Abstract
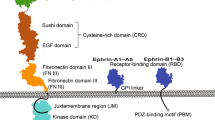
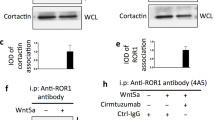
Members of the CD44 family of transmembrane glycoproteins control cell signaling pathways from numerous cell surface receptors, including receptor tyrosine kinases (RTKs). The decisive factor (ligand, RTKs or both) that controls the recruitment of specific CD44 isoforms is still unknown. We investigated this question by using the EGFR signaling pathway, in which one receptor can be activated by a broad range of ligands. By means of siRNA-mediated downregulation of CD44 expression and blocking experiments, we identified CD44v6 as a co-receptor for EGF- and ER-induced ErbB1 activation and for NRG1-induced ErbB3 and ErbB4 activation. In contrast, TGFα is independent of all CD44 isoforms, even though it addresses the same receptor pairs as EGF. Moreover, the heparin-sulfated CD44v3 isoform is required for HB-EGF-induced EGFR signaling. These data suggest that specific CD44 isoforms are recruited in a ligand-dependent manner as co-receptors in the EGFR signaling pathways and that the specificity is determined by the ligand and not by the receptors themselves. The in vivo relevance of this interplay between CD44 isoforms and EGFR ligands is underlined by the decreased metastatic spreading of mammary carcinomas in mice treated with a CD44v6-specific peptide. Most importantly, we found a clear correlation between the presence of CD44v6/ErbB1 complexes in breast cancer patients and lymph node metastases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signaling regulators. Nat Rev Mol Cell Biol 2003;4:33–45.
Naor D, Sionov RV, Ish-Shalom D. CD44: Structure, function and association with the malignant process. In: Vande Woude GF, Klein G, editors. Advances in Cancer Research. 71. San-Diego; 1997. p 243–318.
Orian-Rousseau V. CD44 acts as a signaling platform controlling tumor progression and metastasis. Front Immunol 2015;6:154.
Orian-Rousseau V, Ponta H. Adhesion proteins meet receptors: a common theme? Adv Cancer Res 2008;101:63–92.
Orian-Rousseau V, Sleeman J. CD44 is a multidomain signaling platform that integrates extracellular matrix cues with growth factor and cytokine signals. Adv Cancer Res 2014;123:231–54.
Orian-Rousseau V, Morrison H, Matzke A, Kastilan T, Pace G, Herrlich P, et al. Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol Biol Cell 2007;18:76–83.
Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer 2010;46:1271–7.
Matzke-Ogi A, Jannasch K, Shatirishvili M, Fuchs B, Chiblak S, Morton J, et al. Inhibition of tumor growth and metastasis in pancreatic cancer models by interference with CD44v6 signaling. Gastroenterology. 2016;150:513–25.e10.
Olaku V, Matzke A, Mitchell C, Hasenauer S, Sakkaravarthi A, Pace G, et al. c-Met recruits ICAM-1 as a coreceptor to compensate for the loss of CD44 in Cd44 null mice. Mol Biol Cell 2011;22:2777–86.
Bennett KL, Jackson DG, Simon JC, Tanczos E, Peach R, Modrell B, et al. CD44 isoforms containing exon v3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol 1995;128:687–98.
Sherman L, Wainwright D, Ponta H, Herrlich P. A splice variant of CD44 expressed in the apical ectodermal ridge presents fibroblast growth factors to limb mesenchyme and is required for limb outgrowth. Genes Dev 1998;12:1058–71.
Misra S, Hascall VC, Markwald RR, Ghatak S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front Immunol 2015;6:201.
Tsatas D, Kanagasundaram V, Kaye A, Novak U. EGF receptor modifies cellular responses to hyaluronan in glioblastoma cell lines. J Clin Neurosci 2002;9:282–8.
Bourguignon LY, Zhu H, Chu A, Iida N, Zhang L, Hung MC. Interaction between the adhesion receptor, CD44, and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J Biol Chem 1997;272:27913–8.
Kivisaari AK, Kallajoki M, Ala-aho R, McGrath JA, Bauer JW, Konigova R, et al. Matrix metalloproteinase-7 activates heparin-binding epidermal growth factor-like growth factor in cutaneous squamous cell carcinoma. Br J Dermatol 2010;163:726–35.
Lynch CC, Vargo-Gogola T, Martin MD, Fingleton B, Crawford HC, Matrisian LM. Matrix metalloproteinase 7 mediates mammary epithelial cell tumorigenesis through the ErbB4 receptor. Cancer Res 2007;67:6760–7.
Yu WH, Woessner JF Jr, McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev 2002;16:307–23.
Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005;5:341–54.
Wu WK, Tse TT, Sung JJ, Li ZJ, Yu L, Cho CH. Expression of ErbB receptors and their cognate ligands in gastric and colon cancer cell lines. Anticancer Res 2009;29:229–34.
Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 2014;14:342–56.
Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev 2002;16:3074–86.
Matzke A, Herrlich P, Ponta H, Orian-Rousseau V. A five-amino-acid peptide blocks Met- and Ron-dependent cell migration. Cancer Res 2005;65:6105–10.
Subik K, Lee JF, Baxter L, Strzepek T, Costello D, Crowley P, et al. The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer 2010;4:35–41.
Cook PW, Mattox PA, Keeble WW, Pittelkow MR, Plowman GD, Shoyab M, et al. A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol Cell Biol 1991;11:2547–57.
Volz Y, Koschut D, Matzke-Ogi A, Dietz MS, Karathanasis C, Richert L, et al. Direct binding of hepatocyte growth factor and vascular endothelial growth factor to CD44v6. Biosci Rep. 2015;25...
Tremmel M, Matzke A, Albrecht I, Laib AM, Olaku V, Ballmer-Hofer K, et al. A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood. 2009;114:5236–44.
Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol 1993;120:129–39.
Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2001; Chapter 20: Unit 20 2.
Raymond E, Faivre S, Armand JP, Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs. 2000;60:15–23.Discussion 41-2.
Minuti G, Landi L. MET deregulation in breast cancer. Ann Transl Med 2015;3:181.
Rodig SJ, Shapiro GI. Crizotinib, a small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases. Curr Opin Investig Drugs 2010;11:1477–90.
Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol 2009;21:177–84.
Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 2012;13:767–79.
Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J 2009;418:491–506.
Ellis IR, Schor AM, Schor SL. EGF AND TGF-alpha motogenic activities are mediated by the EGF receptor via distinct matrix-dependent mechanisms. Exp Cell Res 2007;313:732–41.
Wan X, Kim SY, Guenther LM, Mendoza A, Briggs J, Yeung C, et al. Beta4 integrin promotes osteosarcoma metastasis and interacts with ezrin. Oncogene. 2009;28:3401–11.
Pochampalli MR, el Bejjani RM, Schroeder JA, MUC1 is a novel regulator of ErbB1 receptor trafficking. Oncogene. 2007;26:1693–701.
Bennett R Jr, Jarvela T, Engelhardt P, Kostamovaara L, Sparks P, Carpen O, et al. Mucin MUC1 is seen in cell surface protrusions together with ezrin in immunoelectron tomography and is concentrated at tips of filopodial protrusions in MCF-7 breast carcinoma cells. J Histochem Cytochem 2001;49:67–77.
Matsuo I, Kimura-Yoshida C. Extracellular modulation of Fibroblast Growth Factor signaling through heparan sulfate proteoglycans in mammalian development. Curr Opin Genet Dev 2013;23:399–407.
Ilangumaran S, Borisch B, Hoessli DC. Signal transduction via CD44: role of plasma membrane microdomains. Leuk Lymphoma 1999;35:455–69.
Bourguignon LY. CD44-mediated oncogenic signalling and cytoskeleton activation during mammary tumor progression. J Mammary Gland Biol Neoplasia 2001;6:287–97.
Konstantinovsky S, Davidson B, Reich R. Ezrin and BCAR1/p130Cas mediate breast cancer growth as 3-D spheroids. Clin Exp Metastas- 2012;29:527–40.
Bruce B, Khanna G, Ren L, Landberg G, Jirstrom K, Powell C, et al. Expression of the cytoskeleton linker protein ezrin in human cancers. Clin Exp Metastas- 2007;24:69–78.
Diaz LK, Zhou X, Wright ET, Cristofanilli M, Smith T, Yang Y, et al. CD44 expression is associated with increased survival in node-negative invasive breast carcinoma. Clin Cancer Res 2005;11:3309–14.
Foekens JA, Dall P, Klijn JG, Skroch-Angel P, Claassen CJ, Look MP, et al. Prognostic value of CD44 variant expression in primary breast cancer. Int J Cancer 1999;84:209–15.
Morgenstern JP, Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res 1990;18:1068.
König H, Moll J, Ponta H, Herrlich P. Trans-acting factors regulate the expression of CD44 splice variants. EMBO J 1996;15:4030–9.
Acknowledgements
The authors are very thankful to Selma Huber and the animal facility of the Institute of Toxicology and Genetics for their excellent work and help with the animal experiments. This work was supported by a grant from Mildred Scheel Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
These authors disclose the following: V.O.-R. is a co-founder and shareholder of amcure GmbH, which developed a CD44v6 targeting peptide for clinical study. The remaining authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Morath, I., Jung, C., Lévêque, R. et al. Differential recruitment of CD44 isoforms by ErbB ligands reveals an involvement of CD44 in breast cancer. Oncogene 37, 1472–1484 (2018). https://doi.org/10.1038/s41388-017-0030-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-017-0030-1
This article is cited by
-
Exosomes: the key of sophisticated cell–cell communication and targeted metastasis in pancreatic cancer
Cell Communication and Signaling (2022)
-
Decreased CD44v3 expression impairs endometrial stromal cell proliferation and decidualization in women with recurrent implantation failure
Reproductive Biology and Endocrinology (2022)
-
Prognostic and predictive roles of cancer stem cell markers in head and neck squamous cell carcinoma patients receiving chemoradiotherapy with or without nimotuzumab
British Journal of Cancer (2022)
-
Direct interaction of TrkA/CD44v3 is essential for NGF-promoted aggressiveness of breast cancer cells
Journal of Experimental & Clinical Cancer Research (2022)
-
The potential roles of exosomes in pancreatic cancer initiation and metastasis
Molecular Cancer (2020)