Abstract
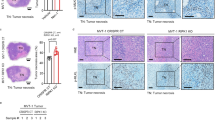
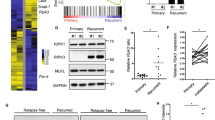
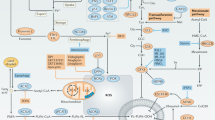
Great efforts have been made in revealing the mechanisms governing cancer resistance and recurrence. The in-situ effects of cell death, caused by hypoxia and metabolic stress, were largely studied in association with inflammation. However, in this work, we focused on the direct effects of necrosis on cancer promotion and on the tumor microenvironment. The conditions leading to cell necrosis, upon nutrient and oxygen deprivation, were recapitulated in-vitro and were used to generate samples for computational proteomic analysis. Under these conditions, we identified clusters of enriched pathways that may be involved in tumor resistance, leading to cancer recurrence. We show that the content of necrotic cells enhances angiogenesis and proliferation of endothelial cells, induces vasculature, as well as increases migration, invasion, and cell-cell interactions. In-vivo studies, where MDA-MB-231 xenografts were exposed to necrotic lysates, resulted in an increase in both proliferation and angiogenesis. Histological analysis of tumor tissues revealed high expression levels of key mediators that were identified by proteomic analysis. Moreover, when cells were injected systemically, coupled with necrotic lysates, a higher number of large lesions was detected in the lung. Finally, using xenografts, we demonstrated that combining an antagonist of a necrotic signal with an anticancer treatment potentiates the prolonged therapeutic effect. This approach suggests a paradigm shift in which targeting late necrotic-secreted factors may increase survival and enhance the efficacy of anticancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Esmatabadi MJ, Bakhshinejad B, Motlagh FM, Babashah S, Sadeghizadeh M. Therapeutic resistance and cancer recurrence mechanisms: unfolding the story of tumour coming back. J Biosci. 2016;41:497–506.
Simard S, Thewes B, Humphris G, Dixon M, Hayden C, Mireskandari S, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv. 2013;7:300–22.
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder, et al. Drug resistance in cancer: an overview. Cancers (Basel). 2014;6:1769–92.
McIntosh A, Freedman G, Eisenberg D, Anderson P. Recurrence rates and analysis of close or positive margins in patients treated without re-excision before radiation for breast cancer. Am J Clin Oncol. 2007;30:146–51.
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Sun Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2016;380:205–15.
Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2014;33:2423–31.
Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–17.
Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–47.
Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–65.
Bredholt G, Mannelqvist M, Stefansson IM, Birkeland E, Bo TH, Oyan AM, et al. Tumor necrosis is an important hallmark of aggressive endometrial cancer and associates with hypoxia, angiogenesis and inflammation responses. Oncotarget. 2015;6:39676–91.
Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–87.
Land WG. The role of damage-associated molecular patterns (DAMPs) in human diseases: part II: DAMPs as diagnostics, prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ Med J. 2015;15:e157–170.
Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–23.
Hernandez C, Huebener P, Schwabe RF. Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene. 2016;35:5931–41.
Sulciner ML, Serhan CN, Gilligan MM, Mudge DK, Chang J, Gartung A, et al. Resolvins suppress tumor growth and enhance cancer therapy. J Exp Med. 2018;215:115–40.
Janakiram NB, Rao CV. Role of lipoxins and resolvins as anti-inflammatory and proresolving mediators in colon cancer. Curr Mol Med. 2009;9:565–79.
Zhang Q, Zhu B, Li Y. Resolution of cancer-promoting inflammation: a new approach for anticancer therapy. Front Immunol. 2017;8:71.
Eales KL, Hollinshead KE, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis. 2016;5:e190.
Caino MC, Chae YC, Vaira V, Ferrero S, Nosotti M, Martin NM, et al. Metabolic stress regulates cytoskeletal dynamics and metastasis of cancer cells. J Clin Invest. 2013;123:2907–20.
Eustace A, Irlam JJ, Taylor J, Denley H, Agrawal S, Choudhury A, et al. Necrosis predicts benefit from hypoxia-modifying therapy in patients with high risk bladder cancer enrolled in a phase III randomised trial. Radiother Oncol. 2013;108:40–47.
Liu R, Li Z, Bai S, Zhang H, Tang M, Lei Y, et al. Mechanism of cancer cell adaptation to metabolic stress: proteomics identification of a novel thyroid hormone-mediated gastric carcinogenic signaling pathway. Mol Cell Proteom. 2009;8:70–85.
Rahman M, Hasan MR. Cancer metabolism and drug resistance. Metabolites. 2015;5:571–600.
Rundqvist H, Johnson RS. Tumour oxygenation: implications for breast cancer prognosis. J Intern Med. 2013;274:105–12.
Tomes L, Emberley E, Niu Y, Troup S, Pastorek J, Strange K, et al. Necrosis and hypoxia in invasive breast carcinoma. Breast Cancer Res Treat. 2003;81:61–69.
Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 2010;40:323–32.
Aalinkeel R, Nair MP, Sufrin G, Mahajan SD, Chadha KC, Chawda RP, et al. Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res. 2004;64:5311–21.
Li W, Li J, Sama AE, Wang H. Carbenoxolone blocks endotoxin-induced protein kinase R (PKR) activation and high mobility group box 1 (HMGB1) release. Mol Med. 2013;19:203–11.
Karsch-Bluman A, Amoyav B, Friedman N, Shoval H, Schwob O, Ella E, et al. High mobility group box 1 antagonist limits metastatic seeding in the lungs via reduction of cell-cell adhesion. Oncotarget. 2017;8:32706–21.
Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med. 2008;205:2791–801.
Herszenyi L, Lakatos G, Hritz I, Varga MZ, Cierny G, Tulassay Z. The role of inflammation and proteinases in tumor progression. Dig Dis. 2012;30:249–54.
Crowley LC, Marfell BJ, Scott AP, Waterhouse NJ. Quantitation of apoptosis and necrosis by Annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb Protoc. 2016;2016:pdb prot087288.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–33.
Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351–9.
Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–96.
Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol Endocrinol Metab. 2008;295:E313–322.
Yang D, Wang J, Xiao M, Zhou T, Shi X. Role of Mir-155 in controlling HIF-1alpha level and promoting endothelial cell maturation. Sci Rep. 2016;6:35316.
Martinive P, Defresne F, Bouzin C, Saliez J, Lair F, Gregoire V, et al. Preconditioning of the tumor vasculature and tumor cells by intermittent hypoxia: implications for anticancer therapies. Cancer Res. 2006;66:11736–44.
Cuvier C, Jang A, Hill RP. Exposure to hypoxia, glucose starvation and acidosis: effect on invasive capacity of murine tumor cells and correlation with cathepsin (L+B) secretion. Clin Exp Metastas-. 1997;15:19–25.
Nagelkerke A, Bussink J, Mujcic H, Wouters BG, Lehmann S, Sweep FC, et al. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013;15:R2.
Ahn SH, Park H, Ahn YH, Kim S, Cho MS, Kang JL, et al. Necrotic cells influence migration and invasion of glioblastoma via NF-kappaB/AP-1-mediated IL-8 regulation. Sci Rep. 2016;6:24552.
Shoval H, Karsch-Bluman A, Brill-Karniely Y, Stern T, Zamir G, Hubert A, et al. Tumor cells and their crosstalk with endothelial cells in 3D spheroids. Sci Rep. 2017;7:10428.
Riffle S, Pandey RN, Albert M, Hegde RS. Linking hypoxia, DNA damage and proliferation in multicellular tumor spheroids. BMC Cancer. 2017;17:338.
Indovina P, Rainaldi G, Santini MT. Hypoxia increases adhesion and spreading of MG-63 three-dimensional tumor spheroids. Anticancer Res. 2008;28:1013–22.
Liu WD, Zhang T, Wang CL, Meng HM, Song YW, Zhao Z, et al. Sphere-forming tumor cells possess stem-like properties in human fibrosarcoma primary tumors and cell lines. Oncol Lett. 2012;4:1315–20.
Maurer AJ, Bonney PA, Toho LC, Glenn CA, Agarwal S, Battiste JD, et al. Tumor necrosis-initiated complement activation stimulates proliferation of medulloblastoma cells. Inflamm Res. 2015;64:185–92.
Forte D, Salvestrini V, Corradi G, Rossi L, Catani L, Lemoli RM, et al. The tissue inhibitor of metalloproteinases-1 (TIMP-1) promotes survival and migration of acute myeloid leukemia cells through CD63/PI3K/Akt/p21 signaling. Oncotarget. 2017;8:2261–74.
Gho YS, Kim PN, Li HC, Elkin M, Kleinman HK. Stimulation of tumor growth by human soluble intercellular adhesion molecule-1. Cancer Res. 2001;61:4253–7.
Kevil CG, Orr AW, Langston W, Mickett K, Murphy-Ullrich J, Patel RP, et al. Intercellular adhesion molecule-1 (ICAM-1) regulates endothelial cell motility through a nitric oxide-dependent pathway. J Biol Chem. 2004;279:19230–8.
Howard K, Lo KK, Ao L, Gamboni F, Edil BH, Schulick R, et al. Intercellular adhesion molecule-1 mediates murine colon adenocarcinoma invasion. J Surg Res. 2014;187:19–23.
Rosette C, Roth RB, Oeth P, Braun A, Kammerer S, Ekblom J, et al. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis. 2005;26:943–50.
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67.
Liu H, Kato Y, Erzinger SA, Kiriakova GM, Qian Y, Palmieri D, et al. The role of MMP-1 in breast cancer growth and metastasis to the brain in a xenograft model. BMC Cancer. 2012;12:583.
Sunami E, Tsuno N, Osada T, Saito S, Kitayama J, Tomozawa S, et al. MMP-1 is a prognostic marker for hematogenous metastasis of colorectal cancer. Oncologist. 2000;5:108–14.
Chung TW, Choi H, Lee JM, Ha SH, Kwak CH, Abekura F, et al. Oldenlandia diffusa suppresses metastatic potential through inhibiting matrix metalloproteinase-9 and intercellular adhesion molecule-1 expression via p38 and ERK1/2 MAPK pathways and induces apoptosis in human breast cancer MCF-7 cells. J Ethnopharmacol. 2017;195:309–17.
Yodkeeree S, Ampasavate C, Sung B, Aggarwal BB, Limtrakul P. Demethoxycurcumin suppresses migration and invasion of MDA-MB-231 human breast cancer cell line. Eur J Pharmacol. 2010;627:8–15.
Polychronidis AC, Tsaroucha AK, Samolis SP, Botaitis SK, Perente SS, Simopoulos CE. Serum levels of intercellular adhesion molecule-1 correlate with advanced and metastatic disease and poor prognosis in gastric cancer. Folia Med (Plovdiv). 2003;45:20–24.
Schroder C, Witzel I, Muller V, Krenkel S, Wirtz RM, Janicke F, et al. Prognostic value of intercellular adhesion molecule (ICAM)-1 expression in breast cancer. J Cancer Res Clin Oncol. 2011;137:1193–201.
Yang M, Liu J, Piao C, Shao J, Du J. ICAM-1 suppresses tumor metastasis by inhibiting macrophage M2 polarization through blockade of efferocytosis. Cell Death Dis. 2015;6:e1780.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS ONE. 2010;5:e13984.
Acknowledgements
This study was kindly supported by grants from the Marie Curie Career Integration Grants (CIG) (No. 0305116), Israel Cancer Association (ICA) (No. 0394691), Israel Foundation of Science (ISF) (No. 0394883), David R. Blum Center for Pharmacy at The Hebrew University, The Shukor Gladi fund, The Frances Brody fund, and Eliyahu Pen Fund. We thank Dr. Gil Hornung and The De Botton Protein Profiling Institute of the Nancy and Stephen Grand Israel National Center for Personalized Medicine, Weizmann Institute of Science, for their support of the proteomic data generating process.
Author contribution
BO: conceived the concept, provided valuable discussions regarding interpreting the experimental data, and was involved in drafting the manuscript. KBA: prepared the manuscript, analyzed all the data (except for the proteomics experiment), performed the S.C. and I.V. in-vivo experiments, and the majority of the in-vitro experiments. FA: performed the computational analysis of the proteomic assay. EA: performed the transfection and siRNA analysis ST: performed the spheroid assembly and spheroid invasion assays. SH: performed the cell migration assays. SO: assisted in the in-vivo experiments. BM: provided valuable scientific interpretation. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Karsch-Bluman, A., Feiglin, A., Arbib, E. et al. Tissue necrosis and its role in cancer progression. Oncogene 38, 1920–1935 (2019). https://doi.org/10.1038/s41388-018-0555-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0555-y
This article is cited by
-
PANoptosis: a potential new target for programmed cell death in breast cancer treatment and prognosis
Apoptosis (2024)
-
Targeting cGAS/STING signaling-mediated myeloid immune cell dysfunction in TIME
Journal of Biomedical Science (2023)
-
Imaging of HER2 detected receptor expression positive breast cancer: from detection to interpretation
Egyptian Journal of Radiology and Nuclear Medicine (2023)
-
Cell death affecting the progression of gastric cancer
Cell Death Discovery (2022)
-
Prognostic value of multi b-value DWI in patients with locally advanced rectal cancer
European Radiology (2022)