-100A System. Premarket approval P100034. U.S. Food and Drug Administration, 2011. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100034. Accessed 7 Feb 2018.
Kessler AF, Frömbling GE, Gross F, Hahn M, Dzokou W, Ernestus R-I, et al. Effects of tumor treating fields (TTFields) on glioblastoma cells are augmented by mitotic checkpoint inhibition. Cell Death Discov. 2018;5:12.
Giladi M, Munster M, Schneiderman RS, Voloshin T, Porat Y, Blat R, et al. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol. 2017;12:206.
Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–202.
Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314:2535–43.
Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318:2306–16.
Toms S, Kim C, Nicholas G, Ram ZJ, Jon O. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial. J Neuro-Oncol. 2019;141:467–73.
Davies AM, Weinberg U, Palti Y. Tumor treating fields: a new frontier in cancer therapy. Ann New Y Acad Sci. 2013;1291:86–95.
Kirson ED, Dbalý V, Tovaryš F, Vymazal J, Soustiel JF, Itzhaki A, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci. 2007;104:10152–7.
Kim EH, Song HS, Yoo SH, Yoon M. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget. 2016;7:65125.
Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12:1528.
Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713.
Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961.
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2016;12:1–222.
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014.
Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–62.
Oncomine. 2006. https://www.oncomine.org/resource/login.html. Accessed 7 Jan 2018.
SurvExpress. 2015. http://bioinformatica.mty.itesm.mx:8080/Biomatec/SurvivaX.jsp. Accessed 7 Jan 2018.
Plastaras JP, Dorsey JF, Carroll K, Kim S-H, Birnbaum MJ, El-Deiry WS. Role of PI3K/Akt signaling in TRAIL-and radiation-induced gastrointestinal apoptosis. Cancer Biol Ther. 2008;7:2047–53.
Stupp R, Taillibert S, Kanner A, Kesari S, Toms SA, Barnett GH, et al. Tumor treating fields (TTFields): A novel treatment modality added to standard chemo-and radiotherapy in newly diagnosed glioblastoma—First report of the full dataset of the EF14 randomized phase III trial. Am. Soc. Clin. Oncol. 2015;33:2000.
Kim EH, Kim YJ, Song HS, Jeong YK, Lee JY, Sung J, et al. Biological effect of an alternating electric field on cell proliferation and synergistic antimitotic effect in combination with ionizing radiation. Oncotarget. 2016;7:62267.
Walter BA, Valera VA, Pinto PA, Merino MJ. Comprehensive microRNA profiling of prostate cancer. J Cancer. 2013;4:350.
Fohlin H, Pérez-Tenorio G, Fornander T, Skoog L, Nordenskjöld B, Carstensen J, et al. Akt2 expression is associated with good long-term prognosis in oestrogen receptor positive breast cancer. Eur J Cancer. 2013;49:1196–204.
Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, et al. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–6.
Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886–918.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756.
Rivas S, Gómez-Oro C, Antón IM, Wandosell F. Role of Akt isoforms controlling cancer stem cell survival, phenotype and self-renewal. Biomedicines. 2018;6:29.
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–23.
Wang Y, Zhang X, Li H, Yu J, Ren X. The role of miRNA-29 family in cancer. Eur J cell Biol. 2013;92:123–8.
Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133.
Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. Cancer J Clin. 2005;55:178–94.
Hippert MM, O’Toole PS, Thorburn A. Autophagy in cancer: good, bad, or both? Cancer Res. 2006;66:9349–51.
Jeong H, Sung J, Oh S-I, Jeong S, Koh EK, Hong S, et al. Inhibition of brain tumor cell proliferation by alternating electric fields. Appl Phys Lett. 2014;105:203703.
Yin J, Oh YT, Kim J-Y, Kim SS, Choi E, Kim TH, et al. Transglutaminase 2 inhibition reverses mesenchymal transdifferentiation of glioma stem cells by regulating C/EBPβ signaling. Cancer Res. 2017;77:4973–84.
Jo Y, Kim E, Sai S, Kim J, Cho J-M, Kim H, et al. Functional Biological Activity of Sorafenib as a Tumor-Treating Field Sensitizer for Glioblastoma Therapy. Int J Mol Sci. 2018;19:3684.
Constantinescu CC, Mukherjee J. Performance evaluation of an Inveon PET preclinical scanner. Phys Med Biol. 2009;54:2885–99.
Ganapathy B, Nandhagopal N, Polizzotti BD, Bennett D, Asan A, Wu Y, et al. Neuregulin-1 administration protocols sufficient for stimulating cardiac regeneration in young mice do not induce somatic, organ, or neoplastic growth. PLoS ONE. 2016;11:e0155456.
Li Y, Wang J, Zhang Z, Yi J, He C, Wang F, et al. Resveratrol compares with melatonin in improving in vitro porcine oocyte maturation under heat stress. J Anim Sci Biotechnol. 2016;7:33.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant (no. NRF-2017R1D1A1B03028923) and a grant from the Korea Institute of Radiological and Medical Sciences (KIRAMS), which was funded by the Ministry of Science, ICT (MSIP) Republic of Korea (50531-2018, 50538-2019).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Statement
Our study was approved by the Animal Ethics Committee (KUIACUC-2018-73, 1 June 2018).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, E.H., Jo, Y., Sai, S. et al. Tumor-treating fields induce autophagy by blocking the Akt2/miR29b axis in glioblastoma cells. Oncogene 38, 6630–6646 (2019). https://doi.org/10.1038/s41388-019-0882-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0882-7
This article is cited by
-
The schemes, mechanisms and molecular pathway changes of Tumor Treating Fields (TTFields) alone or in combination with radiotherapy and chemotherapy
Cell Death Discovery (2022)
-
Whole transcriptome and proteome analyses identify potential targets and mechanisms underlying tumor treating fields against glioblastoma
Cell Death & Disease (2022)
-
An overview on tumor treating fields (TTFields) technology as a new potential subsidiary biophysical treatment for COVID-19
Drug Delivery and Translational Research (2022)
-
Tumour treating fields therapy for glioblastoma: current advances and future directions
British Journal of Cancer (2021)
-
Thymidine decreases the DNA damage and apoptosis caused by tumor‐treating fields in cancer cell lines
Genes & Genomics (2021)