Abstract
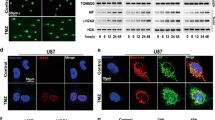
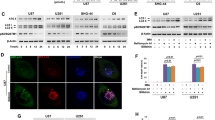
Autophagy is an evolutionarily conserved process regulating cellular homeostasis via digestion of dysfunctional proteins and whole cellular organelles by mechanisms, involving their enclosure into double-membrane vacuoles that are subsequently fused to lysosomes. Glioma stem cells utilize autophagy as a main mechanism of cell survival and stress response. Most recently, we and others demonstrated induction of autophagy in gliomas in response to treatment with chemical drugs, such as temozolomide (TMZ) or oncolytic adenoviruses (Ads). As autophagy has been implicated in the mechanism of Ad-mediated cell killing, autophagy deficiency in some glioma tumors could be the reason for their resistance to oncolysis. Despite the observed connection, the exact relationship between autophagy-activating cell signaling and adenoviral infection remains unclear. Here, we report that inhibition of autophagy in target glioma cells induces their resistance to killing by oncolytic agent CRAd-S-5/3. Furthermore, we found that downregulation of autophagy inducer Beclin-1 inhibits replication-competent Ad-induced oncolysis of human glioma by suppressing cell proliferation and inducing premature senescence. To overcome the autophagy-deficient state of such glioma cells and restore their susceptibility to oncolytic Ad infection, we propose treating glioma tumors with an anticancer drug tamoxifen (TAM) as a means to induce apoptosis in Ad-targeted cancer cells via upregulation of BAX/PUMA genes. In agreement with the above hypothesis, our data suggest that TAM improves susceptibility of Beclin-1-deficient glioma cells to CRAd-S-5/3 oncolysis by means of activating autophagy and pro-apoptotic signaling pathways in the target cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Jiang H, Gomez-Manzano C, Lang FF, Alemany R, Fueyo J. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther. 2009;9:422–7.
Ulasov IV Sonabend AM, Nandi S, Khramtsov A, Han Y, Lesniak MS. Combination of adenoviral virotherapy and temozolomide chemotherapy eradicates malignant glioma through autophagic and apoptotic cell death in vivo. Br J Cancer. 2009;100:1154–64.
Surawicz TS, Davis F, Freels S, Laws ER Jr, Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40:151–60.
Ito H, Daido S, Kanzawa T, Kondo S, Kondo Y. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int J Oncol. 2005;26:1401–10.
Lefranc F, Kiss R. Autophagy, the Trojan horse to combat glioblastomas. Neurosurg Focus. 2006;20:E7.
Lefranc F, Rynkowski M, DeWitte O, Kiss R. Present and potential future adjuvant issues in high-grade astrocytic glioma treatment. Adv Tech Stand Neurosurg. 2009;34:3–35.
Piya S, White EJ, Klein SR, Jiang H, McDonnell TJ, Gomez-Manzano C, et al. The E1B19K oncoprotein complexes with Beclin 1 to regulate autophagy in adenovirus-infected cells. PLoS ONE. 2011;6:e29467.
Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–57.
Tyler MA, Ulasov IV, Lesniak MS. Cancer cell death by design: apoptosis, autophagy and glioma virotherapy. Autophagy. 2009;5:856–7.
Huang X, Bai HM, Chen L, Li B, Lu YC. Reduced expression of LC3B-II and Beclin 1 in glioblastoma multiforme indicates a down-regulated autophagic capacity that relates to the progression of astrocytic tumors. J Clin Neurosci. 2010;17:1515–9.
Pirtoli L, Cevenini G, Tini P, Vannini M, Oliveri G, Marsili S, et al. The prognostic role of Beclin 1 protein expression in high-grade gliomas. Autophagy. 2009;5:930–6.
Giatromanolaki A, Sivridis E, Mitrakas A, Kalamida D, Zois CE, Haider S, et al. Autophagy and lysosomal related protein expression patterns in human glioblastoma. Cancer Biol Ther. 2014;15:1468–78.
Crighton D, Wilkinson S, O’Prey J, Syed N, Smith P, Harrison PR, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–34.
Zhang M, Zhou YF, Gong JY, Gao CB, Li SL. Expression of autophagy-related protein LC3B, p62, and cytoplasmic p53 in human retinoblastoma tissues. Eur Rev Med Pharmacol Sci. 2016;20:3152–60.
Chen QM, Liu J, Merrett JB. Apoptosis or senescence-like growth arrest: influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem J. 2000;347:543–51.
Tepper CG, Seldin MF, Mudryj M. Fas-mediated apoptosis of proliferating, transiently growth-arrested, and senescent normal human fibroblasts. Exp Cell Res. 2000;260:9–19.
Kang HT, Lee KB, Kim SY, Choi HR, Park SC. Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS ONE. 2011;6:e23367.
Mirochnik Y, Veliceasa D, Williams L, Maxwell K, Yemelyanov A, Budunova I, et al. Androgen receptor drives cellular senescence. PLoS ONE. 2012;7:e31052.
Caino MC, Meshki J, Kazanietz MG. Hallmarks for senescence in carcinogenesis: novel signaling players. Apoptosis. 2009;14:392–408.
Schilbach K, Alkhaled M, Welker C, Eckert F, Blank G, Ziegler H, et al. Cancer-targeted IL-12 controls human rhabdomyosarcoma by senescence induction and myogenic differentiation. Oncoimmunology. 2015;4:e1014760.
Abbadie C, Pluquet O, Pourtier A, Epithelial cell senescence: an adaptive response to pre-carcinogenic stresses?. Cell Mol Life Sci. 2017;74:4471–4509.
Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68.
Kumar M, Seeger W, Voswinckel R. Senescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2014;51:323–33.
Lasry A, Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 2015;36:217–28.
Maciel-Baron LA, Morales-Rosales SL, Aquino-Cruz AA, Triana-Martinez F, Galvan-Arzate S, Luna-Lopez A, et al. Senescence associated secretory phenotype profile from primary lung mice fibroblasts depends on the senescence induction stimuli. Age (Dordr). 2016;38:26.
Vater CA, Bartle LM, Dionne CA, Littlewood TD, Goldmacher VS. Induction of apoptosis by tamoxifen-activation of a p53-estrogen receptor fusion protein expressed in E1A and T24 H-ras transformed p53-/- mouse embryo fibroblasts. Oncogene. 1996;13:739–48.
Ulasov IV, Shah N, Kaverina NV, Lee H, Lin B, Lieber A, et al. Tamoxifen improves cytopathic effect of oncolytic adenovirus in primary glioblastoma cells mediated through autophagy. Oncotarget. 2015;6:3977–87.
Fernandez-Cuesta L, Anaganti S, Hainaut P, Olivier M. p53 status influences response to tamoxifen but not to fulvestrant in breast cancer cell lines. Int J Cancer. 2011;128:1813–21.
Criollo A, Dessen P, Kroemer G. DRAM: a phylogenetically ancient regulator of autophagy. Cell Cycle. 2009;8:2319–20.
Yee KS, Wilkinson S, James J, Ryan KM, Vousden KH. PUMA- and Bax-induced autophagy contributes to apoptosis. Cell Death Differ. 2009;16:1135–45.
Jiang H, White EJ, Rios-Vicil CI, Xu J, Gomez-Manzano C, Fueyo J. Human adenovirus type 5 induces cell lysis through autophagy and autophagy-triggered caspase activity. J Virol. 2011;85:4720–9.
Salminen A, Kaarniranta K, Kauppinen A. Beclin 1 interactome controls the crosstalk between apoptosis, autophagy and inflammasome activation: impact on the aging process. Ageing Res Rev. 2013;12:520–34.
Fimia GM, Di Bartolomeo S, Piacentini M, Cecconi F. Unleashing the Ambra1-Beclin 1 complex from dynein chains: Ulk1 sets Ambra1 free to induce autophagy. Autophagy. 2011;7:115–7.
Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003.
Shukla S, Patric IR, Patil V, Shwetha SD, Hegde AS, Chandramouli BA, et al. Methylation silencing of ULK2, an autophagy gene, is essential for astrocyte transformation and tumor growth. J Biol Chem. 2014;289:22306–18.
Ro SH, Jung CH, Hahn WS, Xu X, Kim YM, Yun YS, et al. Distinct functions of Ulk1 and Ulk2 in the regulation of lipid metabolism in adipocytes. Autophagy. 2013;9:2103–14.
Culmsee C, Siewe J, Junker V, Retiounskaia M, Schwarz S, Camandola S, et al. Reciprocal inhibition of p53 and nuclear factor-kappaB transcriptional activities determines cell survival or death in neurons. J Neurosci. 2003;23:8586–95.
Pei L, Shang Y, Jin H, Wang S, Wei N, Yan H, et al. DAPK1-p53 interaction converges necrotic and apoptotic pathways of ischemic neuronal death. J Neurosci. 2014;34:6546–56.
Villalonga-Planells R, Coll-Mulet L, Martinez-Soler F, Castano E, Acebes JJ, Gimenez-Bonafe P, et al. Activation of p53 by nutlin-3a induces apoptosis and cellular senescence in human glioblastoma multiforme. PLoS ONE. 2011;6:e18588.
Tripathi R, Ash D, Shaha C. Beclin-1-p53 interaction is crucial for cell fate determination in embryonal carcinoma cells. J Cell Mol Med. 2014;18:2275–86.
Levine B, Abrams J. p53: the Janus of autophagy? Nat Cell Biol. 2008;10:637–9.
Sasaki T, Lian S, Qi J, Bayliss PE, Carr CE, Johnson JL, et al. Aberrant autolysosomal regulation is linked to the induction of embryonic senescence: differential roles of Beclin 1 and p53 in vertebrate Spns1 deficiency. PLoS Genet. 2014;10:e1004409.
Joshi A, Iyengar R, Joo JH, Li-Harms XJ, Wright C, Marino R, et al. Nuclear ULK1 promotes cell death in response to oxidative stress through PARP1. Cell Death Differ. 2016;23:216–30.
Alers S, Loffler AS, Wesselborg S, Stork B. The incredible ULKs. Cell Commun Signal. 2012;10:7.
Gao W, Shen Z, Shang L, Wang X. Upregulation of human autophagy-initiation kinase ULK1 by tumor suppressor p53 contributes to DNA-damage-induced cell death. Cell Death Differ. 2011;18:1598–607.
Hothi P, Martins TJ, Chen L, Deleyrolle L, Yoon JG, Reynolds B, et al. High-throughput chemical screens identify disulfiram as an inhibitor of human glioblastoma stem cells. Oncotarget. 2012;3:1124–36.
Thomas MA, Broughton RS, Goodrum FD, Ornelles DA. E4orf1 limits the oncolytic potential of the E1B-55K deletion mutant adenovirus. J Virol. 2009;83:2406–16.
Graham FL. Growth of 293 cells in suspension culture. J Gen Virol. 1987;68(Pt 3):937–40.
Liu C, Pham K, Luo D, Reynolds BA, Hothi P, Foltz G, et al. Expression and functional heterogeneity of chemokine receptors CXCR4 and CXCR7 in primary patient-derived glioblastoma cells. PLoS ONE. 2013;8:e59750.
Acknowledgements
This research was supported by Russian Fund of Fundamental Research (number 11 411.0008700. 13.082 and number 13 411. 1008799.13.120 Russia, MAB) and private donors. We are grateful to Dr. Cobbs for providing patient-derived tumor cells and to Dr. Cobbs’s laboratory personnel for their support and technical assistance with experiments.
Author contributions
NVK, MAB, JM, and IVU performed the experiments. AVB, ZGK, AIK, AK, DO, TX, MSL, and IVU analyzed the data and wrote/edited the manuscript. AK and IVU assessed IHC score. TX performed statistical evaluation. MAB, AVB, and IVU designed the experiments, analyzed and interpreted the data, and proofread the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kaverina, N.V., Kadagidze, Z.G., Borovjagin, A.V. et al. Tamoxifen overrides autophagy inhibition in Beclin-1-deficient glioma cells and their resistance to adenovirus-mediated oncolysis via upregulation of PUMA and BAX. Oncogene 37, 6069–6082 (2018). https://doi.org/10.1038/s41388-018-0395-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0395-9
This article is cited by
-
Cellular senescence in glioma
Journal of Neuro-Oncology (2023)
-
Isoforms of autophagy-related proteins: role in glioma progression and therapy resistance
Molecular and Cellular Biochemistry (2022)
-
Protein clearance strategies for disease intervention
Journal of Neural Transmission (2022)
-
The role of P53 up-regulated modulator of apoptosis (PUMA) in ovarian development, cardiovascular and neurodegenerative diseases
Apoptosis (2021)