Abstract
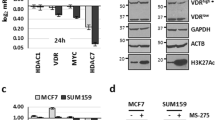
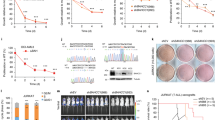
Early Growth Response 1 (EGR1) is a stress response transcription factor with multiple tumour suppressor roles in breast tissue, whose expression is often lost in breast cancers. We have previously shown that the breast cancer oncogene TBX2 (T-BOX2) interacts with EGR1 to co-repress EGR1-target genes including the breast tumour suppressor NDRG1. Here, we show the mechanistic basis of this TBX2 repression complex. We show that siRNA knockdown of TBX2, EGR1, Heterochromatin Protein 1 (HP1) isoforms and the generic HP1-associated corepressor protein KAP1 all resulted in growth inhibition of TBX2-expressing breast cancer cells. We show that TBX2 interacts with HP1 through a conserved HP1-binding motif in its N-terminus, which in turn leads to the recruitment of KAP1 and other associated proteins. Mutation of the TBX2 HP1 binding domain abrogates the TBX2-HP1 interaction and loss of repression of target genes such as NDRG1. Chromatin-immunoprecipitation (ChIP) assays showed that TBX2 establishes a repressive chromatin mark, specifically H3K9me3, around the NDRG1 proximal promoter coincident with the recruitment of the DNA methyltransferase DNMT3B and histone methyltransferase (HMT) complex components (G9A, Enhancer of Zeste 2 (EZH2) and Suppressor of Zeste 12 (SUZ12)). Knockdown of G9A, EZH2 or SUZ12 resulted in upregulation of TBX2/EGR1 co-regulated targets accompanied by a dramatic inhibition of cell proliferation. We show that a generic inhibitor of HMT activity, DzNep, phenocopies expression of an inducible dominant negative TBX2. Knockdown of TBX2, KAP1 or HP1 inhibited NDRG1 promoter decoration specifically with the H3K9me3 repression mark. Correspondingly, treatment with a G9A inhibitor effectively reversed TBX2 repression of NDRG1 and synergistically downregulated cell proliferation following TBX2 functional inhibition. These data demonstrate that TBX2 promotes suppression of normal growth control mechanisms through recruitment of a large repression complex to EGR1-responsive promoters leading to the uncontrolled proliferation of breast cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, et al. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206:379–90.
Roylance R, Gorman P, Harris W, Liebmann R, Barnes D, Hanby A, et al. Comparative genomic hybridization of breast tumors stratified by histological grade reveals new insights into the biological progression of breast cancer. Cancer Res. 1999;59:1433–6.
Tirkkonen M, Johannsson O, Agnarsson BA, Olsson H, Ingvarsson S, Karhu R, et al. Distinct somatic genetic changes associated with tumor progression in carriers of BRCA1 and BRCA2 germ-line mutations. Cancer Res. 1997;57:1222–7.
Sinclair CS, Adem C, Naderi A, Soderberg CL, Johnson M, Wu K, et al. TBX2 is preferentially amplified in BRCA1- and BRCA2-related breast tumors. Cancer Res. 2002;62:3587–91.
Barlund M, Monni O, Kononen J, Cornelison R, Torhorst J, Sauter G, et al. Multiple genes at 17q23 undergo amplification and overexpression in breast cancer. Cancer Res. 2000;60:5340–4.
Jacobs JJL, Keblusek P, Robanus-Maandag E, Kristel P, Lingbeek M, Nederlof PM, et al. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19(ARF)) and is amplified in a subset of human breast cancers. Nat Genet. 2000;26:291–9.
Prince S, Carreira S, Vance KW, Abrahams A, Goding CR. Tbx2 directly represses the expression of the p21WAF1 cyclin-dependent kinase inhibitor. Cancer Res. 2004;64:1669–74.
Vance KW, Carreira S, Brosch G, Goding CM. Tbx2 is overexpressed and plays an important role in maintaining proliferation and suppression of senescence in melanomas. Cancer Res. 2005;65:2260–8.
Ismail A, Bateman A. Expression of TBX2 promotes anchorage-independent growth and survival in the p53-negative SW13 adrenocortical carcinoma. Cancer Lett. 2009;278:230–40.
Abrahams A, Parker MI, Prince S. The T-box transcription factor Tbx2: Its role in development and possible implication in cancer. IUBMB Life 2010;62:92–102.
Wang B, Lindley LE, Fernandez-Vega V, Rieger ME, Sims AH, Briegel KJ. The T box transcription factor TBX2 promotes epithelial-mesenchymal transition and invasion of normal and malignant breast epithelial cells. PLoS ONE. 2012;7. https://doi.org/10.1371/journal.pone.0041355.
Redmond KL, Crawford NT, Farmer H, D’Costa ZC, O’Brien GJ, Buckley NE, et al. T-box 2 represses NDRG1 through an EGR1-dependent mechanism to drive the proliferation of breast cancer cells. Oncogene. 2010;29:3252–62.
De Belle I, Huang RP, Fan Y, Liu C, Mercola D, Adamson ED. P53 and Egr-1 additively suppress transformed growth in HT1080 cells but Egr-1 counteracts p53-dependent apoptosis. Oncogene. 1999;18:3633–42.
Vance KW, Shaw HM, Rodriguez M, Ott S, Goding CR. The retinoblastoma protein modulates Tbx2 functional specificity. Mol Biol Cell. 2010;21:2770–9.
Martin N, Benhamed M, Nacerddine K, Demarque MD, Van Lohuizen M, Dejean A, et al. Physical and functional interaction between PML and TBX2 in the establishment of cellular senescence. EMBO J. 2012;31:95–109.
Li Y, Kirschmann DA, Wallrath LL. Does heterochromatin protein 1 always follow code? Proc Natl Acad Sci. 2002;99:16462–9.
Sims RJ, Nishioka K, Reinberg D. Histone lysine methylation: a signature for chromatin function. Trends Genet. 2003;19:629–39.
Frietze S, O’Geen H, Blahnik KR, Jin VX, Farnham PJ. ZNF274 recruits the histone methyltransferase SETDB1 to the 39 ends of ZNF genes. PLoS ONE. 2010;5. https://doi.org/10.1371/journal.pone.0015082.
Friedman JR, Fredericks WJ, Jensen DE, Speicher DW, Huang XP, Neilson EG, et al. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 1996;10:2067–78.
Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–4.
Kwon SH, Florens L, Swanson SK, Washburn MP, Abmayr SM, Workman JL. Heterochromatin protein 1 (HP1) connects the FACT histone chaperone complex to the phosphorylated CTD of RNA polymerase II. Genes Dev. 2010;24:2133–45.
Della Ragione F, Cucciolla V, Criniti V, Indaco S, Borriello A, Zappia V. p21Cip1 gene expression is modulated by Egr1. A novel regulatory mechanism involved in the resveratrol antiproliferative effect. J Biol Chem. 2003. https://doi.org/10.1074/jbc.M300771200.
Cowieson NP, Partridge JF, Allshire RC, McLaughlin PJ. Dimerisation of a chromo shadow domain and distinctions from the chromodomain as revealed by structural analysis. Curr Biol. 2000;10:517–25.
Wu H, Min J, Lunin VV, Antoshenko T, Dombrovski L, Zeng H, et al. Structural biology of human H3K9 methyltransferases. PLoS ONE. 2010;5. https://doi.org/10.1371/journal.pone.0008570.
De La Cruz CC, Kirmizis A, Simon MD, Isono KI, Koseki H, Panning B. The polycomb group protein SUZ12 regulates histone H3 lysine 9 methylation and HP1α distribution. Chromosom Res. 2007;15:299–314.
Adem C, Soderberg CL, Hafner K, Reynolds C, Slezak JM, Sinclair CS, et al. ERBB2, TBX2, RPS6KB1, and MYC alterations in breast tissues of BRCA1 and BRCA2 mutation carriers. Genes Chromosom Cancer. 2004;41:1–11.
Krones-Herzig A, Adamson E, Mercola D. Early growth response 1 protein, an upstream gatekeeper of the p53 tumor suppressor, controls replicative senescence. Proc Natl Acad Sci. 2003;100:3233–8.
Vormer TL, Foijer F, Wielders CLC, te Riele H. Anchorage-independent growth of pocket protein-deficient murine fibroblasts requires bypass of G2 arrest and can be accomplished by expression of TBX2. Mol Cell Biol. 2008;28:7263–73.
Hartman ZC, Poage GM, Den Hollander P, Tsimelzon A, Hill J, Panupinthu N, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013;73:3470–80.
Moore HM, Gonzalez ME, Toy KA, Cimino-Mathews A, Argani P, Kleer CG. EZH2 inhibition decreases p38 signaling and suppresses breast cancer motility and metastasis. Breast Cancer Res Treat. 2013;138:741–52.
Yu H, Simons DL, Segall I, Carcamo-Cavazos V, Schwartz EJ, Yan N, et al. PRC2/EED-EZH2 complex is up-regulated in breast cancer lymph node metastasis compared to primary tumor and correlates with tumor proliferation in situ. PLoS ONE. 2012;7. https://doi.org/10.1371/journal.pone.0051239.
Granit RZ, Gabai Y, Hadar T, Karamansha Y, Liberman L, Waldhorn I, et al. EZH2 promotes a bi-lineage identity in basal-like breast cancer cells. Oncogene. 2013;32:3886–95.
Dong C, Wu Y, Yao J, Wang Y, Yu Y, Rychahou PG, et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J Clin Invest. 2012;122:1469–86.
Purcell DJ, Khalid O, Ou CY, Little GH, Frenkel B, Baniwal SK, et al. Recruitment of coregulator G9a by Runx2 for selective enhancement or suppression of transcription. J Cell Biochem. 2012;113:2406–14.
Wozniak RJ, Klimecki WT, Lau SS, Feinstein Y, Futscher BW. 5-Aza-2′-deoxycytidine-mediated reductions in G9A histone methyltransferase and histone H3 K9 di-methylation levels are linked to tumor suppressor gene reactivation. Oncogene. 2007;26:77–90.
Roll JD, Rivenbark AG, Jones WD, Coleman WB. DNMT3b overexpression contributes to a hypermethylator phenotype in human breast cancer cell lines. Mol Cancer. 2008;7. https://doi.org/10.1186/1476-4598-7-15.
Buckley NE, Hosey AM, Gorski JJ, Purcell JW, Mulligan JM, Harkin DP, et al. BRCA1 regulates IFN- signaling through a mechanism involving the type I IFNs. Mol Cancer Res. 2007;5:261–70.
Hosey AM, Gorski JJ, Murray MM, Quinn JE, Chung WY, Stewart GE, et al. Molecular basis for estrogen receptor α deficiency in BRCA1-linked breast cancer. J Natl Cancer Inst. 2007;99:1683–94.
Acknowledgements
Special thanks to Dr James Murray (Trinity College Dublin) for the kind gift of sheep anti-human NDRG1 antibody and Professor Max Costa (University of New York) for NDRG1 promoter luciferase constructs. This work was supported by grants from the Breast Cancer Campaign and Breast Cancer Now (NEC, AMCI, AMCC, ZCD’C, NEB, PBM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Crawford, N.T., McIntyre, A.J., McCormick, A. et al. TBX2 interacts with heterochromatin protein 1 to recruit a novel repression complex to EGR1-targeted promoters to drive the proliferation of breast cancer cells. Oncogene 38, 5971–5986 (2019). https://doi.org/10.1038/s41388-019-0853-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0853-z
This article is cited by
-
Expression and prognosis analysis of TBX2 subfamily in human lung carcinoma
Discover Oncology (2024)
-
DNMT3B overexpression downregulates genes with CpG islands, common motifs, and transcription factor binding sites that interact with DNMT3B
Scientific Reports (2022)
-
Combined genomic and proteomic approaches reveal DNA binding sites and interaction partners of TBX2 in the developing lung
Respiratory Research (2021)
-
EGR1 promotes stemness and predicts a poor outcome of uterine cervical cancer by inducing SOX9 expression
Genes & Genomics (2021)
-
Bioinformatic analysis of the expression and prognostic value of chromobox family proteins in human breast cancer
Scientific Reports (2020)