Abstract
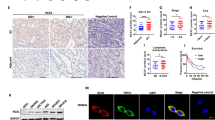
Helicobacter pylori (H. pylori) is the major stomach carcinogen, but the molecular mechanism responsible for the pathogenesis of cancer development mediated by H. pylori infection is still unclear. Aquaporin 3 (AQP3), overexpressed in gastric carcinoma, has a crucial role in gastric carcinogenesis and progression. However, the triggers and precise regulations for AQP3 upregulation during gastric carcinogens also remain unknown. Here we report that H. pylori infection-mediated carcinogenesis may be mechanistically depended on the upregulation of AQP3 expression via reactive oxygen species (ROS) pathway activation in the stomach. The retrospective analyses of clinical samples from patients with gastric carcinoma and other different stages of gastric diseases indicated that AQP3 expression was positively associated with gastric mucosal disease progression and H. pylori infection status as well. Furthermore, H. pylori infection significantly upregulated AQP3 and HIF-1α expression and increased ROS amount in human gastric epithelial AGS and GES-1 cells. Blockage of ROS with inhibitors, NAC and DPI, markedly decreased the expression of AQP3 and HIF-1α in both AGS and GES-1 cells simultaneously. Furthermore, the increased AQP3 in cells was mechanistically due to the transcriptional regulation by HIF-1α. In addition, H. pylori infection exerted production of proinflammatory cytokines IL-6, IL-8, and TNF depending on AQP3 level. Importantly, these in vitro novel findings were further investigated in vivo in a mouse H. pylori infectious model. Our current studies identify the mechanistic link between H. pylori infection and AQP3 upregulation in the pathogenesis of gastric carcinoma, which involves the activation of the ROS–HIF-1α axis and the exacerbated ROS–HIF-1α–AQP3–ROS loop.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fichtner-Feigl S, Kesselring R, Strober W. Chronic inflammation and the development of malignancy in the GI tract. Trends Immunol. 2015;36:451–9.
de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–15.
Thompson PA, Khatami M, Baglole CJ, Sun J, Harris SA, Moon EY, et al. Environmental immune disruptors, inflammation and cancer risk. Carcinogenesis. 2015;36(Suppl 1):S232–253.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32.
Yamaguchi N, Kakizoe T. Synergistic interaction between Helicobacter pylori gastritis and diet in gastric cancer. Lancet Oncol. 2001;2:88–94.
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9.
International Agency for Research on Cancer. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241.
Backert S, Neddermann M, Maubach G, Naumann M. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2016;1(21Suppl):19–25.
Alzahrani S, Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol. 2014;20:12767–80.
Wan XK, Yuan SL, Tao HX, Diao LP, Wang YC, Cao C, et al. The upregulation of TRAF1 induced by Helicobacter pylori plays an antiapoptotic effect on the infected cells. Helicobacter. 2016;21:554–64.
Gajewski A, Mnich E, Szymański K, Hinc K, Obuchowski M, Moran AP, et al. Helicobacter pylori antigens, acetylsalicylic acid, LDL and 7-ketocholesterol - their potential role in destabilizing the gastric epithelial cell barrier. An in vitro model of Kato III cells. Acta Biochim Pol. 2016;63:145–52.
Li X, Liu S, Luo J, Liu A, Tang S, Liu S, et al. Helicobacter pylori induces IL-1β and IL-18 production in human monocytic cell line through activation of NLRP3 inflammasome via ROS signaling pathway. Pathog Dis. 2015;73:(pii):ftu024.
Hartung ML, Gruber DC, Koch KN, Grüter L, Rehrauer H, Tegtmeyer N, et al. H. pylori-induced DNA strand breaks are introduced by nucleotide excision repair endonucleases and promote NF-kB target gene expression. Cell Rep. 2015;13:70–79.
Koeppel M, Garcia-Alcalde F, Glowinski F, Schlaermann P, Meyer TF. Helicobacter pylori infection causes characteristic DNA damage patterns in human cells. Cell Rep. 2015;11:1703–17013.
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–54.
Gobert AP, Wilson KT. Human and Helicobacter pylori interactions determine the outcome of gastric diseases. Curr Top Microbiol. 2017;400:27–52.
Handa O, Naito Y, Yoshikawa T. Helicobacter pylori: a ROS-inducing bacterial species in the stomach. Inflamm Res. 2010;59:997–1003.
Gobert AP, Wilson KT. Polyamine- and NADPH-dependent generation of ROS during Helicobacter pylori infection: a blessing in disguise. Free Radic Biol Med. 2017;105:16–27.
Shimizu T, Chiba T, Marusawa H. Helicobacter pylori-mediated genetic instability and gastric carcinogenesis. Curr Top Microbiol. 2017;400:305–23.
Kang MJ, Song EJ, Kim BY, Kim DJ, Park JH. Helicobacter pylori induces vascular endothelial growth factor production in gastric epithelial cells through hypoxia-inducible factor-1α-dependent pathway. Helicobacter. 2014;19:476–83.
Verkman AS, Anderson MO, Papadopoulos MC. Aquaporins: important but elusive drug targets. Nat Rev Drug Discov. 2014;13:259–77.
Shen L, Zhu Z, Huang Y, Shu Y, Sun M, Xu H, et al. Expression profile of multiple aquaporins in human gastric carcinoma and its clinical significance. Biomed Pharmacother. 2010;64:313–8.
Huang Y, Zhu Z, Sun M, Wang J, Guo R, Shen L, et al. Critical role of Aquaporin-3 in the human epidermal growth factor-induced migration and proliferation in the human gastric adenocarcinoma cells. Cancer Biol Ther. 2010;9:1000–7.
Wang J, Gui Z, Deng L, Sun M, Guo R, Zhang W, et al. c-Met upregulates aquaporin 3 expression in human gastric carcinoma cells via the ERK signalling pathway. Cancer Lett. 2012;319:109–17.
Chen J, Wang T, Zhou YC, Gao F, Zhang ZH, Xu H, et al. Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer. J Exp Clin Cancer Res. 2014;33:38.
Zhou Y, Wang Y, Wen J, Zhao H, Dong X, Zhang Z, et al. Aquaporin 3 promotes the stem-like properties of gastric cancer cells via Wnt/GSK-3β/β-catenin pathway. Oncotarget. 2016;7:16529–41.
Zhao H, Yang X, Zhou Y, Zhang W, Wang Y, Wen J, et al. Potential role of aquaporin 3 in gastric intestinal metaplasia. Oncotarget. 2015;6:38926–33.
Dong X, Wang Y, Zhou Y, Wen J, Wang S, Shen L. Aquaporin 3 facilitates chemoresistance in gastric cancer cells to cisplatin via autophagy. Cell Death Discov. 2016;2:16087.
Wang G, Gao F, Zhang W, Chen J, Wang T, Zhang G, et al. Involvement of aquaporin 3 in Helicobacter pylori-related gastric diseases. PLoS ONE. 2012;7:e49104.
Miller EW, Dickinson BC, Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci USA. 2010;107:15681–6.
Bienert GP, Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys Acta. 2014;1840:1596–604.
Satooka H, Hara-Chikuma M. Aquaporin-3 controls breast cancer cell migration by regulating hydrogen peroxide transport and its downstream cell signaling. Mol Cell Biol. 2016;36:1206–18.
Thiagarajah JR, Chang J, Goettel JA, Verkman AS, Lencer WI. Aquaporin-3 mediates hydrogen peroxide-dependent responses to environmental stress in colonic epithelia. Proc Natl Acad Sci USA. 2017;114:568–73.
Nookaew I, Thorell K, Worah K, Wang S, Hibberd ML, Sjövall H, et al. Transcriptome signatures in Helicobacter pylori-infected mucosa identifies acidic mammalian chitinase loss as a corpus atrophy marker. BMC Med Genom. 2013;6:41.
Park JH, Kim TY, Jong HS, Kim TY, Chun YS, Park JW, et al. Gastric epithelial reactive oxygen species prevent normoxic degradation of hypoxia-inducible factor-1 alpha in gastric cancer cells. Clin Cancer Res. 2003;9:433–40.
Dong X, Hu F, Gao W, Cheng H, Liu X, Xu L. et al. Detection of Helicobacter pylori by immunoblot: a multiple-center study. Zhonghua Yi Xue Za Zhi. 2016;96:265–9.
Suarez G, Romero-Gallo J, Piazuelo MB, Wang G, Maier RJ, Forsberg LS, et al. Modification of Helicobacter pylori peptidoglycan enhances NOD1 activation and promotes cancer of the stomach. Cancer Res. 2015;75:1749–59.
Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–90.
Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–17.
Bhattacharyya A, Chattopadhyay R, Hall EH, Mebrahtu ST, Ernst PB, Crowe SE. Mechanism of hypoxia-inducible factor 1 alpha-mediated Mcl1 regulation in Helicobacter pylori-infected human gastric epithelium. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1177–86.
Shida M, Kitajima Y, Nakamura J, Yanagihara K, Baba K, Wakiyama K, et al. Impaired mitophagy activates mtROS/HIF-1α interplay and increases cancer aggressiveness in gastric cancer cells under hypoxia. Int J Oncol. 2016;48:1379–90.
Grandjean G, de Jong PR, James BP, Koh MY, Lemos R, Kingston J, et al. Definition of a novel feed-forward mechanism for glycolysis-HIF1α signaling in hypoxic tumors highlights aldolase A as a therapeutic target. Cancer Res. 2016;76:4259–69.
Yuan LW, Yamashita H, Seto Y. Glucose metabolism in gastric cancer: the cutting-edge. World J Gastroenterol. 2016;22:2046–59.
Nakamura J, Kitajima Y, Kai K, Hashiguchi K, Hiraki M, Noshiro H, et al. HIF-1 alpha is an unfavorable determinant of relapse in gastric cancer patients who underwent curative surgery followed by adjuvant 5-FU chemotherapy. Int J Cancer. 2010;127:1158–71.
Nam SY, Ko YS, Jung J, Yoon J, Kim YH, Choi YJ, et al. A hypoxia-dependent upregulation of hypoxia-inducible factor-1 by nuclear factor-κB promotes gastric tumour growth and angiogenesis. Br J Cancer. 2011;104:166–74.
Li YN, Xi MM, Guo Y, Hai CX, Yang WL, Qin XJ. NADPH oxidase-mitochondria axis-derived ROS mediate arsenite-induced HIF-1α stabilization by inhibiting prolyl hydroxylases activity. Toxicol Lett. 2014;224:165–74.
Lee TI, Johnstone SE, Young RA. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat Protoc. 2006;1:729–48.
Acknowledgements
We are grateful to Prof. Wenxi Wu (Department of General Surgery, First Affiliated Hospital, Nanjing Medical University) for his helpful suggestions and critical review of the manuscript.
Funding
This project was partially supported by the National Natural Science Foundation of China (Grant No. 81272711 to LS, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, JX10231801 to LS), and the Key Medical Talents Program of Jiangsu Province (ZDRCA2016014 to LS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wen, J., Wang, Y., Gao, C. et al. Helicobacter pylori infection promotes Aquaporin 3 expression via the ROS–HIF-1α–AQP3–ROS loop in stomach mucosa: a potential novel mechanism for cancer pathogenesis. Oncogene 37, 3549–3561 (2018). https://doi.org/10.1038/s41388-018-0208-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0208-1
This article is cited by
-
Gut microbes involvement in gastrointestinal cancers through redox regulation
Gut Pathogens (2023)
-
Comprehensive insight into altered host cell-signaling cascades upon Helicobacter pylori and Epstein–Barr virus infections in cancer
Archives of Microbiology (2023)
-
Helicobacter pylori infection activates Wnt/β-catenin pathway to promote the occurrence of gastritis by upregulating ASCL1 and AQP5
Cell Death Discovery (2022)
-
Siah2–GRP78 interaction regulates ROS and provides a proliferative advantage to Helicobacter pylori-infected gastric epithelial cancer cells
Cellular and Molecular Life Sciences (2022)
-
Clinical value and molecular mechanism of AQGPs in different tumors
Medical Oncology (2022)