Abstract
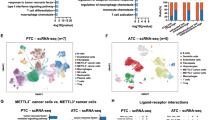
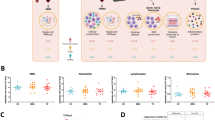
PTEN hamartoma tumor syndrome (PHTS) is caused by inactivating germline PTEN mutations with subsequent activation of Akt-mTOR signaling, leading to an increased risk of developing thyroid carcinoma (TC). Activation of Akt-mTOR signaling is essential for innate immune cell activation and reprogramming of TC-induced macrophages. Here, we aim to assess the effect of PTEN mutations on innate immune cell function in PHTS patients, especially in the context of TC, using a unique ex vivo model. Monocyte-derived cytokine responses were assessed in 29 PHTS patients and 29 controls. To assess the functional profile of TC-induced-macrophages, a co-culture model with two TC cell lines was performed. Rapamycin, a lactate transport blocker and metformin were used as modulators of the Akt-mTOR pathway and cell metabolism. Monocytes from PHTS patients showed increased production of IL-6, TNF-α, IL-8 and MCP-1, and higher lactate production. After co-culture with TC cell lines, TC-induced macrophages showed significantly increased production of cytokines in both patients and controls, especially after co-culture with a PTEN-deficient TC cell line; these effects were abolished after use of rapamycin or a lactate transport blocker. Metformin blocked the production of anti-inflammatory cytokines. In conclusion, innate immune cells from PHTS patients have increased lactate production and a more proinflammatory phenotype, especially after co-culture with PTEN-deficient TC. Metformin promotes a proinflammatory phenotype by blocking anti-inflammatory cytokine response, whereas rapamycin reduces production of proinflammatory cytokines. This indicates that PHTS patients may benefit from treatment with mTOR blocking agents to limit the inflammatory response in the tumor microenvironment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nieuwenhuis MH, Kets CM, Murphy-Ryan M, Yntema HG, Evans DG, Colas C, et al. Cancer risk and genotype-phenotype correlations in PTEN hamartoma tumor syndrome. Fam Cancer. 2014;13:57–63.
Pilarski R, Burt R, Kohlman W, Pho L, Shannon KM, Swisher E. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607–16.
Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39.
Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–44.
Newton RH, Turka LA. Regulation of T cell homeostasis and responses by pten. Front Immunol. 2012;3:151.
Amer M, Mostafa FF, Attwa EM, Ibrahim S. Cowden’s syndrome: a clinical, immunological, and histopathological study. Int J Dermatol. 2011;50:516–21.
Chen HH, Handel N, Ngeow J, Muller J, Huhn M, Yang HT. et al. Immune dysregulation in patients with PTEN hamartoma tumor syndrome: Analysis of FOXP3 regulatory T cells. The Journal of Allergy and Clinical Immunology. 2016;139:607–20.
Heindl M, Handel N, Ngeow J, Kionke J, Wittekind C, Kamprad M, et al. Autoimmunity, intestinal lymphoid hyperplasia, and defects in mucosal B-cell homeostasis in patients with PTEN hamartoma tumor syndrome. Gastroenterology. 2012;142:1093–6.e6.
Xie S, Chen M, Yan B, He X, Chen X, Li D. Identification of a role for the PI3K/AKT/mTOR signaling pathway in innate immune cells. PLoS ONE. 2014;9:e94496.
Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684.
Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–32.
Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 2016;17:2562–71.
Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. 2012;109:17537–42.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61.
Arts RJW, Plantinga TS, Tuit S, Ulas T, Heinhuis B, Tesselaar M. et al. Transcriptional and metabolic reprogramming induce an inflammatory phenotype in non-medullary thyroid carcinoma-induced macrophages. OncoImmunology. 2016;5:e122972500
Caillou B, Talbot M, Weyemi U, Pioche-Durieu C, Al Ghuzlan A, Bidart JM, et al. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS ONE. 2011;6:e22567.
Ryder M, Gild M, Hohl TM, Pamer E, Knauf J, Ghossein R, et al. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLoS ONE. 2013;8:e54302.
Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63.
Xu J, Ji J, Yan XH. Cross-talk between AMPK and mTOR in regulating energy balance. Crit Rev Food Sci Nutr. 2012;52:373–81.
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74.
Bridges HR, Jones AJ, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J. 2014;462:475–87.
Han B, Cui H, Kang L, Zhang X, Jin Z, Lu L, et al. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the mTOR pathway. Tumour Biol. 2015;36:6295–304.
Jang EK, Kim WG, Kwon H, Choi YM, Jeon MJ, Kim TY, et al. Metformin is associated with a favorable outcome in diabetic patients with cervical lymph node metastasis of differentiated thyroid cancer. Eur Thyroid J. 2015;4:181–8.
Klubo-Gwiezdzinska J, Costello J Jr., Patel A, Bauer A, Jensen K, Mete M, et al. Treatment with metformin is associated with higher remission rate in diabetic patients with thyroid cancer. J Clin Endocrinol Metab. 2013;98:3269–79.
Phadngam S, Castiglioni A, Ferraresi A, Morani F, Follo C, Isidoro C. PTEN dephosphorylates AKT to prevent the expression of GLUT1 on plasmamembrane and to limit glucose consumption in cancer cells. Oncotarget. 2016;7:84999–5020.
Morani F, Phadngam S, Follo C, Titone R, Aimaretti G, Galetto A, et al. PTEN regulates plasma membrane expression of glucose transporter 1 and glucose uptake in thyroid cancer cells. J Mol Endocrinol. 2014;53:247–58.
Fu Y, Maianu L, Melbert BR, Garvey WT. Facilitative glucose transporter gene expression in human lymphocytes, monocytes, and macrophages: a role for GLUT isoforms 1, 3, and 5 in the immune response and foam cell formation. Blood Cells, Mol & Dis. 2004;32:182–90.
Aguirre-Gamboa R, Joosten I, Urbano PCM, van der Molen RG, van Rijssen E, van Cranenbroek B, et al. Differential Effects of Environmental and Genetic Factors on T and B Cell Immune Traits. Cell Rep. 2016;17:2474–87.
Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–22.
Sarrazy V, Viaud M, Westerterp M, Ivanov S, Giorgetti-Peraldi S, Guinamard R, et al. Disruption of glut1 in hematopoietic stem cells prevents myelopoiesis and enhanced glucose flux in atheromatous plaques of ApoE(-/-) mice. Circ Res. 2016;118:1062–77.
Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell. 2018;172:147–61.e12.
Guerin V, Bene MC, Judlin P, Beurey J, Landes P, Faure G. Cowden disease in a young girl: gynecologic and immunologic overview in a case and in the literature. Obstet Gynecol. 1989;73(5 Pt 2):890–2.
Saccardi A, Bacci S, Romagnoli P, Ravina A, Ficarra G. Cowden’s syndrome: a case report with clinical, histopathologic and immunological studies. Minerva Stomatol. 1994;43:417–22.
Starink TM, van der Veen JP, Arwert F, de Waal LP, de Lange GG, Gille JJ, et al. The Cowden syndrome: a clinical and genetic study in 21 patients. Clin Genet. 1986;29:222–33.
Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–32.
Molnarfi N, Gruaz L, Dayer JM, Burger D. Opposite regulation of IL-1beta and secreted IL-1 receptor antagonist production by phosphatidylinositide-3 kinases in human monocytes activated by lipopolysaccharides or contact with T cells. J Immunol (Baltim, Md: 1950). 2007;178:446–54.
Hou P, Liu D, Shan Y, Hu S, Studeman K, Condouris S, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13:1161–70.
Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51.
Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6:202–16.
Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490:412–6.
Mehta A, Kim YJ, Robert L, Tsoi J, Comin-Anduix B, Berent-Maoz B, et al. Immunotherapy Resistance by Inflammation-Induced Dedifferentiation. Cancer Discov. 2018;8:935–43.
Kozicky LK, Sly LM. Phosphatase regulation of macrophage activation. Semin Immunol. 2015;27:276–85.
Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098.
Netea MG, Joosten LAB, van der Meer JWM. Hypothesis: stimulation of trained immunity as adjunctive immunotherapy in cancer. J Leukoc Biol. 2017;102:1323–32.
Bekkering S, Blok BA, Joosten LA, Riksen NP, van Crevel R, Netea MG. In vitro experimental model of trained innate immunity in human primary monocytes. Clin Vaccin Immunol: Cvi. 2016;23:926–33.
Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–41.
Acknowledgements
This work was supported by a grant from the Netherlands Cancer Foundation (KWF) [grant number #10559, 2017]. MGN was supported by a Spinoza grant of the Netherlands Organization for Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sloot, Y.J.E., Rabold, K., Netea, M.G. et al. Effect of PTEN inactivating germline mutations on innate immune cell function and thyroid cancer-induced macrophages in patients with PTEN hamartoma tumor syndrome. Oncogene 38, 3743–3755 (2019). https://doi.org/10.1038/s41388-019-0685-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0685-x
This article is cited by
-
PTEN hamartoma tumour syndrome: case report based on data from the Iranian hereditary colorectal cancer registry and literature review
Diagnostic Pathology (2023)
-
Emerging role of PTEN loss in evasion of the immune response to tumours
British Journal of Cancer (2020)
-
Interplay between thyroid cancer cells and macrophages: effects on IL-32 mediated cell death and thyroid cancer cell migration
Cellular Oncology (2019)