Abstract
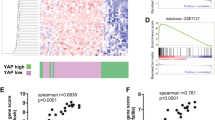
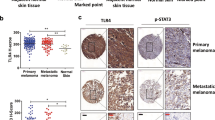
Due to its high proclivity to metastasize, and despite the recent development of targeted and immune therapy strategies, melanoma is still the deadliest form of skin cancer. Therefore, understanding the molecular mechanisms underlying melanoma invasion remains crucial. We previously characterized Tspan8 for its ability to prompt melanoma cell detachment from their microenvironment and trigger melanoma cell invasiveness, but the signaling events by which Tspan8 regulates the invasion process still remain unknown. Here, we demonstrated that β-catenin stabilization is a molecular signal subsequent to the onset of Tspan8 expression, and that, in turn, β-catenin triggers the direct transcriptional activation of Tspan8 expression, leading to melanoma invasion. Moreover, we showed that β-catenin activation systematically correlates with a high expression of Tspan8 protein in melanoma lesions from transgenic Nras; bcat* mice, as well as in deep penetrating naevi, a type of human pre-melanoma neoplasm characterized by a combined activation of β-catenin and MAP kinase signaling. Overall, our data suggest that β-catenin and Tspan8 are part of a positive feedback loop, which sustains a high Tspan8 expression level, conferring to melanoma cells the invasive properties required for tumor progression and dissemination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Shain AH, Bastian BC. The genetic evolution of melanoma. N Engl J Med. 2016;374:995–6.
Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14:463–82.
Szala S, Froehlich M, Scollon M, Kasai Y, Steplewski Z, Koprowski H, et al. Molecular cloning of cDNA for the carcinoma-associated antigen GA733-2. Proc Natl Acad Sci USA. 1990;87:3542–6.
Hemler ME. Tetraspanin proteins promote multiple cancer stages. Nat Rev Cancer. 2014;14:49–60.
Zoller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9:40–55.
Pan SJ, Wu YB, Cai S, Pan YX, Liu W, Bian LG, et al. Over-expression of tetraspanin 8 in malignant glioma regulates tumor cell progression. Biochem Biophys Res Commun. 2015;458:476–82.
Kanetaka K, Sakamoto M, Yamamoto Y, Yamasaki S, Lanza F, Kanematsu T, et al. Overexpression of tetraspanin CO-029 in hepatocellular carcinoma. J Hepatol. 2001;35:637–42.
Bhansali M, Zhou J, Shemshedini L. TM4SF3 and AR: a nuclear complex that stabilizes both proteins. Mol Endocrinol. 2016;30:13–25.
Zhou Z, Ran YL, Hu H, Pan J, Li ZF, Chen LZ, et al. TM4SF3 promotes esophageal carcinoma metastasis via upregulating ADAM12m expression. Clin Exp Metastas-. 2008;25:537–48.
Greco C, Bralet MP, Ailane N, Dubart-Kupperschmitt A, Rubinstein E, Le Naour F, et al. E-cadherin/p120-catenin and tetraspanin Co-029 cooperate for cell motility control in human colon carcinoma. Cancer Res. 2010;70:7674–83.
Guo Q, Xia B, Zhang F, Richardson MM, Li M, Zhang JS, et al. Tetraspanin CO-029 inhibits colorectal cancer cell movement by deregulating cell-matrix and cell-cell adhesions. PLoS One. 2012;7:e38464.
Herlevsen M, Schmidt DS, Miyazaki K, Zoller M. The association of the tetraspanin D6.1A with the alpha6beta4 integrin supports cell motility and liver metastasis formation. J Cell Sci. 2003;116:4373–90.
Berthier-Vergnes O, El Kharbili M, de la Fouchardiere A, Pointecouteau T, Verrando P, Wierinckx A, et al. Gene expression profiles of human melanoma cells with different invasive potential reveal TSPAN8 as a novel mediator of invasion. Br J Cancer. 2011;104:155–65.
Gesierich S, Paret C, Hildebrand D, Weitz J, Zgraggen K, Schmitz-Winnenthal FH, et al. Colocalization of the tetraspanins, CO-029 and CD151, with integrins in human pancreatic adenocarcinoma: impact on cell motility. Clin Cancer Res. 2005;11:2840–52.
Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–78.
Yue S, Mu W, Erb U, Zoller M. The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget. 2015;6:2366–84.
Claas C, Seiter S, Claas A, Savelyeva L, Schwab M, Zoller M. Association between the rat homologue of CO-029, a metastasis-associated tetraspanin molecule and consumption coagulopathy. J Cell Biol. 1998;141:267–80.
Kanetaka K, Sakamoto M, Yamamoto Y, Takamura M, Kanematsu T, Hirohashi S. Possible involvement of tetraspanin CO-029 in hematogenous intrahepatic metastasis of liver cancer cells. J Gastroenterol Hepatol. 2003;18:1309–14.
Gesierich S, Berezovskiy I, Ryschich E, Zoller M. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res. 2006;66:7083–94.
Ailane N, Greco C, Zhu Y, Sala-Valdes M, Billard M, Casal I, et al. Effect of an anti-human Co-029/tspan8 mouse monoclonal antibody on tumor growth in a nude mouse model. Front Physiol. 2014;5:364.
Park CS, Kim TK, Kim HG, Kim YJ, Jeoung MH, Lee WR, et al. Therapeutic targeting of tetraspanin8 in epithelial ovarian cancer invasion and metastasis. Oncogene. 2016;35:4540–8.
Maisonial-Besset A, Witkowski T, Navarro-Teulon I, Berthier-Vergnes O, Fois G, Zhu Y, et al. Tetraspanin 8 (TSPAN 8) as a potential target for radio-immunotherapy of colorectal cancer. Oncotarget. 2017;8:22034–47.
Rodia MT, Ugolini G, Mattei G, Montroni I, Zattoni D, Ghignone F, et al. Systematic large-scale meta-analysis identifies a panel of two mRNAs as blood biomarkers for colorectal cancer detection. Oncotarget. 2016;7:30295–306.
Agaesse G, Barbollat-Boutrand L, Sulpice E, Bhajun R, El Kharbili M, Berthier-Vergnes O, et al. A large-scale RNAi screen identifies LCMR1 as a critical regulator of Tspan8-mediated melanoma invasion. Oncogene. 2017;36:446–57.
Agaesse G, Barbollat-Boutrand L, El Kharbili M, Berthier-Vergnes O, Masse I. p53 targets TSPAN8 to prevent invasion in melanoma cells. Oncogenesis. 2017;6:e309.
El Kharbili M, Robert C, Witkowski T, Danty-Berger E, Barbollat-Boutrand L, Masse I, et al. Tetraspanin 8 is a novel regulator of ILK-driven beta1 integrin adhesion and signaling in invasive melanoma cells. Oncotarget. 2017;8:17140–55.
Delmas V, Beermann F, Martinozzi S, Carreira S, Ackermann J, Kumasaka M, et al. Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 2007;21:2923–35.
Gallagher SJ, Rambow F, Kumasaka M, Champeval D, Bellacosa A, Delmas V, et al. Beta-catenin inhibits melanocyte migration but induces melanoma metastasis. Oncogene. 2013;32:2230–8.
Yeh I, Lang UE, Durieux E, Tee MK, Jorapur A, Shain AH, et al. Combined activation of MAP kinase pathway and beta-catenin signaling cause deep penetrating nevi. Nat Commun. 2017;8:644.
Abe M, Sugiura T, Takahashi M, Ishii K, Shimoda M, Shirasuna K. A novel function of CD82/KAI-1 on E-cadherin-mediated homophilic cellular adhesion of cancer cells. Cancer Lett. 2008;266:163–70.
Seubert B, Cui H, Simonavicius N, Honert K, Schafer S, Reuning U, et al. Tetraspanin CD63 acts as a pro-metastatic factor via beta-catenin stabilization. Int J Cancer. 2015;136:2304–15.
Li L, Yang D, Cui D, Li Y, Nie Z, Wang J, et al. Quantitative proteomics analysis of the role of tetraspanin-8 in the drug resistance of gastric cancer. Int J Oncol. 2018;52:473–84.
Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, et al. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci USA. 2009;106:1193–8.
Arozarena I, Bischof H, Gilby D, Belloni B, Dummer R, Wellbrock C. In melanoma, beta-catenin is a suppressor of invasion. Oncogene. 2011;30:4531–43.
Kageshita T, Hamby CV, Ishihara T, Matsumoto K, Saida T, Ono T. Loss of beta-catenin expression associated with disease progression in malignant melanoma. Br J Dermatol. 2001;145:210–6.
Maelandsmo GM, Holm R, Nesland JM, Fodstad O, Florenes VA. Reduced beta-catenin expression in the cytoplasm of advanced-stage superficial spreading malignant melanoma. Clin Cancer Res. 2003;9:3383–8.
Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA. Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res. 2005;11:8606–14.
Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–2.
Larue L, Delmas V. The WNT/Beta-catenin pathway in melanoma. Front Biosci. 2006;11:733–42.
Conde-Perez A, Gros G, Longvert C, Pedersen M, Petit V, Aktary Z, et al. A caveolin-dependent and PI3K/AKT-independent role of PTEN in beta-catenin transcriptional activity. Nat Commun. 2015;6:8093.
Eichhoff OM, Weeraratna A, Zipser MC, Denat L, Widmer DS, Xu M, et al. Differential LEF1 and TCF4 expression is involved in melanoma cell phenotype switching. Pigment Cell Melanoma Res. 2011;24:631–42.
Murakami T, Toda S, Fujimoto M, Ohtsuki M, Byers HR, Etoh T, et al. Constitutive activation of Wnt/beta-catenin signaling pathway in migration-active melanoma cells: role of LEF-1 in melanoma with increased metastatic potential. Biochem Biophys Res Commun. 2001;288:8–15.
Sinnberg T, Menzel M, Ewerth D, Sauer B, Schwarz M, Schaller M, et al. beta-Catenin signaling increases during melanoma progression and promotes tumor cell survival and chemoresistance. PLoS One. 2011;6:e23429.
Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE, et al. beta-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell. 2011;20:741–54.
Grossmann AH, Yoo JH, Clancy J, Sorensen LK, Sedgwick A, Tong Z, et al. The small GTPase ARF6 stimulates beta-catenin transcriptional activity during WNT5A-mediated melanoma invasion and metastasis. Sci Signal. 2013;6:ra14.
Aktary Z, Bertrand JU, Larue L. The WNT-less wonder: WNT-independent beta-catenin signaling. Pigment Cell Melanoma Res. 2016;29:524–40.
Pan SJ, Zhan SK, Pan YX, Liu W, Bian LG, Sun B, et al. Tetraspanin 8-rictor-integrin alpha3 complex is required for glioma cell migration. Int J Mol Sci. 2015;16:5363–74.
Cavard C, Terris B, Grimber G, Christa L, Audard V, Radenen-Bussiere B, et al. Overexpression of regenerating islet-derived 1 alpha and 3 alpha genes in human primary liver tumors with beta-catenin mutations. Oncogene. 2006;25:599–608.
Le Naour F, Andre M, Greco C, Billard M, Sordat B, Emile JF, et al. Profiling of the tetraspanin web of human colon cancer cells. Mol Cell Proteom. 2006;5:845–57.
Masse I, Barbollat-Boutrand L, Molina M, Berthier-Vergnes O, Joly-Tonetti N, Martin MT, et al. Functional interplay between p63 and p53 controls RUNX1 function in the transition from proliferation to differentiation in human keratinocytes. Cell Death Dis. 2012;3:e318.
Delmas V, Martinozzi S, Bourgeois Y, Holzenberger M, Larue L. Cre-mediated recombination in the skin melanocyte lineage. Genesis. 2003;36:73–80.
Acknowledgements
We thank Elise Malandain for technical assistance and Cyril Py for immunohistochemical processing. We thank C. Boucheix for the gift of TS29 monoclonal antibody.
Author contributions
IM and OBV conceived the idea and designed research. MEL, GA, LBB, OBV and IM performed experimental research. MEL, GA and LBB analyzed data under the supervision of IM and OBV. RMP performed bioinformatics analysis. ADLF provided tumor specimens and performed immunohistochemistry analyzes. LL performed mouse experiments. MEL, GA, JC and AP were involved in critical revision of the manuscript. IM and OBV prepared figures and wrote the paper.
Funding
This work was supported by a grant from the French Society for Dermatological Research (SRD), a grant from La Ligue Contre le Cancer (Comité Ardèche) and a grant from the association “Vaincre le Mélanome”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
El Kharbili, M., Agaësse, G., Barbollat-Boutrand, L. et al. Tspan8-β-catenin positive feedback loop promotes melanoma invasion. Oncogene 38, 3781–3793 (2019). https://doi.org/10.1038/s41388-019-0691-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0691-z
This article is cited by
-
The versatile roles of testrapanins in cancer from intracellular signaling to cell–cell communication: cell membrane proteins without ligands
Cell & Bioscience (2023)
-
Tetraspanin Tspan8 restrains interferon signaling to stabilize intestinal epithelium by directing endocytosis of interferon receptor
Cellular and Molecular Life Sciences (2023)
-
TSPAN8 regulates EGFR/AKT pathway to enhance metastasis in gastric cancer
Molecular Biology Reports (2023)
-
Tetraspanin8 expression predicts an increased metastatic risk and is associated with cancer-related death in human cutaneous melanoma
Molecular Cancer (2021)
-
SOX9 is a critical regulator of TSPAN8-mediated metastasis in pancreatic cancer
Oncogene (2021)