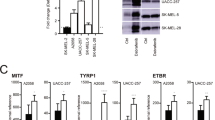
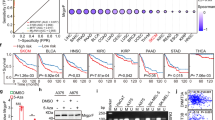
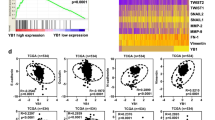
Abstract
Despite emergence of new systemic therapies, metastatic melanoma remains a challenging and often fatal form of skin cancer. The renin–angiotensin system (RAS) is a major physiological regulatory pathway controlling salt–water equilibrium, intravascular volume and blood pressure. Biological effects of the RAS are mediated by the vasoactive hormone angiotensin II (AngII) via two receptor subtypes, AT1R (encoded by AGTR1) and AT2R (encoded by AGTR2). We report decreasing expression and increasing CpG island methylation of AGTR1 in metastatic versus primary melanoma and detection in serum of methylated genomic DNA from the AGTR1 CpG island in metastatic melanoma implying that AGTR1 encodes a tumour suppressor function in melanoma. Consistent with this hypothesis, antagonism of AT1R using losartan or shRNA-mediated knockdown in melanoma cell lines expressing AGTR1 resulted in acquisition of the ability to proliferate in serum-free conditions. Conversely, ectopic expression of AGTR1 in cell lines lacking endogenous expression inhibits proliferation irrespective of the presence of AngII implying a ligand-independent suppressor function for AT1R. Treatment of melanoma cell lines expressing endogenous AT2R with either AngII or the AT2R-selective agonist Y6AII induces proliferation in serum-free conditions whereas the AT2R-specific antagonists PD123319 and EMA401 inhibit melanoma growth and angiogenesis and potentiate inhibitors of BRAF and MEK in cells with BRAF V600 mutations. Our results demonstrate that the RAS has both oncogenic and tumour suppressor functions in melanoma. Pharmacological inhibition of AT2R may provide therapeutic opportunities in melanomas expressing this receptor and AGTR1 CpG island methylation in serum may serve as a novel biomarker of metastatic melanoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Flaherty KT. Next generation therapies change the landscape in melanoma. F1000 Med Rep. 2011;3:8.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2012;364:2507–16.
Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–14.
Sullivan RJ, Flaherty KT. New strategies in melanoma: entering the era of combinatorial therapy. Clin Cancer Res. 2015;21:2424–35.
Hanaizi Z, Van Zwieten-Boot B, Calvo G, Lopez AS, Van Dartel M, Camarero J, et al. The European Medicines Agency review of ipilimumab (Yervoy) for the treatment of advanced (unresectable or metastatic) melanoma in adults who have received prior therapy: summary of the scientific assessment of the Committee for Medicinal Products for Human Use. Eur J Cancer. 2012;48:237–42.
Homet Moreno B, Parisi G, Robert L, Ribas A. Anti-PD-1 therapy in melanoma. Semin Oncol. 2015;42:466–73.
Wolchok JD. PD-1 blockers. Cell. 2015;162:937.
Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, et al. Angiotensin receptors: Interpreters of pathophysiological angiotensinergic stimuli. Pharmacol Rev. 2015;67:754–819.
Rhodes DR, Ateeq B, Cao Q, Tomlins SA, Mehra R, Laxman B, et al. AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist. Proc Natl Acad Sci USA. 2009;106:10284–9.
Ateeq B, Tomlins SA, Chinnaiyan AM. AGTR1 as a therapeutic target in ER-positive and ERBB2-negative breast cancer cases. Cell Cycle. 2009;8:3794–5.
Chen X, Meng Q, Zhao Y, Liu M, Li D, Yang Y, et al. Angiotensin II type 1 receptor antagonists inhibit cell proliferation and angiogenesis in breast cancer. Cancer Lett. 2013;328:318–24.
Guo R, Gu J, Zhang Z, Wang Y, Gu C. MicroRNA-410 functions as a tumor suppressor by targeting angiotensin II type 1 receptor in pancreatic cancer. IUBMB Life. 2015;67:42–53.
Otake AH, Mattar AL, Freitas HC, Machado CM, Nonogaki S, Fujihara CK, et al. Inhibition of angiotensin II receptor 1 limits tumor-associated angiogenesis and attenuates growth of murine melanoma. Cancer Chemother Pharmacol. 2010;66:79–87.
Reis IM, Ramachandran K, Speer C, Gordian E, Singal R. Serum GADD45a methylation is a useful biomarker to distinguish benign vs malignant prostate disease. Br J Cancer. 2015;113:460–8.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2011;363:711–23.
Lo Nigro C, Wang H, McHugh A, Lattanzio L, Matin R, Harwood C, et al. Methylated tissue factor pathway inhibitor 2 (TFPI2) DNA in serum is a biomarker of metastatic melanoma. J Invest Dermatol. 2013;133:1278–85.
Hoshimoto S, Kuo CT, Chong KK, Takeshima TL, Takei Y, Li MW, et al. AIM1 and LINE-1 epigenetic aberrations in tumor and serum relate to melanoma progression and disease outcome. J Invest Dermatol. 2012;132:1689–97.
Hatzimichael E, Syed N, Lo Nigro C, Rao B, Crook T. How detection of epigenetic alterations of blood-borne DNA could improve melanoma diagnosis. Expert Rev Mol Diagn. 2014;14:639–42.
Brunner D, Appl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. ALTEX. 2010;27:53–62.
Magnani F, Pappas CG, Crook T, Magafa V, Cordopatis P, Ishiguro S, et al. Electronic sculpting of ligand-GPCR subtype selectivity: the case of angiotensin II. ACS Chem Biol. 2014;9:1420–5.
Anand U, Facer P, Yiangou Y, Sinisi M, Fox M, McCarthy T, et al. Angiotensin II type 2 receptor (AT2R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons. Eur J Pain. 2013;17:1012–26.
Beaumont KA, Mohana-Koumaran N, Hass NK. Modeling melanoma in vitro and in vivo. Healthcare. 2014;2:27–46.
Patton EE, Mitchell DL, Nairn RS. Genetic and environmental melanoma models in fish. Pigment Cell Melanoma Res. 2010;23:314–37.
Carpentier AF, Ferrari D, Bailon O, Ursu R, Banissi C, Dubessy AL, et al. Steroid-sparing effects of angiotensin-II inhibitors in glioblastoma patients. Eur J Neurol. 2012;19:1337–42.
Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506.
Miura S, Karnik SS, Saku K. Constitutively active homo-oligomeric angiotensin II type 2 receptor induces cell signalling independent of receptor conformation and ligand stimulation. J Biol Chem. 2005;280:18237–44.
Hesselink JMK, Schatman ME. EMA401: an old antagonist of the AT2R for a new indication in neuropathic pain. J Pain Res. 2017;10:439–43.
Campbell DJ. Endogenous angiotensin II levels and the mechanism of action of angiotensin-converting enzyme inhibitors and angiotensin receptor type 1 antagonists. Clin Exp Pharmacol Physiol. 1996;3(Suppl):S125–31.
Nussberger J1, Wuerzner G, Jensen C, Brunner HR. Angiotensin II suppression in humans by the orally active renin inhibitor aliskiren (SPP100): comparison with enalapril. Hypertension. 2002;39:E1–8.
Yoon C, Yang HS, Jeon I, Chang Y, Park SM. Use of angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers and cancer risk: a meta-analysis of observational studies. CMAJ. 2011;183:E1073–84.
Bar J, Ding K, Zhao H, Han L, Laurie SA, Seymour L. et al. Angiotensin-converting enzyme and aldosterone serum levels as prognostic and predictive biomarkers for cediranib in NCIC Clinical Trials Group Study BR.24. Clin Lung Cancer. 2015;16:e189–201.
Moreno-Muñoz D, de la Ha ba-Rodríguez JR, Conde F, López-Sánchez LM, Valverde A, Hernández V. et al. Genetic variants in the renin-angiotensin system predict response to bevacizumab in cancer patients. Eur J Clin Invest. 2015;45:1325–32.
Syed N, Smith P, Sullivan A, Spender LC, Dyer M, Karran L. et al. Transcriptional silencing of Polo-like kinase 2 (SNK/PLK2) is a frequent event in B-cell malignancies. Blood. 2006;107:250–6.
Shah R, Smith P, Purdie C, Quinlan P, Baker L, Aman P. et al. The prolyl 3-hydroxylases P3H2 and P3H3 are novel targets for epigenetic silencing in breast cancer. Br J Cancer. 2009;100:1687–96.
Corkery DP, Dellaire G, Berman JN. Leukaemia xenotransplantation in zebrafish—chemotherapy response assay in vivo. Br J Haematol. 2011;15:786–9.
Acknowledgements
The work was supported by The Chief Scientific Officer of Scotland, The Anonymous Trust, Tenovus Scotland (to Dr. T.C.), Melanoma Focus (to C.P.), The Leng Foundation (to C.P.) and The Brain Tumour Research Campaign (to N.S.). Antonio Vega-Rioja is under contract Proyectos I + D + I para jovenes investigadores from de Economica y Competitividad (SAF2014–60649-JN) and co-funded by Fondo Europeo de Regional-FEDER. Tim Crook is a Scottish Senior Clinical Fellow in Medical Oncology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Renziehausen, A., Wang, H., Rao, B. et al. The renin angiotensin system (RAS) mediates bifunctional growth regulation in melanoma and is a novel target for therapeutic intervention. Oncogene 38, 2320–2336 (2019). https://doi.org/10.1038/s41388-018-0563-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0563-y
This article is cited by
-
Recombinant ACE2 protein protects against acute lung injury induced by SARS-CoV-2 spike RBD protein
Critical Care (2022)
-
Synergy of epidermal growth factor (EGFR) and angiotensin II (AT1R) receptor determines composition and temporal pattern of transcriptome variation
Cellular and Molecular Life Sciences (2022)