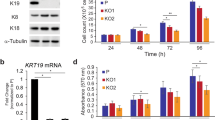
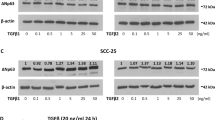
Abstract
Keratin intermediate filament (IF) is one component of cellular architectures, which provides necessary mechanical support to conquer environmental stresses. Recent findings reveal its involvement in mechano-transduction and the associated stem cell reprogramming, suggesting the possible roles in cancer development. Here, we report t(12;17)(q13.13;q21.2) chromosomal rearrangement as the most common fusion event in OSCC, resulting in a variety of inter-keratin fusions. Junction site mapping verified 9 in-frame K6-K14 variants, three of which were correlated with lymph node invasion, late tumor stages (T3/T4) and shorter disease-free survival times. When expressed in OSCC cells, those fusion variants disturbed wild-type K14 organization through direct interaction or aggregate formation, leading to perinuclear structure loss and nuclear deformation. Protein array analyses showed the ability of K6-K14 variant 7 (K6-K14/V7) to upregulate TGF-β and G-CSF signaling, which contributed to cell stemness, drug tolerance, and cell aggressiveness. Notably, K6-K14/V7-expressing cells easily adapted to a soft 3-D culture condition in vitro and formed larger, less differentiated tumors in vivo. In addition to the anti-mechanical-stress activity, our data uncover oncogenic functionality of novel keratin filaments caused by gene fusions during OSCC development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3:770–81.
Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82.
Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21:632–41.
Sewell A, Brown B, Biktasova A, Mills GB, Lu Y, Tyson DR, et al. Reverse-phase protein array profiling of oropharyngeal cancer and significance of PIK3CA mutations in HPV-associated head and neck cancer. Clin Cancer Res. 2014;20:2300–11.
Souto GR, Caliari MV, Lins CE, de Aguiar MC, de Abreu MH, Mesquita RA. Tobacco use increase the number of aneuploid nuclei in the clinically healthy oral epithelium. J Oral Pathol Med. 2010;39:605–10.
Li R, Faden DL, Fakhry C, Langelier C, Jiao Y, Wang Y, et al. Clinical, genomic, and metagenomic characterization of oral tongue squamous cell carcinoma in patients who do not smoke. Head Neck. 2015;37:1642–9.
Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129:705–33.
Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403–39.
Karantza V. Keratins in health and cancer: more than mere epithelial cell markers. Oncogene. 2011;30:127–38.
Loschke F, Seltmann K, Bouameur JE, Magin TM. Regulation of keratin network organization. Curr Opin Cell Biol. 2015;32:56–64.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89.
Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104.
Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–51.
Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci USA. 2016;113:E854–63.
Willipinski-Stapelfeldt B, Riethdorf S, Assmann V, Woelfle U, Rau T, Sauter G, et al. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005;11:8006–14.
Joosse SA, Hannemann J, Spötter J, Bauche A, Andreas A, Müller V, et al. Changes in keratin expression during metastatic progression of breast cancer: impact on the detection of circulating tumor cells. Clin Cancer Res. 2012;18:993–1003.
Seltmann K, Fritsch AW, Kas JA, Magin TM. Keratins significantly contribute to cell stiffness and impact invasive behavior. Proc Natl Acad Sci USA. 2013;110:18507–12.
Kawai T, Yasuchika K, Ishii T, Katayama H, Yoshitoshi EY, Ogiso S, et al. Keratin 19, a cancer stem cell marker in human hepatocellular carcinoma. Clin Cancer Res. 2015;21:3081–91.
Reinholz MM, Kitzmann KA, Tenner K, Hillman D, Dueck AC, Hobday TJ, et al. Cytokeratin-19 and mammaglobin gene expression in circulating tumor cells from metastatic breast cancer patients enrolled in North Central Cancer Treatment Group trials, N0234/336/436/437. Clin Cancer Res. 2011;17:7183–93.
Wang GY, Wang J, Mancianti ML, Epstein EH Jr. Basal cell carcinomas arise from hair follicle stem cells in Ptch1( + /-) mice. Cancer Cell. 2011;19:114–24.
Bu W, Chen J, Morrison GD, Huang S, Creighton CJ, Huang J, et al. Keratin 6a marks mammary bipotential progenitor cells that can give rise to a unique tumor model resembling human normal-like breast cancer. Oncogene. 2011;30:4399–409.
Fortier AM, Asselin E, Cadrin M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin1 up-regulation. J Biol Chem. 2013;288:11555–71.
Zaphiropoulos PG. Trans-splicing in higher eukaryotes: implications for cancer development? Front Genet. 2011;2:92.
Hsu DM, Agarwal S, Benham A, Coarfa C, Trahan DN, Chen Z, et al. G-CSF receptor positive neuroblastoma subpopulations are enriched in chemotherapy-resistant or relapsed tumors and are highly tumorigenic. Cancer Res. 2013;73:4134–46.
Agarwal S, Lakoma A, Chen Z, Hicks J, Metelitsa LS, Kim ES, et al. G-CSF promotes neuroblastoma tumorigenicity and metastasis via STAT3-dependent cancer stem cell activation. Cancer Res. 2015;75:2566–79.
Fuxe J, Vincent T, Garcia de Herreros A. Transcriptional crosstalk between TGF-beta and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363–74.
Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51.
Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J Vis Exp. 2011;51:1–4.
de Silva Rudland S, Platt-Higgins A, Winstanley JH, Jones NJ, Barraclough R, West C, et al. Statistical association of basal cell keratins with metastasis-inducing proteins in a prognostically unfavorable group of sporadic breast cancers. Am J Pathol. 2011;179:1061–72.
Chen J, Cheng X, Merched-Sauvage M, Caulin C, Roop DR, Koch PJ. An unexpected role for keratin 10 end domains in susceptibility to skin cancer. J Cell Sci. 2006;119:5067–76.
Sawant MS, Leube RE. Consequences of keratin phosphorylation for cytoskeletal organization and epithelial functions. Int Rev Cell Mol Biol. 2017;330:171–225.
Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362–5.
Snider NT, Omary MB. Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol. 2014;15:163–77.
Beil M, Micoulet A, von Wichert G, Paschke S, Walther P, Omary MB, et al. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nat Cell Biol. 2003;5:803–11.
Feng X, Coulombe PA. A role for disulfide bonding in keratin intermediate filament organization and dynamics in skin keratinocytes. J Cell Biol. 2015;209:59–72.
Feng X, Coulombe PA. Complementary roles of specific cysteines in keratin 14 toward the assembly, organization, and dynamics of intermediate filaments in skin keratinocytes. J Biol Chem. 2015;290:22507–19.
van Helvert S, Storm C, Friedl P. Mechanoreciprocity in cell migration. Nat Cell Biol. 2018;20:8–20.
McGregor AL, Hsia CR, Lammerding J. Squish and squeeze-the nucleus as a physical barrier during migration in confined environments. Curr Opin Cell Biol. 2016;40:32–40.
Iwaya K, Mukai K. Accumulation of ubiquitin-conjugated cytokeratin fragments in tumor cells. Semin Cancer Biol. 2005;15:309–18.
Sugimoto K, Ideguchi M, Kimura T, Kajiwara K, Imoto H, Sadahiro H, et al. Epithelioid/rhabdoid glioblastoma: a highly aggressive subtype of glioblastoma. Brain Tumor Pathol. 2016;33:137–46.
Ohmoto T, Yoshitani N, Nishitsuji K, Takayama T, Yanagisawa Y, Takeya M, et al. CD44-expressing undifferentiated carcinoma with rhabdoid features of the pancreas: molecular analysis of aggressive invasion and metastasis. Pathol Int. 2015;65:264–70.
Przybycin CG, McKenney JK, Reynolds JP, Campbell S, Zhou M, Karafa MT, et al. Rhabdoid differentiation is associated with aggressive behavior in renal cell carcinoma: a clinicopathologic analysis of 76 cases with clinical follow-up. Am J Surg Pathol. 2014;38:1260–5.
Kuroiwa K, Kinoshita Y, Shiratsuchi H, Oshiro Y, Tamiya S, Oda Y, et al. Renal cell carcinoma with rhabdoid features: an aggressive neoplasm. Histopathology. 2002;41:538–48.
Gemenetzidis E, Gammon L, Biddle A, Emich H, Mackenzie IC. Invasive oral cancer stem cells display resistance to ionising radiation. Oncotarget. 2015;6:43964–77.
Baillie R, Tan ST, Itinteang T. Cancer stem cells in oral cavity squamous cell carcinoma: a review. Front Oncol. 2017;7:112.
Yoshihara K, Wang Q, Torres-Garcia W, Zheng S, Vegesna R, Kim H, et al. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene. 2015;34:4845–54.
Guo T, Gaykalova DA, Considine M, Wheelan S, Pallavajjala A, Bishop JA, et al. Characterization of functionally active gene fusions in human papillomavirus related oropharyngeal squamous cell carcinoma. Int J Cancer. 2016;139:373–82.
Sheu JJ, Hua CH, Wan L, Lin YJ, Lai MT, Tseng HC, et al. Functional genomic analysis identified epidermal growth factor receptor activation as the most common genetic event in oral squamous cell carcinoma. Cancer Res. 2009;69:2568–76.
Kang NY, Yun SW, Ha HH, Park SJ, Chang YT. Embryonic and induced pluripotent stem cell staining and sorting with the live-cell fluorescence imaging probe CDy1. Nat Protoc. 2011;6:1044–52.
Sheu JJ, Lee CC, Hua CH, Li CI, Lai MT, Lee SC, et al. LRIG1 modulates aggressiveness of head and neck cancers by regulating EGFR-MAPK-SPHK1 signaling and extracellular matrix remodeling. Oncogene. 2014;33:1375–84.
Acknowledgements
The authors thank technical assistance from Ms. Lesley Lin at China Medical University Hospital and Mr. Ming-Der Pong at National Sun Yatsen University. The authors also thank critical comments from Prof. Mien-Chie Hung at MD Anderson Cancer Center/U.S., Prof. Chung Chang at National Sun Yatsen University/Taiwan, Prof. Ming-Jer Tang at National Cheng Kung University/Taiwan, and Prof. Ka-Lok Ng at Asia University/Taiwan. This study was supported by grants from Ministry of Science and Technology (MOST), Taiwan with numbers: NSC-102-2628-B-039-001-MY3, MOST 103-2314-B-039-009-MY3, and MOST 106-2314-B-110-001-MY3, and the NSYSU-KMU joint research projects: 107-P012 and 105-P021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsai, FJ., Lai, MT., Cheng, J. et al. Novel K6-K14 keratin fusion enhances cancer stemness and aggressiveness in oral squamous cell carcinoma. Oncogene 38, 5113–5126 (2019). https://doi.org/10.1038/s41388-019-0781-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0781-y
This article is cited by
-
MicroRNA-485-5p targets keratin 17 to regulate oral cancer stemness and chemoresistance via the integrin/FAK/Src/ERK/β-catenin pathway
Journal of Biomedical Science (2022)
-
Susceptibility of cytoskeletal-associated proteins for tumor progression
Cellular and Molecular Life Sciences (2022)
-
High expression of keratin 6C is associated with poor prognosis and accelerates cancer proliferation and migration by modulating epithelial–mesenchymal transition in lung adenocarcinoma
Genes & Genomics (2020)