Abstract
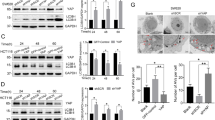
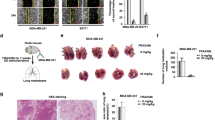
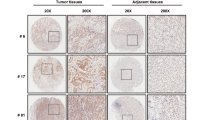
X-linked inhibitor of apoptosis protein (XIAP) possesses a critical role in promotion of cell survival and maintenance of cellular homeostasis. In cancer, elevated XIAP expression has been associated with malignancy, poor prognosis, and treatment resistance. However, the underlying mechanisms of these effects remain unclear. XIAP has previously been proposed to promote tumor growth through suppression of autophagy. In this study, we examined the expression of XIAP and p62, two critical mediators of autophagy, in breast and colon cancer. We observed a negative correlation between XIAP and p62 expression in normal and cancer tissues of breast and colon, and that the ratio of XIAP and p62 expression determines the cancer phenotype. In vitro, we observed that XIAP interacted with p62 and also that XIAP depletion resulted in increased expression of p62. XIAP functioned as an ubiquitination E3 ligase towards p62 and suppressed p62 expression through ubiquitin–proteasomal degradation. Furthermore, XIAP enhanced cancer cell proliferation, viability, and colony formation in vitro via suppression of p62. In addition, we demonstrated that XIAP-enhanced tumor growth is dependent on depletion of p62 in vivo. Herein, we have therefore delineated a novel mechanism by which XIAP contributes to development and progression of breast and colon carcinoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–68.
Holcik M, Gibson H, Korneluk RG. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–61.
Holcik M, Korneluk RG. XIAP, the guardian angel. Nat Rev Mol Cell Biol. 2001;2:550–6.
Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–94.
Fulda S. Molecular pathways: targeting inhibitor of apoptosis proteins in cancer—from molecular mechanism to therapeutic application. Clin Cancer Res. 2014;20:289–95.
LaCasse EC, et al. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–75.
Huang X, Wu Z, Mei Y, Wu M. XIAP inhibits autophagy via XIAP-Mdm2-p53 signalling. EMBO J. 2013;32:2204–16.
Merlo P, Cecconi F. XIAP: inhibitor of two worlds. EMBO J. 2013;32:2187–8.
Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta. 2009;1793:664–73.
Ichimura Y, Komatsu M. Selective degradation of p62 by autophagy. Semin Immunopathol. 2010;32:431–6.
Moscat J, Diaz-Meco MT. p62: a versatile multitasker takes on cancer. Trends Biochem Sci. 2012;37:230–6.
Puissant A, Fenouille N, Auberger P. When autophagy meets cancer through p62/SQSTM1. Am J Cancer Res. 2012;2:397–413.
Gao C, et al. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat Cell Biol. 2010;12:781–90.
Linares JF, Amanchy R, Greis K, Diaz-Meco MT, Moscat J. Phosphorylation of p62 by cdk1 controls the timely transit of cells through mitosis and tumor cell proliferation. Mol Cell Biol. 2011;31:105–17.
Duran A, et al. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13:343–54.
Amin MB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–9.
Komatsu M. Potential role of p62 in tumor development. Autophagy. 2011;7:1088–90.
Rosenfeldt MT, Ryan KM. The multiple roles of autophagy in cancer. Carcinogenesis. 2011;32:955–63.
Takahashi Y, et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. 2007;9:1142–51.
Liang C, et al. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–99.
Morselli E, et al. Anti- and pro-tumor functions of autophagy. Biochim Biophys Acta. 2009;1793:1524–32.
Qu X, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–20.
Mathew R, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–75.
Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, autopsy, and cancer. Cell. 2009;137:1001–4.
Moscat J, Karin M, Diaz-Meco MT. p62 in cancer: signaling adaptor beyond autophagy. Cell. 2016;167:606–9.
Lee HM, et al. Autophagy negatively regulates keratinocyte inflammatory responses via scaffolding protein p62/SQSTM1. J Immunol. 2011;186:1248–58.
Pandey V, et al. Artemin stimulates oncogenicity and invasiveness of human endometrial carcinoma cells. Endocrinology. 2010;151:909–20.
Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12:1–222.
Schlafli AM, Berezowska S, Adams O, Langer R, Tschan MP. Reliable LC3 and p62 autophagy marker detection in formalin fixed paraffin embedded human tissue by immunohistochemistry. Eur J Histochem. 2015;59:2481.
Kong DK, et al. Deficiency of the transcriptional regulator p8 results in increased autophagy and apoptosis, and causes impaired heart function. Mol Biol Cell. 2010;21:1335–49.
Wu WY, Kim H, Zhang CL, Meng XL, Wu ZS. Clinical significance of autophagic protein LC3 levels and its correlation with XIAP expression in hepatocellular carcinoma. Med Oncol. 2014;31:108.
Hung TH, Chen SF, Li MJ, Yeh YL, Hsieh TT. Differential effects of concomitant use of vitamins C and E on trophoblast apoptosis and autophagy between normoxia and hypoxia-reoxygenation. PLoS ONE. 2010;5:e12202
Acknowledgements
We deeply thank Dr. Bert Vogelstein (Johns Hopkins University) for XIAP−/− HCT116 and XIAP−/− DLD1 cell lines, Dr. Noboru Mizushima (The University of Tokyo) for Atg5−/− MEF cell lines, and Dr. Tao Zhu (University of Science & Technology of China) for breast cell lines. Especially, the author Xing Huang would like to express deepest thanks to Dr. Mian Wu (University of Science & Technology of China) for the technological training and ideological inspiration in his lab. This work was supported by grants from National Natural Science Foundation of China (81502975, 81472493, and 81572305), and China Postdoctoral Science Foundation (2016T90413 and 2015M581693). The research was funded in part by Jiangsu Planned Projects for Postdoctoral Research Funds (1501002A), Fundamental Research Funds for the Central Universities (2242016R20027 and 2242016K41045), Anhui provincial academic and technical leader reserve candidate (#2016H074), Key Program of Outstanding Young Talents in Higher Education Institutions of Anhui (#gxyqZD2016046) and the Shenzhen Development and Reform Commission Subject Construction Project ([2017]1434).
Author Contributions
X.H. and Z.W. conceived the project, designed, and conducted the majority of the experiments, with the assistance from X.-n.W., X.-d.Y., and W.-y.W. X.H., P.E.L., and Z.W. performed the data analysis and interpretation. X.H., X.-d.Y., P.E.L., and Z.W. wrote the manuscript; other authors discussed the results and commented on the manuscript. X.H. and Z.W. supervised the study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
These authors contributed equally: Xing Huang, Xiao-nan Wang
Rights and permissions
About this article
Cite this article
Huang, X., Wang, Xn., Yuan, Xd. et al. XIAP facilitates breast and colon carcinoma growth via promotion of p62 depletion through ubiquitination-dependent proteasomal degradation. Oncogene 38, 1448–1460 (2019). https://doi.org/10.1038/s41388-018-0513-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0513-8
This article is cited by
-
Role of protein degradation systems in colorectal cancer
Cell Death Discovery (2024)
-
Molecular basis for nuclear accumulation and targeting of the inhibitor of apoptosis BIRC2
Nature Structural & Molecular Biology (2023)
-
The involvement of E3 ubiquitin ligases in the development and progression of colorectal cancer
Cell Death Discovery (2023)
-
Lentiviral Gene Transfer Corrects Immune Abnormalities in XIAP Deficiency
Journal of Clinical Immunology (2023)
-
miR-431-5p regulates cell proliferation and apoptosis in fibroblast-like synoviocytes in rheumatoid arthritis by targeting XIAP
Arthritis Research & Therapy (2020)