Abstract
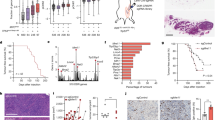
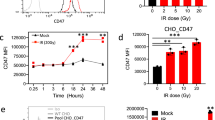
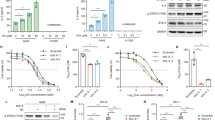
Programmed death-ligand 1 (PD-L1) is a key factor influencing cancer immunotherapy; however, the regulation of PD-L1 expression in cancer cells remains unclear, particularly regarding DNA damage, repair and its signalling. Herein, we demonstrate that oxidative DNA damage induced by exogenously applied hydrogen peroxide (H2O2) upregulates PD-L1 expression in cancer cells. Further, depletion of the base excision repair (BER) enzyme DNA glycosylase augments PD-L1 upregulation in response to H2O2. PD-L1 upregulation in BER-depleted cells requires ATR/Chk1 kinase activities, demonstrating that PD-L1 upregulation is mediated by DNA damage signalling. Further analysis of The Cancer Genome Atlas revealed that the expression of PD-L1 is negatively correlated with that of the BER/single-strand break repair (SSBR) and tumours with low BER/SSBR gene expression show high microsatellite instability and neoantigen production. Hence, these results suggest that PD-L1 expression is regulated in cancer cells via the DNA damage signalling and neoantigen–interferon-γ pathway under oxidative stress.
Highlights
-
Exogenous oxidative DNA damage upregulates PD-L1 expression in cancer cells.
-
BER deficiency augments PD-L1 upregulation following oxidative DNA damage.
-
Tumour samples with BER/SSBR mutations show high microsatellite instability, neoantigen and PD-L1 expression.
-
PD-L1 and BER/SSBR gene expressions are negatively correlated in clinical specimens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All relevant data are available from the corresponding author(s) upon reasonable request.
References
Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39.
Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24:26.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39.
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65.
Roger A, Finet A, Boru B, Beauchet A, Mazeron JJ, Otzmeguine Y. et al. Efficacy of combined hypo-fractionated radiotherapy and anti-PD-1 monotherapy in difficult-to-treat advanced melanoma patients. Oncoimmunology. 2018;7:e1442166
Qin Q, Nan X, Miller T, Fisher R, Teh B, Pandita S. et al. Complete local and abscopal responses from a combination of radiation and nivolumab in refractory Hodgkinas lymphoma. Radiat Res. 2018;190:322–29.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29.
Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol. 2015;23:32–38.
Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–801.
Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–58.
Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7:188–201.
Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19:1189–201.
Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13.
Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–23.
Shen J, Ju Z, Zhao W, Wang L, Peng Y, Ge Z, et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med. 2018;24:556–62.
Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:13587–98.
Chen MF, Chen PT, Chen WC, Lu MS, Lin PY, Lee KD. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget. 2016;7:7913–24.
Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwara Y, Isono M, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017;8:1751.
Mouw KW, Konstantinopoulos PA. From checkpoint to checkpoint: DNA damage ATR/Chk1 checkpoint signalling elicits PD-L1 immune checkpoint activation. Br J Cancer. 2018;118:933–5.
Vendetti FP, Karukonda P, Clump DA, Teo T, Lalonde R, Nugent K. et al. ATR kinase inhibitor AZD6738 potentiates CD8.T cell-dependent antitumor activity following radiation. J Clin Invest. 2018;128:3926–40.
Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–8.
Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Fiaschi T, Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol. 2012;2012:762825.
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked?. Free Radic Biol Med. 2010;49:1603–16.
Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–85.
Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10.
Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet. 2008;9:619–31.
Chen BP, Li M, Asaithamby A. New insights into the roles of ATM and DNA-PKcs in the cellular response to oxidative stress. Cancer Lett. 2012;327:103–10.
Wallace SS, Murphy DL, Sweasy JB. Base excision repair and cancer. Cancer Lett. 2012;327:73–89.
Karahalil B, Bohr VA, Wilson DM 3rd. Impact of DNA polymorphisms in key DNA base excision repair proteins on cancer risk. Hum Exp Toxicol. 2012;31:981–1005.
O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60:547–60.
Hutter C, Zenklusen JC. The Cancer Genome Atlas: creating lasting value beyond its data. Cell. 2018;173:283–5.
Tubbs A, Nussenzweig A, Endogenous DNA. Damage as a source of genomic instability in cancer. Cell. 2017;168:644–56.
Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–15.
Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–96.
Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–6.
Umar A, Boyer JC, Thomas DC, Nguyen DC, Risinger JI, Boyd J, et al. Defective mismatch repair in extracts of colorectal and endometrial cancer cell lines exhibiting microsatellite instability. J Biol Chem. 1994;269:14367–70.
Aquilina G, Hess P, Branch P, MacGeoch C, Casciano I, Karran P, et al. A mismatch recognition defect in colon carcinoma confers DNA microsatellite instability and a mutator phenotype. Proc Natl Acad Sci USA. 1994;91:8905–9.
Fishel R, Ewel A, Lee S, Lescoe MK, Griffith J. Binding of mismatched microsatellite DNA sequences by the human MSH2. Protein Sci. 1994;266:1403–5.
Klein HL, Kreuzer KN. Replication, recombination, and repair: going for the gold. Mol Cell. 2002;9:471–80.
Ensminger M, Iloff L, Ebel C, Nikolova T, Kaina B, Lbrich M. DNA breaks and chromosomal aberrations arise when replication meets base excision repair. J Cell Biol. 2014;206:29–43.
Feng W, Jasin M. BRCA2 suppresses replication stress-induced mitotic and G1 abnormalities through homologous recombination. Nat Commun. 2017;8:525.
Shibata A. Regulation of repair pathway choice at two-ended DNA double-strand breaks. Mutat Res. 2017;803–5:51–55.
Weren RD, Ligtenberg MJ, Kets CM, de Voer RM, Verwiel ET, Spruijt L, et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat Genet. 2015;47:668–71.
Shibata A, Moiani D, Arvai AS, Perry J, Harding SM, Genois MM, et al. DNA double-strand break repair pathway choice is directed by distinct MRE11 nuclease activities. Mol Cell. 2014;53:7–18.
McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9.
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77:e108–e110.
Cortes-Ciriano I, Lee S, Park WY, Kim TM, Park PJ. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun. 2017;8:15180.
Acknowledgements
We thank Yoshimi Omi, Akiko Shibata, Yoko Hayashi, Shiho Nakanishi, Yukihiko Yoshimatsu and Yuka Hirota for assisting with the lab work.
Funding
This work was supported by the Program of the network-type Joint Usage/Research Center for Radiation Disaster Medical Science of Hiroshima University, Nagasaki University and Fukushima Medical University. This work was also supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan for programs for Leading Graduate Schools, Cultivating Global Leaders in Heavy Ion Therapeutics and Engineering.
Author contributions
A.S. designed the experiments and wrote the paper with T.B.M.P. The experiments including immunoblotting, qPCR and flow cytometry were performed by T.B.M.P., H.S., Y.H. and A.S. The dataset of TCGA was developed by T.Y. The TCGA analysis was performed by T.B.M.P. and Y.H. under the supervision of T.Y. Acquired data was analysed and interpreted by T.B.M.P., H.S., T.Y. and A.S. The manuscript was reviewed by T.O., K.D.H. and T.N. Administrative, technical or material support was provided by T.O., S. G. and T.N. The study was supervised by A.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Permata, T.B.M., Hagiwara, Y., Sato, H. et al. Base excision repair regulates PD-L1 expression in cancer cells. Oncogene 38, 4452–4466 (2019). https://doi.org/10.1038/s41388-019-0733-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0733-6
This article is cited by
-
Targeting the NF-κB pathway as a potential regulator of immune checkpoints in cancer immunotherapy
Cellular and Molecular Life Sciences (2024)
-
Cholesterol removal improves performance of a model biomimetic system to co-deliver a photothermal agent and a STING agonist for cancer immunotherapy
Nature Communications (2023)
-
Crosstalk between immune checkpoint and DNA damage response inhibitors for radiosensitization of tumors
Strahlentherapie und Onkologie (2023)
-
DNA damage repair and cancer immunotherapy
Genome Instability & Disease (2023)
-
The role of DNA damage repair (DDR) system in response to immune checkpoint inhibitor (ICI) therapy
Journal of Experimental & Clinical Cancer Research (2022)