Abstract
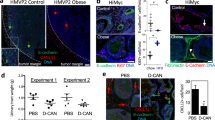
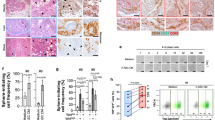
Fat tissue, overgrowing in obesity, promotes the progression of various carcinomas. Clinical and animal model studies indicate that adipose stromal cells (ASC), the progenitors of adipocytes, are recruited by tumors and promote tumor growth as tumor stromal cells. Here, we investigated the role of ASC in cancer chemoresistance and invasiveness, the attributes of tumor aggressiveness. By using human cell co-culture models, we demonstrate that ASC induce epithelial-mesenchymal transition (EMT) in prostate cancer cells. Our results for the first time demonstrate that ASC interaction renders cancer cells more migratory and resistant to docetaxel, cabazitaxel, and cisplatin chemotherapy. To confirm these findings in vivo, we compared cancer aggressiveness in lean and obese mice grafted with prostate tumors. We show that obesity promotes EMT in cancer cells and tumor invasion into the surrounding fat tissue. A hunter-killer peptide D-CAN, previously developed for targeted ASC ablation, suppressed the obesity-associated EMT and cancer progression. Importantly, cisplatin combined with D-CAN was more effective than cisplatin alone in suppressing growth of mouse prostate cancer allografts and xenografts even in non-obese mice. Our data demonstrate that ASC promote tumor aggressiveness and identify them as a target of combination cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, et al. Annual Report to the Nation on the status of cancer, 1975-2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338–66.
Lengyel E, Makowski L, DiGiovanni J, Kolonin MG. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer. 2018;4:374–84.
Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–101.
Zhang Y, Daquinag AC, Amaya-Manzanares F, Sirin O, Tseng C, Kolonin MG. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72:5198–208.
Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Comm. 2013;4:1795.
Zhang T, Tseng C, Daquinag AC, Corn PG, Troncoso P, Pettaway C, et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumor microenvironment. Nat Comm. 2016;7:11674–90.
Zhang Y, Daquinag A, Traktuev DO, Amaya F, Simmons PJ, March KL, et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259–66.
Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–65.
Daquinag AC, Dadbin A, Snyder B, Wang X, Sahin A, Uneo NT, et al. Non-glycanated decorin is a drug target on human adipose stromal cells. Mol Ther - Oncolytics. 2017;6:1–9.
Daquinag AC, Tseng C, Zhang Y, Amaya-Manzanares F, Florez F, Dadbin A, et al. Targeted pro-apoptotic peptides depleting adipose stromal cells inhibit tumor growth. Mol Ther. 2016;1:34–40.
Daquinag AC, Salameh A, Zhang Y, Tong Q, Kolonin MG. Depletion of white adipocyte progenitors induces beige adipocyte differentiation and suppresses obesity development. Cell Death & Diff. 2015;22:351–63.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15.
Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–29.
Saha A, Blando J, Fernandez I, Kiguchi K, DiGiovanni J. Linneg Sca-1high CD49fhigh prostate cancer cells derived from the Hi-Myc mouse model are tumor-initiating cells with basal-epithelial characteristics and differentiation potential in vitro and in vivo. Oncotarget. 2016;7:25194–207.
Saha A, Ahn S, Blando J, Su F, Kolonin MG, DiGiovanni J. Proinflammatory CXCL12-CXCR4/CXCR7 signaling axis drives Myc-induced prostate cancer in obese mice. Cancer Res. 2017;77:5158–68.
Nowicka A, Marini FC, Solley TN, Elizondo PB, Zhang Y, Sharp HJ, et al. Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS One. 2013;8:e81859.
Duong MN, Cleret A, Matera EL, Chettab K, Mathe D, Valsesia-Wittmann S, et al. Adipose cells promote resistance of breast cancer cells to trastuzumab-mediated antibody-dependent cellular cytotoxicity. Breast Cancer Res. 2015;17:57–63.
Orecchioni S, Gregato G, Martin-Padura I, Reggiani F, Braidotti P, Mancuso P, et al. Complementary populations of human adipose CD34+progenitor cells promote growth, angiogenesis, and metastasis of breast cancer. Cancer Res. 2013;73:5880–91.
Rowan BG, Gimble JM, Sheng M, Anbalagan M, Jones RK, Frazier TP, et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS One. 2014;9:e89595.
Lu Y, Yang Y, Liu Y, Hao Y, Zhang Y, Hu Y, et al. Upregulation of PAG1/Cbp contributes to adipose-derived mesenchymal stem cells promoted tumor progression and chemoresistance in breast cancer. Biochem Biophys Res Commun. 2017;494:719–27.
Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–31.
Tan EHP, Sng MK, How ISB, Chan JSK, Chen J, Tan CK, et al. ROS release by PPARbeta/delta-null fibroblasts reduces tumor load through epithelial antioxidant response. Oncogene. 2018;37:2067–78.
Acknowledgements
We thank A. Daquinag, Z. Gao, and W. Cao for discussions and technical support.
Funding
This study was supported by NIH grant R01 CA196259 (to JD and MK), R21 CA216745 (to MK) and the Harry E. Bovay, Jr. Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Su, F., Ahn, S., Saha, A. et al. Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance. Oncogene 38, 1979–1988 (2019). https://doi.org/10.1038/s41388-018-0558-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0558-8
This article is cited by
-
Stromal cells in the tumor microenvironment: accomplices of tumor progression?
Cell Death & Disease (2023)
-
Obesity and prostate cancer — microenvironmental roles of adipose tissue
Nature Reviews Urology (2023)
-
Phosphorylation of IWS1 by AKT maintains liposarcoma tumor heterogeneity through preservation of cancer stem cell phenotypes and mesenchymal-epithelial plasticity
Oncogenesis (2023)
-
CXCR4 and CXCR7 signaling promotes tumor progression and obesity-associated epithelial-mesenchymal transition in prostate cancer cells
Oncogene (2022)
-
White adipose tissue-derived factors and prostate cancer progression: mechanisms and targets for interventions
Cancer and Metastasis Reviews (2022)