Abstract
Aim
The purpose of this study was to investigate the association of dietary patterns with the risk of insulin resistance (IR), diabetes mellitus (DM), and central obesity in China.
Methods
We performed a cross-sectional study on 1432 participants, aged 40–65 years in Hangzhou, Zhejiang province, China. Dietary intake was assessed using a semi-quantitative food frequency questionnaire.
Results
Factor analysis extracted four major dietary patterns: vegetable-fruits, rice–meat, seafood–eggs, and sweet–fast. The vegetable-fruits pattern was inversely associated with HOMA-IR (p < 0.001 in both genders), while sweet–fast food pattern was significantly associated with higher HOMA-IR (p = 0.002 in male, and p < 0.001 in female). The vegetables–fruits pattern was inversely correlated with visceral fat area (VFA) (p = 0.029 in males, and p = 0.017 in females), while sweet–fast food pattern presented a significant direct association (p < 0.001 in male) with VFA in males. There was no association observed between the rice–meat pattern or the seafood–eggs pattern and HOMA-IR or VFA. After adjustment for potential confounding factors, participants in the highest tertile of vegetable-fruits pattern showed a significantly lower risk of DM in both males and females (OR: 0.30, 95% CI: 0.13–0.70 in male, and OR: 0.28, 95% CI: 0.11–0.72 in female), and lower risk of central obesity was observed in males (OR: 0.50, 95% CI: 0.29–0.86 in male). Conversely, participants in the highest tertile of sweet–fast food pattern had higher risk of DM (OR: 2.58, 95% CI: 1.23–5.88 in male), and central obesity (OR: 2.85, 95% CI: 1.67–4.86 in male) only in male. While neither the rice–meat pattern nor the seafood–eggs pattern showed significant association with DM or central obesity in both genders.
Conclusions
Our findings indicated low risk of IR, DM, and central obesity with vegetable-fruits pattern while inverse relation with sweet–fast food pattern.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) has become a major public health concern worldwide due to its growing epidemic prevalence. In China, the prevalence of DM dramatically increased among adults from 0.67% in 1980 to 10.9% in 20131,2,3,4,5,6. There are two major subtypes of DM: type 1 and type 2. Type 2 diabetes (T2DM) is the most common type of DM, affecting 90% of people with diabetes, and is mainly linked to insulin resistance (IR) and relative insufficiency of insulin secretion. The probability of DM was higher in overweight and obese individuals than individuals with normal body mass index (BMI), and was even higher in participants with central obesity7. It is well known that obesity has a multifactorial etiology which includes genetic, environmental, and dietary factors. Of these, dietary factors are thought to play a key role.
Approximately 90% of T2DM occurring is attributed to excess weight8. Previous studies have reported that body composition, especially body fat, is closely related to glucose metabolism in humans9,10. Compared with subcutaneous adipose tissue, visceral adipose tissue is known to release more proinflammatory and proatherogenic factors, leading to exacerbation of oxidative stress and IR. Studies have demonstrated that magnetic resonance imaging (MRI) and computed tomography (CT) are the reference methods to measure the accumulation of visceral fat11. Dietary patterns, especially western dietary pattern, have been shown to increase weight and body fat percentage12,13. However, few reports have demonstrated the association between dietary patterns and body fat distribution.
Traditional approach that investigates diet and disease associations mainly focuses on a single nutrient or food. However, no one consumes just one single nutrient or food, making it more difficult to separate the effect of individual dietary components. The effect of different combinatorial foods on the body can be studied by dietary pattern analyses. Cumulative studies have revealed that vegetables and fruits14, low-fat dairy products15, and regular alcohol consumption16,17 might reduce the risk of IR. In contrast, the western dietary pattern defined by high energy and fat, and low dietary fiber intake increased the risk of glucose tolerance abnormalities18,19,20.
Due to the rapid social and economic development, dietary patterns, and lifestyles have changed substantially in China. Therefore, the aim of the study was to identify the major Chinese dietary patterns of individuals aged 40–65 years and evaluate their association with body fat distribution, and DM.
Research design and methods
Study design
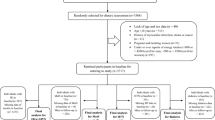
During the period of March to May 2010, a population-based cross-sectional survey was carried out in the Caihe communities of Hangzhou, Zhejiang province, China. Totally 1432 Han Chinese participants aged 40–65 years were included in the present study. Subjects who had a previous history of stroke or ischemic heart disease, and who were previously diagnosed with DM were excluded. The study was conducted in accordance with the Declaration of Helsinki, and all procedures were approved by the ethics committee of Sir Run Run Shaw Hospital. All participants signed an informed consent before data collection.
Demographic characteristics
Demographic and lifestyle covariates, such as smoking status, alcohol intake, and physical activity were collected by a questionnaire. We identified three categories for smoking: current smoker, former smoker, and non- smoker according to the participants’ cigarette use answer. A participant was defined as “drinker” in case of having drunk alcoholic beverage once per week on average during the last 4 weeks, excluding those who drank alcoholic beverage only during festivals.
Dietary assessment
A validated semi-quantitative food frequency questionnaire (FFQ) with 81 items was used to assess the food and nutritional intakes by trained dietitians during a structured interview21,22. Participants were asked to recall their frequency of intakes of each food item in the previous 4 weeks. The frequency of food intake was measured using seven categories which were as follows: (1) never; (2) <1 time/ week; (3) 1 time/ week; (4) 2–3 times/ week; (5) 4–6 times/ week; (6) 1 time/day; (7) ≥2 times/day. Eighty-one food items were gathered in 21 predefined food groups on the basis of similarity in nutrient profiles, culinary use among the foods and the grouping scheme used in other studies. The amount of each food item consumed on average was calculated by multiplying the frequency of intake by portion size of the food, using China Food Composition Table (2009) as the database.
Laboratory measurements
Participants were requested not to eat after 20:00 pm, and received a medical checkup from 7:00 to 12:00 am the next day. Venous blood samples were obtained at 0 and 2 hours after a 75 g glucose load for an oral glucose tolerance test (OGTT). Serum glucose concentrations, triglyceride (TG), total cholesterol (TC), high density lipoprotein–cholesterol (HDL-c), and low density lipoprotein–cholesterol (LDL-c) were determined by an autoanalyzer (Aeroset, Chicago, IL, USA). Glycosylated hemoglobin A1c (HbA1c) in the whole blood was measured by high-performance liquid chromatography (Hemglobin Testing System; Bio-Rad, Hercules, CA, USA). Insulin in the serum was measured by radioimmunology using an insulin detection kit (Beijing North Institute of Biological Technology, China). Homeostasis model of insulin resistance (HOMA-IR) sore was calculated using the following equation: [fasting serum insulin (FINS; mU/L) × fasting serum glucose (FPG; mmol/L)/22.5]23.
Anthropometric measurement
BMI was defined as body weight divided by height squared (kg/m2). Waist circumference (WC) was measured at a level halfway between the lower rib cage and iliac crest. Hip circumference was measured at the maximal horizontal girth between the waist and thigh, and the waist–to-hip ratio (WHR) was calculated and recorded for each participant. Both measurements were performed while the participant was standing. Body fat percentage (Fat%) was determined with a bioelectrical impedance analysis system (TBF-300, Tanita Co, Tokyo, Japan). Blood pressure was calculated as the average of three readings, using a mercury sphygmomanometer.
Abdominal adipose tissue was measured using a whole-body imaging system (SMT-100, Shimadzu Co, Kyoto, Japan). Spinecho sequences, TR-500 and TE-200, with a matrix size of 256 × 256 were performed for all participants. Visceral fat area (VFA) and subcutaneous adipose tissue area (SFA) were determined at the abdominal level between L4 and L5 vertebral disc space in the supine position with MRI, which were calculated with the workstation provided by the manufacturer.
Diagnosis of diabetes and central obesity
DM was diagnosed according to the World Health Organization criteria. Subjects without a history of DM were administered with a 75 g OGTT. We coded participants as DM when they had glucose level above the diagnostic threshold, which is fasting glucose level≥7 mmol/L, and or 2-h glucose level ≥11.1 mmol/L. And VFA ≥ 80 cm2 was defined as central obesity24.
Statistical analysis
Sample characteristics were presented as mean ± standard deviation (SD) for normally distributed variables and frequency and percentage for categorical variables. Glucose, insulin, HOMA-IR, TG, VFA, SFA, and total energy intake were reported as median (range) due to skewness and transformed lg(x) before analysis. Sweet–fast food pattern score underwent lg(x + 10) transformation to achieve a normal distribution. To compare the general characteristics according to DM status in patients and controls, continuous variables were tested by student’s-test and Pearson’s χ2 test for categorical variables. We used the factor analysis (principal component) to extract the participants’ dietary patterns among the 21 predefined food groups. To improve interpretability, the factors were rotated with an orthogonal rotation (varimax rotation) to minimize the correlation between factors. Four patterns were selected after evaluating the eigenvalue (≥1.5), scree plot, and factor interpretability. Items were remained in a factor if they had an absolute correlation of >±0.25 with that pattern. Correlation analysis and linear regression analysis were used to investigate the association between each dietary pattern score and HOMA-IR, as well as VFA. Factor scores for each pattern were divided into tertiles for further analysis. Multivariate logistic regression analysis was used to examine the relationships between dietary pattern with DM status and central obesity.
All analyses were performed by use of SPSS software (version 16.0 for Windows, SPSS Inc., Chicago, IL, USA). Statistical significance was considered when two-sided p-values < 0.05.
Results
Subject characteristics
The subject characteristics are presented in Table 1. A total of 575 males and 857 females were surveyed. The prevalence of newly diagnosed DM was observed to be 10.6% in males and 6.1% in females, respectively. There were no significant differences in education, family income, smoking, drinking, physical activity, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), 2h insulin (2h INS), TC, LDL-c, SFA, total energy intake between participants with and without DM in both males and females. WC, WHR, Fat%, FPG, 2h postprandia glucose (2h PG), FINS, HOMA-IR, HbA1C, HDL-c, VFA were significantly higher in the DM than control group. Compared with participants who did not have DM, those who had DM tended to be older and with higher TG in females, but not in males.
Dietary patterns
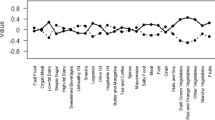
We derived four major dietary patterns among 21 predefined food groups, and the associated factor loading scores with ≤−0.25 or ≥0.25 are shown in Table 2. The “vegetables-fruits” dietary pattern was represented by a high consumption of vegetables, beans, mushrooms, tubers, fruits, coarse cereals, seaweeds, wheat, nuts, and dairy products. The “rice–meat” pattern was characterized by high intake of red meat, white rice, poultry, animal organ meat, condiments, eggs, and beans. The “seafood-eggs” pattern included eggs, seafood, dairy products, nuts, fruits, and tea, and low intake of beverages. The “sweet-fast food” pattern was loaded heavily on fast foods, beverages, desserts, alcoholic beverages, and was inversely related on white rice and dairy products. Each dietary pattern showed a 12.33%, 12.29%, 8.41%, and 6.07% of the variation in food intake, respectively.
Correlation of dietary pattern score with insulin resistance and fat accumulation
Table 3 showed the association between each dietary pattern scores with HOMA-IR. The vegetables–fruits pattern showed an inverse association (R = −0.250; p < 0.001 in male; and R = −0.152, p < 0.001 in female) with HOMA-IR. While sweet–fast food pattern presented a significant and positive association (R = 0.162, p = 0.002 in male, and R = 0.225, p < 0.001 in female) with HOMA-IR, when adjusted for age, smoking, drinking, education, economic income, total energy intake, physical activity, and BMI. In contrast, we found no significant association in the adjusted analysis for either the rice–meat pattern or the seafood–eggs pattern. Analyses of association between each dietary pattern score with VFA were also presented in Table 3. Vegetable-fruits pattern was negatively correlated with VFA in both males and females (R = −0.111, p = 0.029 in male, and R = −0.099 p = 0.017 in female). While sweet–fast food pattern was closely correlated with VFA in males but not in females (R = 0.200, p < 0.001 in male, and R = 0.012, p = 0.775 in female) after adjustment for age, smoking, drinking, education, economic income, total energy intake, and physical activity. Similarly, there was no association observed between the rice–meat pattern or the seafood–eggs pattern and VFA.
The effect of dietary patterns on the risk of DM and central obesity
The risks of DM and central obesity were analyzed across the dietary pattern score tertiles (Table 4). Those participants in the highest tertile of vegetable-fruits pattern showed a significantly lower risk of DM in both males and females (OR: 0.30, 95% CI: 0.13–0.70 in male, and OR: 0.28, 95% CI: 0.11–0.72 in female), but lower risk of central obesity was observed only in males (OR: 0.50, 95% CI: 0.29–0.86 in male, OR: 1.48, 95% CI: 0.93–2.38 in female). Conversely, participants in the highest tertile of the sweet–fast food pattern demonstrated a higher risk of DM (OR: 2.58, 95% CI: 1.23–5.88 in male, and OR: 0.73, 95% CI: 0.29–1.85 in female), and central obesity (OR: 2.85,95% CI: 1.67–4.86 in male, and OR: 1.04, 95% CI: 0.66–1.64 in female) only in males. Neither the rice–meat pattern nor the seafood–eggs pattern showed significant association with DM or central obesity in both males and females.
Discussion
In the present study, we investigated the association of dietary patterns with the risk of DM and central obesity in the middle- aged Chinese population. We extracted four major dietary patterns using factor analysis: vegetables–fruits food pattern, rice–meat pattern, seafood–eggs pattern, and sweet–fast food pattern. Vegetables–fruits food pattern and sweet–fast food pattern were associated with DM and central obesity; however, neither rice–meat food pattern nor seafood–eggs pattern were associated with DM or central obesity.
The vegetables–fruits pattern is generally considered as a healthy pattern. Previous studies have reported that the fruits and vegetables, particularly intake of high green leafy vegetables, contribute to a reduced risk of T2DM and obesity25,26,27,28. The protective effects of this pattern may be attributable to the healthy constituents, which contain abundant dietary fiber but a low glycemic index29,30. In addition, vegetables and fruits were considered as potent antioxidants31, and some studies have supported this hypothesis32,33. Overall, these findings suggested that vegetables and fruits provided benefits for decreasing the prevalence of DM and central obesity among Chinese population.
Sweet–fast food pattern characterized by high consumption of desserts, fast foods, and beverages was significantly associated with a higher prevalence of DM and central obesity. The results are consistent with the existing studies. Fast food tends to be energy-dense, high in glycemic load, poor in fiber and low in micronutrients. Positive association between the fast food and obesity and T2DM have been observed in several previous studies34,35,36. In addition, it was assessed that people who consumed more sweetened beverages and desserts showed a higher risk of developing central obesity, IR and DM28,37,38,39, because of high glycemic index and glycemic load. However, the study participants were restricted to the middle- aged Chinese population, who rarely consume fast foods, dessert, and drinks, and this may partly affect the outcomes.
In our study, we found no association of rice–meat pattern or seafood–eggs pattern with DM or central obesity. Red meat and white rice were the major components of rice–meat pattern. Red meat, which contains huge amount of saturated fat and cholesterol, is considered to be an energy-dense food and excess consumption may contribute to a surplus intake of energy, which may in turn increase the risk of T2DM and obesity40,41. However, few studies showed no relationship between red meat and T2DM or obesity22. This may be due to high content of zinc in red meat. Zinc content is highest in animal-source foods, and low in refined cereals and vegetables42. Zinc deficiency may aggravate IR in non-insulin dependent DM over a 6 year follow-up study43. According to a previous systematic review conducted among the Asian populations, rice which had a high glycemic index was positively associated with the risk of developing T2DM and weight gain30,44,45. However, studies aiming to investigate the association between rice intake and propensity to develop obesity are still inconclusive45,46,47. Furthermore, study participants with obesity and DM risk might have been advised to limit their consumption of carbohydrate and fat, which would influence the results.
Egg and seafood had a high factor loading in the seafood–eggs pattern. Egg is a nutrient-dense food containing high-quality protein and amount of beneficial nutrients. However, dietary cholesterol from eggs can also lead to a modest increase in blood liquids. The results of previous studies focused on egg consumption and IR and T2DM were controversial. A meta-analysis by Djousse et al. showed no significant effect of egg consumption on T2DM48. While the China National Nutrition Survey reported that egg consumption more than one time per day increased the T2DM occurrence in females and that TG and TC were significantly higher in females taking two or more eggs per week49. On the other hand, a study conducted in Korean reported that consumption of more than one egg per day could decrease the risk of metabolic syndrome (MetS)50. Different results on the association were likely due to differences in dietary patterns across countries. Data from previous studies in relation to the intake of fish with DM risk also are inconclusive. Ecological studies in several countries have shown protective effects of fish intake against the body weight gain and the development of DM51,52. However, the beneficial effects were not evident in other studies53,54, which was consistent with our study. A recent systematic review and meta-analysis of fish consumption and risk of T2DM suggested heterogeneity between geographical regions in the observed association55. The different associations of fish consumption with DM and obesity in different populations could be partly explained by diverse fish preparation methods, different type and amount of cooking fat used, and various kind of condiments.
In the present study, we found that the effect of food patterns might be greater in males than in females for DM and central obesity. Difference between the genders in the physiological response to different food patterns is uncertain, one plausible explanation is the different anatomy and physiology between males and females. For example, female may convert more α-linoleic acid into docosahexanoic acid than male do. In addition, the study participants are middle-aged Chinese population, and at times few female participants are involved in peri- and postmenopausal period. Previous studies proved that menopause-related loss of estrogen and increased androgens appear to be key hormonal changes that is associated with the development of MetS, which were independent of aging and other standard cardiovascular disease risk factors56. The study power of stratified analysis was limited due to the number of participants. Additional large population are needed in the future.
There were several potential limitations of this study that need to be highlighted. Firstly, because our study is a cross-sectional design study, no cause-and-effect relationships between dietary patterns and the risk of DM and central obesity may be established. Secondly, several arbitrary and subjective decisions in the use of factor analysis should be taken into consideration. Thirdly, even though we have adjusted for confounding factors that are related to the risk of DM and obesity, we cannot exclude the influence of uncontrolled confounders on our observed results. Finally, the study sample was composed of the middle-aged Chinese population in Hangzhou, Zhejiang Province, eastern China, and these results cannot be generalized to the entire Chinese population. In conclusion, this study has identified that vegetables–fruits food pattern was significantly associated with a lower risk of DM and central obesity, while the reverse was apparent for sweet–fast food pattern among Chinese population aged 40–65 years. Nevertheless, further prospective studies are needed to understand the causal relationships between dietary patterns and the risk of central obesity and DM.
References
National Diabetes Research Group. A mass survey of diabetes mellitus in a population of 300,000 in 14 provinces and municipalities in China (in Chinese). Zhonghua Nei Ke Za Zhi 20, 678–683 (1981).
Pan, X. R., Yang, W. Y., Li, G. W. & Liu, J. Prevalence of diabetes and its risk factors in China, 1994. National Diabetes Prevention and Control Cooperative Group. Diabetes Care 20, 1664–1669 (1997).
Yang, S. H., Dou, K. F. & Song, W. J. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 362, 2425–2426 (2010).
Xu, Y. et al. Prevalence and control of diabetes in Chinese adults. JAMA 310, 948–959 (2013).
Lu, C. & Sun, W. Prevalence of diabetes in Chinese adults. JAMA 311, 199–200 (2014).
Wang, L. et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 317, 2515–2523 (2017).
Yin, M., Augustin, B., Shu, C., Qin, T. & Yin, P. Probit models to investigate prevalence of total diagnosed and undiagnosed diabetes among aged 45 years or older adults in China. PloS ONE 11, e0164481 (2016).
Hossain, P., Kawar, B. & El Nahas, M. Obesity and diabetes in the developing world-a growing challenge. N. Engl. J. Med. 356, 213–215 (2007).
Gur, E. B. et al. Ultrasonographic visceral fat thickness in the first trimester can predict metabolic syndrome and gestational diabetes mellitus. Endocrine 47, 478–484 (2014).
Kahn, S. E. et al. Obesity, body fat distribution, insulin sensitivity and Islet beta-cell function as explanations for metabolic diversity. J. Nutr. 131, 354S–360S (2001).
Alberti, K. G., Zimmet, P. & Shaw, J. Metabolic syndrome-a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. J. Br. Diabet. Assoc. 23, 469–480 (2006).
Rodriguez-Monforte, M., Flores-Mateo, G. & Sanchez, E. Dietary patterns and CVD: a systematic review and meta-analysis of observational studies. Br. J. Nutr. 114, 1341–1359 (2015).
Schrijvers, J. K., McNaughton, S. A., Beck, K. L. & Kruger, R. Exploring the dietary patterns of young new Zealand women and associations with BMI and body frat. Nutrients https://doi.org/10.3390/nu8080450 (2016).
Spence, M., McKinley, M. C. & Hunter, S. J. Session 4: CVD, diabetes and cancer: diet, insulin resistance and diabetes: the right (pro)portions. Proc. Nutr. Soc. 69, 61–69 (2010).
Tremblay, A. & Gilbert, J. A. Milk products, insulin resistance syndrome and type 2 diabetes. J. Am. Coll. Nutr. 28(Suppl 1), 91S–102S (2009).
Gunji, T. et al. Alcohol consumption is inversely correlated with insulin resistance, independent of metabolic syndrome factors and fatty liver diseases. J. Clin. Gastroenterol. 45, 808–813 (2011).
Flanagan, D. E. et al. Alcohol consumption and insulin resistance in young adults. Eur. J. Clin. Invest. 30, 297–301 (2000).
He, Y. et al. Dietary patterns and glucose tolerance abnormalities in Chinese adults. Diabetes Care 32, 1972–1976 (2009).
Villegas, R. et al. Dietary patterns are associated with lower incidence of type 2 diabetes in middle-aged women: the Shanghai Women’s Health Study. Int. J. Epidemiol. 39, 889–899 (2010).
Zuo, H. et al. Dietary patterns are associated with insulin resistance in Chinese adults without known diabetes. Br. J. Nutr. 109, 1662–1669 (2013).
Jia, Q. et al. Dietary patterns are associated with prevalence of fatty liver disease in adults. Eur. J. Clin. Nutr. 69, 914–921 (2015).
Zhang, M. et al. Associations between dietary patterns and impaired fasting glucose in Chinese men: a cross-sectional study. Nutrients 7, 8072–8089 (2015).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Bao, Y. et al. Optimal waist circumference cutoffs for abdominal obesity in Chinese. Atherosclerosis 201, 378–384 (2008).
Li, M., Fan, Y., Zhang, X., Hou, W. & Tang, Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: meta-analysis of prospective cohort studies. BMJ Open 4, e005497 (2014).
Schwingshackl, L. et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 32, 363–375 (2017).
Schwingshackl, L. et al. Fruit and vegetable consumption and changes in anthropometric variables in adult populations: a systematic review and meta-analysis of prospective cohort studies. PLoS ONE 10, e0140846 (2015).
van Eekelen, E. et al. Sweet snacks are positively and fruits and vegetables are negatively associated with visceral or liver fat content in middle-aged men and women. J. Nutr. 149, 304–313 (2019).
Murakami, K. et al. Dietary fiber intake, dietary glycemic index and load, and body mass index: a cross-sectional study of 3931 Japanese women aged 18-20 years. Eur. J. Clin. Nutr. 61, 986–995 (2007).
Sugiyama, M., Tang, A. C., Wakaki, Y. & Koyama, W. Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur. J. Clin. Nutr. 57, 743–752 (2003).
Hamer, M. & Chida, Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: systematic review and meta-analysis. J. Hypertension 25, 2361–2369 (2007).
Salonen, J. T. et al. Increased risk of non-insulin dependent diabetes mellitus at low plasma vitamin E concentrations: a four year follow up study in men. BMJ 311, 1124–1127 (1995).
Reunanen, A., Knekt, P., Aaran, R. K. & Aromaa, A. Serum antioxidants and risk of non-insulin dependent diabetes mellitus. Eur. J. Clin. Nutr. 52, 89–93 (1998).
Li, M., Dibley, M. J., Sibbritt, D. W. & Yan, H. Dietary habits and overweight/obesity in adolescents in Xi’an City, China. Asia Pac. J. Clin. Nutr. 19, 76–82 (2010).
Shu, L. et al. Association between dietary patterns and the indicators of obesity among Chinese: a cross-sectional study. Nutrients 7, 7995–8009 (2015).
Mazidi, M. & Speakman, J. R. Association of fast-food and full-service restaurant densities with mortality from cardiovascular disease and stroke, and the prevalence of diabetes mellitus. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.117.007651 (2018).
Ma, J. et al. Sugar-sweetened beverage but not diet soda consumption is positively associated with progression of insulin resistance and prediabetes. J. Nutr. 146, 2544–2550 (2016).
O’Connor, L. et al. Intakes and sources of dietary sugars and their association with metabolic and inflammatory markers. Clin. Nutr. 37, 1313–1322 (2018).
Karatzi, K. et al. Dietary patterns and breakfast consumption in relation to insulin resistance in children. The Healthy Growth Study. Public Health Nutr. 17, 2790–2797 (2014).
Vergnaud, A. C. et al. Meat consumption and prospective weight change in participants of the EPIC-PANACEA study. Am. J. Clin. Nutr. 92, 398–407 (2010).
Aune, D., Ursin, G. & Veierod, M. B. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia 52, 2277–2287 (2009).
Brown, K. H. et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 25, S99–S203 (2004).
Vashum, K. P. et al. Is dietary zinc protective for type 2 diabetes? Results from the Australian longitudinal study on women’s health. BMC Endocr. Disord. 13, 40 (2013).
Lambert, E. et al. Change in sympathetic nerve firing pattern associated with dietary weight loss in the metabolic syndrome. Front. Physiol. 2, 52 (2011).
Shi, Z., Taylor, A. W., Hu, G., Gill, T. & Wittert, G. A. Rice intake, weight change and risk of the metabolic syndrome development among Chinese adults: the Jiangsu Nutrition Study (JIN). Asia Pac. J. Clin. Nutr. 21, 35–43 (2012).
Kim, J., Jo, I. & Joung, H. A rice-based traditional dietary pattern is associated with obesity in Korean adults. J. Acad. Nutr. Dietetics 112, 246–253 (2012).
Kolahdouzan, M. et al. The association between dietary intake of white rice and central obesity in obese adults. ARYA Atheroscler. 9, 140–144 (2013).
Djousse, L., Khawaja, O. A. & Gaziano, J. M. Egg consumption and risk of type 2 diabetes: a meta-analysis of prospective studies. Am. J. Clin. Nutr. 103, 474–480 (2016).
Shi, Z., Yuan, B., Zhang, C., Zhou, M. & Holmboe-Ottesen, G. Egg consumption and the risk of diabetes in adults, Jiangsu, China. Nutrition 27, 194–198 (2011).
Shin, S. et al. Egg consumption and risk of metabolic syndrome in Korean adults: results from the Health Examinees Study. Nutrients https://doi.org/10.3390/nu9070687 (2017).
Feskens, E. J. et al. Dietary factors determining diabetes and impaired glucose tolerance. A 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care 18, 1104–1112 (1995).
Nkondjock, A. & Receveur, O. Fish-seafood consumption, obesity, and risk of type 2 diabetes: an ecological study. Diabetes Metab. 29, 635–642 (2003).
Xun, P. C. & He, K. Fish consumption and incidence of diabetes meta-analysis of data from 438,000 individuals in 12 independent prospective cohorts with an average 11-year follow-up. Diabetes Care 35, 930–938 (2012).
Ramadeen, A. et al. N-3 polyunsaturated fatty acid supplementation does not reduce vulnerability to atrial fibrillation in remodeling atria. Heart Rhythm. Off. J. Heart Rhythm. Soc. 9, 1115 (2012).
Wallin, A. et al. Fish consumption, dietary long-chain n-3 fatty acids, and risk of type 2 diabetes: systematic review and meta-analysis of prospective studies. Diabetes Care 35, 918–929 (2012).
Janssen, I., Powell, L. H., Crawford, S., Lasley, B. & Sutton-Tyrrell, K. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch. Intern. Med. 168, 1568–1575 (2008).
Acknowledgements
This research was supported by grants from the Administration of Traditional Chinese Medicine of Zhejiang Province (grant no. 2015ZA058) and Zhejiang Provincial Medical and Health Science Project (grant no. 2016141525 & 2018253234).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, X., Chen, Y., Lu, W. et al. Association of dietary patterns with the newly diagnosed diabetes mellitus and central obesity: a community based cross-sectional study. Nutr. Diabetes 10, 16 (2020). https://doi.org/10.1038/s41387-020-0120-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-020-0120-y
This article is cited by
-
Association of dietary patterns with general and central obesity among Chinese adults: a longitudinal population-based study
BMC Public Health (2023)
-
Traditional Fermented Foods as an Adjuvant Treatment to Diabetes
Current Geriatrics Reports (2020)