Abstract
Background
Predisposition to obesity and type 2 diabetes can arise during foetal development and in early postnatal life caused by imbalances in maternal nutritional overload. We aimed to investigate the effects of maternal and postnatal intake of chocolate and soft drink on hypothalamic anti-oxidative stress markers, inflammation and peripheral glucose homeostasis.
Methods
Pregnant Sprague-Dawley rats were fed ad libitum chow diet only (C) or with chocolate and high sucrose soft drink supplements (S). At birth, litter size was adjusted into 10 male offspring per dam. After weaning at 3 weeks of age, offspring from both dietary groups were assigned to either S or C diet, giving four groups until the end of the experiment at 26 weeks of age.
Results
Offspring exposed to maternal S had up-regulated hypothalamic anti-oxidative markers such as SOD2 and catalase at 3 weeks of age as an indication of oxidative stress. However, at 12 weeks of age these anti-oxidative markers tended to decrease while pro-inflammatory markers such as TNF and IL-1β became up-regulated of all offspring exposed to S diet during some point of their life. Thus, despite an increase in anti-oxidative stress response, offspring exposed to maternal S had a reduced ability to counteract hypothalamic inflammation. At the same time point, postnatal S resulted in increased adiposity, reduced glucose tolerance and insulin sensitivity with no effect on body weight. However, at 25 weeks of age, the impaired glucose tolerance was reversible to the response of the control regardless of increased adiposity and body weight pointing towards a compensatory response of the insulin sensitivity or insulin secretion.
Conclusion
Indications of hypothalamic oxidative stress was observed prior to the inflammatory response in offspring exposed to maternal S. Both maternal and postnatal S induced hypothalamic inflammation prior to increased weight gain and thus contributing to obese phenotype.
Similar content being viewed by others
Introduction
Obesity is an important risk factor for metabolic diseases such as Type 2 Diabetes and cardiovascular diseases, and is associated with higher rates of mortality1. The primary cause of obesity is when food intake exceeds energy expenditure. Today’s abundance and easy access of palatable diets and snacks rich in fat and sugars contribute to the problem2,3. Consequently, many pregnant women have a substantial intake of fat- and sugar-rich diets which can affect the foetal development and predispose progeny to obesity4,5. Increased nutrient supply to the foetus can alter the development of neuronal circuits of the brain6,7,8,9,10 as well as other essential organs11 that are critical to regulation of growth and energy homeostasis4,5.
The hypothalamus is the predominant brain area that controls energy balance. Growing evidence suggests that the hypothalamus is one of the first tissues to show signs of impairments upon high fat diet (HFD) exposure. For instance, HFD has been shown to induce hypothalamic inflammatory responses resulting in a dysfunctional regulation of energy balance12,13,14 and glucose homeostasis15,16,17,18,19. There are several suggested mechanisms behind diet-induced inflammation including mitochondrial dysfunction, reactive oxygen species (ROS), and endoplasmic reticulum (ER) stress associated with unfolded protein response20,21,22. Oxidative stress is defined as a persistent imbalance between the production of ROS and the naturally occurring antioxidant defence mechanism23.
Enzymes as catalase and the two anti-oxidative superoxide dismutases (SODs) are considered to be the first line of defence mechanism and play an important role against oxidative stress24. SOD1 and SOD2 are exclusively expressed in the intracellular cytoplasmic spaces and in the mitochondrial spaces, respectively24. When ROS homeostasis is disrupted, excessive ROS, which is highly reactive to lipids, proteins and nuclei acids, are accumulated in the mitochondria and cytoplasm. This may cause cellular damage and/or inflammation, thus leading to tissue impairment and diseases15,21,23,25. For instance, excessive ROS in response to HFD have been shown to induce inflammation and neuronal cell death26, contributing to development of obesity12 and neurodegenerative diseases18,26,27. In the view of inflammation, cellular oxidative stress may activate the nuclear factor-κB (NFκB) and c-Jun N-terminal kinase (JNK) pathway which are the most well characterized cellular inflammatory pathways15,18,21,25. Also, the pro-inflammatory marker tumor necrosis factor alpha (TNFα) released from non-neuronal cell types such as microglia contributes to induction of JNK and NF-κB pathways28.
Only limited focus has been given to development of hypothalamic oxidative stress and inflammation during foetal and postnatal life. Maternal obesity and a maternal HFD appear to be risk factors for induction of early onset of hypothalamic inflammation in the offspring. In a mice study, offspring exposed to maternal high-fat diet (HFD) had increased activation of several hypothalamic inflammatory pathways such as IKKβ and JNK1 associated with obesity and reduced glucose tolerance in offspring aged 3 weeks15. Other studies have shown increased hypothalamic IL-1β expression in rat offspring as a consequence of maternal obesity29 and in non-human primate foetuses as a consequence of maternal HFD30.
In the present study, we investigated the effects of maternal and postnatal chocolate and soft drink supplementation (S) on hypothalamic anti-oxidative stress markers and inflammation, adiposity and glucose tolerance in rat offspring. To evaluate hypothalamic oxidative stress and inflammation, we measured real-time PCR mRNA expression of anti-oxidative stress and inflammatory markers in hypothalamus extract from offspring at 3 and 12 weeks of age. Furthermore, assessment of glucose homeostasis was performed by an oral glucose tolerance test (OGTT) at 12 and 25 weeks of age in the offspring.
Materials and methods
Study design
Data in the present manuscript arise from a previously published study. Figure 1 illustrates the study design (for further details see ref.32). For the present publication, hypothalamic samples for gene expression analysis were collected from the offspring upon euthanisation with isofluran followed by decapitation at the different developmental stages at 3 weeks (n = 10/group) and 12 weeks of age (n = 8/group). Blood was collected at 3, 12 and 25 weeks of age (n = 10/group).
Twenty-eight pregnant Sprague-Dawley rats were either fed ad libitum chow diet only (C) or chow with chocolate and high sucrose soft drink supplement (S) from one day after copulation plug and until weaning of offspring 3 weeks after parturition. Litter size was adjusted into 10 male offspring per mother. After weaning, offspring from both dietary groups were assigned to either S or C diet, giving four groups until the end of the experiment at 26 weeks of age, namely CC, CS, SC, and SS. For details of animals and procedures, see Experimental materials and methods (ref.32)
Diets and food intake
All rats had free access to water from drinking nipples and were fed a standard laboratory chow diet ad libitum (Altromin 1320, Brogaarden, Denmark). The chow diet contained a caloric density of 2.8 kcal/g (24% protein, 11% fat, and 65% carbohydrates). The high-fat/high-sucrose supplementary diet consisted of various chocolate bars on an average caloric density of approximately 5.4 kcal/g (8% protein, 33% fat, 59% carbohydrates of which 53% sucrose is present). The soft drink was produced according to European standards. It consisted of a caloric density of on average 1.96 kcal/ml (0% protein, 0% fat, 100% sucrose), and was given ad libitum in bottles (at least 80 ml per day) and changed every third day. Chocolate and soft drink intake were recorded by manual weighing once a week. The maternal intake of chocolate and soft drink were estimated to be approximately 40 kcal/week during gestation and 50 kcal/week during lactation. Thus, the intake of chocolate and soft drink was 20 and 15–20% out of the total caloric intake, respectively (for further details, see ref.31). For each offspring, chow and chocolate intake were recorded by manual weighing once a day during five days at 11 and 24 weeks of age. The energy intake was calculated in kcal ingested daily per rat and presented as daily energy consumption per group. For further details, see ref.32.
Body composition
Body composition was determined by the Echo Medical System per rat QMR scanner (EchoMRI 2004, Houston, USA) at 3, 12 and 25 weeks of age, as previously described in ref.31.
Oral glucose tolerance test (OGTT)
OGTT was performed at 12 and 25 weeks of age in the offspring. Before subjecting the rats to the OGTT, offspring were semi-fasted overnight, only receiving 10 g of chow diet overnight. Chocolate and soft drink were also removed as well as clean cages were given to the rats. The semi-fasting condition was done to induce a mild degree of fasting in the rats with access to 50% of their daily energy requirements for 16 h before the OGTT. The offspring were given an oral glucose bolus (500 mg glucose/ml) of 2 g glucose/kg body weight (BW) at 12 weeks of age, and a dose of 4 g glucose/kg BW at 25 weeks of age. Blood for glucose and insulin measurements was sampled at specific time points (t = −2 (baseline) and at 15, 30, 60, 120, 180 and 240 min after glucose bolus). Blood for corticosterone determination was sampled 15 min after glucose administration. All blood samples were collected from the tongue vein. Calculation of the areas under the curve (AUC) for plasma concentration of glucose and insulin were evaluated to define glucose tolerance. Plasma concentration of corticosterone was evaluated 15 min after oral glucose load as a measure for stress-responsiveness of the animals.
Blood analysis
Blood samples used for glucose, insulin, plasma triglycerides (TG), and free fatty acids (FFA) determination were analysed as previously described31.
RNA extraction and real time PCR
Cellular RNA and PCR reactions were performed on the whole rat hypothalamus at 3 and 12 weeks of age as previously described in ref.31. The target genes were tumor necrosis factor alpha (TNFα) (Rn99999017_m1), interleukin 1 beta (IL-1β) (Rn00580432_m1), Nuclear Factor-κB (NFκB) (Rn01399566_m1), superoxide dismutase 1 (SOD1) (Rn01477289_m1), superoxide dismutase 2 (SOD2) (Rn00690588_g1), catalase (Rn00560930_m1) and18S ribosomal RNA (Mm04277571_s1) (Applied Biosystems). Level of expression of each target gene of interest was presented as a percentage of the expression of the appropriate housekeeping gene 18S ribosomal RNA. 18S ribosomal RNA was chosen as the most appropriate reference gene for the hypothalamus as it was not affected by the treatments.
Statistics
The statistical analysis was performed in the statistical program R. Data were performed by fitting data as mixed linear models (“lme”) and analysed either as one or two factor study designs (ANOVA). The fixed factors “Maternal diet”, “offspring diet”, and “dam lactation” were used as a random factor. All variables were visually assessed and statistically tested by “Shapiro-Wilk normality test” for normal distribution. Also, homogeneity of variance were assessed by visual inspection of residual plot and tested through “Bartlett test of homogeneity of variances”. The replicate of the statistical analysis was referred to individual offspring in each group. Data are presented as means ± standard errors of mean (SEM). A p-value of 0.05 or less was considered statistically significant.
Results
Pregnant and lactating mothers
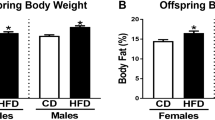
Maternal body weight and percentage of body fat mass was not affected by the S-diet during gestation. However, by the end of lactation percentage of body fat mass was increased in S fed mothers (p < 0.01) but there were no effect on body weight. No effect was observed on blood glucose, plasma insulin, TG and FFA levels during gestation and lactation (data not shown). Therefore, the possible changes in foetus development were a direct effect of the maternal intake of the S-diet. Furthermore, pregnancy length, litter size and offspring birth weight did not differ between C- and S-diet fed mothers (data not shown).
Body weight, fat mass and plasma lipids
At weaning (3 weeks of age), no differences in body weight, TG and FFA were observed between the offspring groups (Table 1). However, the percentage of body fat mass was increased in offspring from S-fed mothers (Table 1). At 12 weeks of age, still no significant difference in body weight was observed between treatment groups, but the postnatal S diet resulted in increased body fat mass and plasma FFA in CS and SS compared to CC and SC offspring, and in SC compared to CC (p < 0.05) (Table 1). Furthermore, plasma TG was increased in SS compared to CC (Table 1). At 25 weeks of age, postnatal S diet resulted in increased body weight, body fat mass and plasma lipids of TG and FFA in CS and SS offspring compared to SC and CC offspring fed postnatal chow (Table 1).
Hypothalamus anti-oxidative stress and inflammation
At 3 weeks of age, offspring from S fed mothers had increased mRNA levels of the hypothalamic anti-oxidative markers SOD2 (p < 0.05) and catalase (p < 0.05) compared to offspring from mothers fed the chow diet. Moreover, the mRNA level of SOD1 was slightly increased in S offspring compared to C offspring, but failed to reach statistical significance (p = 0.08). No significant difference was observed in the inflammatory markers between the groups (Fig. 2). At 12 weeks of age, the mRNA levels of hypothalamic anti-oxidative markers did not differ between the groups. However, mRNA levels of the pro-inflammatory markers TNF-α and IL-1β were increased in CS and SS offspring fed postnatal S diet compared to CC offspring (p < 0.01, p < 0.05). The mRNA level of IL-1β was significantly increased in CS, SC and SS offspring compared to CC offspring (p < 0.01, p < 0.001) (Fig. 3).
a SOD1, b SOD2, c catalase, d TNFα, e IL-1β, f NFκB. Data are expressed as means ± SEM, n = 8–10, analysed by linear mixed models (lme), unpaired t-test in R. (*p < 0.05, **p < 0.01, ***p < 0.001). CC maternal C–post-weaning C, CS maternal C–post-weaning S, SC maternal S–post-weaning C, SS maternal C–post-weaning S. C Chow diet, S Chow diet supplemented with chocolate and soft drink ad libitum
Glucose homeostasis and lipid plasma profile
At the oral glucose tolerance test (OGTT) at 12 weeks of age, the area under the curve (AUC) for blood glucose was larger in CS and SS offspring than in CC offspring (p < 0.05, p < 0.001). Furthermore, SS offspring had increased AUC of blood glucose compared to SC (p < 0.001) and CS (p < 0.05) (Fig. 4a). AUC of plasma insulin was increased in both CS and SS offspring compared to SC (p < 0.001) and CC offspring (p < 0.001, p < 0.01). We also observed that SC offspring had a reduced AUC of plasma insulin compared to CC (p < 0.05) (Fig. 4b). During the OGTT, we measured the levels of the stress hormone corticosterone 15 min after the glucose load. SS offspring had increased plasma corticosterone levels compared to SC (p < 0.01), CS (p < 0.05) and CC (0.001). CS offspring also had higher levels of corticosterone compared to CC offspring (p < 0.05) (Fig. 4c).
a OGGT glucose response, b OGGT insulin response, c OGGT, corticosterone. Data are expressed as means ± SEM, n = 8–10, analysed by linear mixed models (lme), unpaired t-test in R. (*p < 0.05, **p < 0.01, ***p < 0.001). CC: maternal C–post-weaning C, CS maternal C–post-weaning S, SC maternal S–post-weaning C, SS maternal C–post-weaning S, C Chow diet, S Chow diet supplemented with chocolate and soft drink ad libitum
The glucose tolerance at 25 weeks of age showed that CS offspring had significantly higher AUC of blood glucose compared to SC offspring (p < 0.05) (Fig. 5a). No significant differences were observed in AUC of plasma insulin between the groups (Fig. 5b). The corticosterone levels showed that SS offspring had higher response than SC (p < 0.05) and CC (p < 0.05), while CS had higher corticosterone levels than SC (p < 0.05) (Fig. 5c).
a OGGT glucose response, b OGGT insulin response, c OGGT, corticosterone. Data are expressed as means ± SEM, n = 8–10, analysed by linear mixed models (lme), unpaired t-test in R. (*p < 0.05, **p < 0.01, ***p < 0.001). CC maternal C–post-weaning C, CS maternal C–post-weaning S, SC maternal S–post-weaning C, SS maternal C–post-weaning S, C Chow diet, S Chow diet supplemented with chocolate and soft drink ad libitum
Discussion
The hypothalamus is suggested to be one of the first tissues to show signs of inflammation upon HFD exposure predisposing to obesity12,19,33,34. The present study supported the hypothesis that early changes in the hypothalamus can predispose for obesity, as the hypothalamic inflammatory response preceded increased weight gain32. Only limited focus has been given to hypothalamic oxidative stress and inflammation in recent publications on metabolic programming. We demonstrated that offspring exposed to maternal chocolate and soft drink (S) had increased early anti-oxidative markers, such as SOD2 and catalase, which is likely a response to protect against cellular reactive oxidative stress (ROS) and damage23. However, at 12 weeks of age expression of the anti-oxidative markers tended to decrease, which may indicate a reduced ability to eliminate ROS. At the same time, pro-inflammatory markers, such as TNFα and IL-1β, were up-regulated in the hypothalamus of all offspring exposed to the S diet during some point of their life (more pronounced in the offspring fed with postnatal S diet). Hypothalamic expression of NFκB was not affected by the increase of TNFα and IL-1β as previously found18,28,35,36. However, since astrogliosis was observed in the adult offspring fed with postnatal S diet32, increased activation of the NFκB pathway may likely appear later as JNK and NF-κB pathways often drive development of astrogliosis37,38. Nevertheless, the study results indicated that hypothalamic oxidative stress likely appears before inflammation and the rise in anti-oxidative stress response could not counteract hypothalamic inflammation in the offspring exposed to the maternal S diet. In addtition to hypothalamic inflammation in the offspring aged 12 weeks, the increased adiposity and plasma FFA may also affect hypothalamic neuronal circuits through increased systemic cytokines and adipokines and contributing to the obese phenotype12,39,40,41. At 12 weeks of age, hypothalamic inflammation was not associated with impaired glucose tolerance or insulin resistance as previously indicated15,16,17,18,19. Impaired glucose tolerance was only observed in CS and SS offspring and not in SC offspring at 12 weeks of age. Also, no indications of impaired glucose tolerance was observed at 25 weeks of age, although CS and SS offspring had developed astrogliosis32. Thus, neuronal areas responsible for peripheral glucose homeostasis was not affected by the intake of S diet at 25 weeks of age. The impaired glucose tolerance at 12 weeks of age could rather be an effect of reduced insulin sensitivity as insulin secretion was increased. The increased insulin secretion may be a result of increased β-cell mass as this has been observed in rats fed with a candy supplement42. At 25 weeks of age, a higher glucose bolus (4 mg/kg) was used in the OGTT to ensure a sufficient glucose response and to obtain a clear difference in insulin secretion in the offspring. The findings revealed that offspring in every group had an increase in insulin capacity to lower the glucose bolus. This was indicated by a higher insulin peak and same glucose profile compared to the 12 weeks offspring, suggesting that the glucose handling was not decreased at 25 weeks of age but rather reversible. During the OGTT, the glucose tolerance and insulin secretion in CS and SS offspring were comparable to CC offspring despite obesity, suggesting a compensatory response compared to the findings at 12 weeks of age. This may either have occurred as a result of up-regulated peripheral insulin sensitivity or increased pancreatic insulin secretory capacity. It has been reviewed previously that maternal HFD was associated with impaired glucose regulation in the offspring at different ages43,44,45,46, but long-term impaired glucose tolerance or insulin resistance has not been observed (25 weeks of age)46 resembling the results in the present study. Furthermore, Fernandez-Twinn and colleagues demonstrated that offspring exposed to a maternal low-protein diet developed improved glucose tolerance in young adulthood (20 weeks of age) followed by an age-dependent loss of glucose tolerance at 21 months of age47. This could also be true for offspring exposed to the maternal HFD46 or maternal S diet in the present study. In addition, during the OGTT, increased plasma corticosterone levels were observed 15 min after glucose bolus in CS and SS offspring at both 12 and 25 weeks of age suggesting that offspring exposed to postnatal S were more stress-sensitive; however, it did not have an effect on glucose tolerance at 25 weeks of age. Also, in the litterature, there are generally conflicting data in both obese humans and rodent models of obesity, as to whether obesity is associated with an increased stress sensitivity48.
Interestingly, SC offspring fed chow diet after weaning was not able to prevent the increase in hypothalamic inflammatory response, adiposity and FFA at 12 weeks. This is consistent with a study of HFD-fed rats that were switched back to a standard chow diet for 8 weeks. These rats lost their excess weight, whereas they maintained an increase in hypothalamic inflammatory markers49. However, it cannot be ruled out that a hypothalamic inflammatory state may be reversible in HFD-fed rats over a longer period of time with standard chow diet than 8–9 weeks. In the present study, adiposity and plasma lipids were normalized, and no signs of astrogliosis was found, in SC offspring at 26 weeks of age, indicating that metabolic changes induced by maternal S diet could apparently be reversed by exposure to a healthy nutrient after weaning, which is in agreement with previous findings50,51,52,53. Nevertheless, despite normalization of the metabolic profile, SC offspring may be prone to develop metabolic disorders later in life compared to offspring exposed to a maternal healthy diet, as early metabolic changes may have long-lasting effects54,55.
In conclusion, we demonstrated that indications of hypothalamic oxidative stress was observed prior to the inflammatory response in offspring exposed to a maternal S diet. Furthermore, both maternal and postnatal S induced hypothalamic inflammation at 12 weeks of age associated with increased adiposity and plasma FFA. This metabolic profile appeared prior to weight gain, thus contributing to the obese phenotype in adulthood. Hypothalamic inflammation was not reversed at 12 weeks of age by switching to chow after weaning, indicating an increased sensitivity to hypothalamic changes in response to maternal S. No correlation was found between hypothalamic inflammation and impaired glucose tolerance and insulin secretion. At 12 weeks of age, reduced glucose tolerance was found in offspring exposed to the postnatal S diet; however, this was reversed at 25 weeks of age, which points towards a compensatory response of the insulin sensitivity or insulin secretion.
References
WHO. Obesity and overweight. World Health Organization. http://www.who.int/mediacentre/factsheets/fs311/en/. 2013.
Bellisle, F., Drewnowski, A., Anderson, G. H., Westerterp-Plantenga, M. & Martin, C. K. Sweetness, satiation, and satiety. J. Nutr. 142, 1149S–1154SS (2012).
Schulze, M. B. et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 292, 927–934 (2004).
Ainge, H., Thompson, C., Ozanne, S. E. & Rooney, K. B. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int. J. Obes. 35, 325–335 (2011).
Rooney, K. & Ozanne, S. E. Maternal over-nutrition and offspring obesity predisposition: targets for preventative interventions. Int. J. Obes. 35, 883–890 (2011).
Bouret, S. G. Early life origins of obesity: role of hypothalamic programming. J. Pediatr. Gastroenterol. Nutr. 48, S31–S38 (2009).
Steculorum, S. M., Vogt, M. C. & Bruning, J. C. Perinatal programming of metabolic diseases: role of insulin in the development of hypothalamic neurocircuits. Endocrinol. Metab. Clin. North. Am. 42, 149–164 (2013).
Glavas, M. M. et al. Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet. Endocrinology 151, 1598–1610 (2010).
Stachowiak, E. K. et al. Maternal obesity affects gene expression and cellular development in fetal brains. Nutr. Neurosci. 16, 96–103 (2013).
Chang, G. Q., Gaysinskaya, V., Karatayev, O. & Leibowitz, S. F. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J. Neurosci. 28, 12107–12119 (2008).
Langley-Evans, S. C. Metabolic programming in pregnancy: studies in animal models. Genes Nutr. 2, 33–38 (2007).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012).
Lumeng, C. N. & Saltiel, A. R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 (2011).
White, C. L. et al. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol. Dis. 35, 3–13 (2009).
Rother, E. et al. Hypothalamic JNK1 and IKKbeta activation and impaired early postnatal glucose metabolism after maternal perinatal high-fat feeding. Endocrinology 153, 770–781 (2012).
Calegari, V. C. et al. Inflammation of the hypothalamus leads to defective pancreatic islet function. J. Biol. Chem. 286, 12870–12880 (2011).
Arruda, A. P. et al. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology 152, 1314–1326 (2011).
Gregor, M. F. & Hotamisligil, G. S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 (2011).
Jais, A. & Bruning, J. C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Invest. 127, 24–32 (2017).
Dandona, P., Aljada, A. & Bandyopadhyay, A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 25, 4–7 (2004).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Wisse, B. E. & Schwartz, M. W. Does hypothalamic inflammation cause obesity? Cell. Metab. 10, 241–242 (2009).
Maritim, A. C., Sanders, R. A. & Watkins, J. B. III Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 17, 24–38 (2003).
Zelko, I. N., Mariani, T. J. & Folz, R. J. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 33, 337–349 (2002).
Zhang, X. et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
Zhang, X., Dong, F., Ren, J., Driscoll, M. J. & Culver, B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp. Neurol. 191, 318–325 (2005).
Valko, M. et al. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 39, 44–84 (2007).
Romanatto, T. et al. TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient—effects on leptin and insulin signaling pathways. Peptides 28, 1050–1058 (2007).
Bilbo, S. D. & Tsang, V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 24, 2104–2115 (2010).
Grayson, B. E. et al. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 151, 1622–1632 (2010).
Kjaergaard M., Nilsson C., Rosendal A., Nielsen M. O., Raun K. Maternal chocolate and sucrose soft drink intake induces hepatic steatosis in rat offspring associated with altered lipid gene expression profile. Acta Physiol. 19, 2013 https://doi.org/10.1111/apha.12138.:10.
Kjaergaard, M. et al. Differential hypothalamic leptin sensitivity in obese rat offspring exposed to maternal and postnatal intake of chocolate and soft drink. Nutr. Diabetes 7, e242 (2017).
de Souza, C. T. et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146, 4192–4199 (2005).
Velloso, L. A., Araujo, E. P. & de Souza, C. T. Diet-induced inflammation of the hypothalamus in obesity. Neuroimmunomodulation 15, 189–193 (2008).
Morgan, M. J. & Liu, Z. G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 21, 103–115 (2011).
Muriach, M., Flores-Bellver, M., Romero, F. J. & Barcia, J. M. Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid. Med. Cell Longev. 2014, 102158 (2014).
Sparacio, S. M., Zhang, Y., Vilcek, J. & Benveniste, E. N. Cytokine regulation of interleukin-6 gene expression in astrocytes involves activation of an NF-kappa B-like nuclear protein. J. Neuroimmunol. 39, 231–242 (1992).
Sofroniew, M. V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647 (2009).
Lam, T. K., Schwartz, G. J. & Rossetti, L. Hypothalamic sensing of fatty acids. Nat. Neurosci. 8, 579–584 (2005).
Bruce-Keller, A. J., Keller, J. N. & Morrison, C. D. Obesity and Vulnerability of the CNS. Biochim. Biophys. Acta 1792, 395–400 (2009).
Kershaw, E. E. & Flier, J. S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89, 2548–2556 (2004).
Raun, K. et al. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes 56, 8–15 (2007).
Buckley, A. J. et al. Altered body composition and metabolism in the male offspring of high fat-fed rats. Metabolism 54, 500–507 (2005).
Samuelsson, A. M. et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51, 383–392 (2008).
Taylor, P. D. et al. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am. J. Physiol. 288, R134–R139 (2005).
Howie, G. J., Sloboda, D. M., Kamal, T. & Vickers, M. H. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J. Physiol. 587, 905–915 (2009).
Fernandez-Twinn, D. S. et al. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R368–R373 (2005).
Levin, B. E., Richard, D., Michel, C. & Servatius, R. Differential stress responsivity in diet-induced obese and resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R1357–R1364 (2000).
Wang, X. et al. Increased hypothalamic inflammation associated with the susceptibility to obesity in rats exposed to high-fat diet. Exp. Diabetes Res. 2012, 847246 (2012).
Enriori, P. J. et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell. Metab. 5, 181–194 (2007).
Chen, H. & Morris, M. J. Differential responses of orexigenic neuropeptides to fasting in offspring of obese mothers. Obesity 17, 1356–1362 (2009).
Page, K. C., Malik, R. E., Ripple, J. A. & Anday, E. K. Maternal and postweaning diet interaction alters hypothalamic gene expression and modulates response to a high-fat diet in male offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1049–R1057 (2009).
Ong Z. Y., Muhlhausler B. S. Consuming a low-fat diet from weaning to adulthood reverses the programming of food preferences in male, but not female, offspring of ‘junk food’-fed rat dams. Acta Physiol. 10; 2014 Jan;210(1):127-41. https://doi.org/10.1111/apha.12132. Epub 2013 Jul 10.
Barker, D. J. Fetal origins of coronary heart disease. BMJ 311, 171–174 (1995).
Hales, C. N. et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303, 1019–1022 (1991).
Acknowledgements
We express our thanks to all the animal technicians in the Animal Unit and the laboratory technicians of Novo Nordisk A/S and the Faculty of Health and Medical Sciences for excellent assistance during this study. For funding details see Ref. 32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kirsten Raun is a full-time employee at Novo Nordisk and also a minor stock owner through an employee offering program.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kjaergaard, M., Nilsson, C., Nielsen, M.O. et al. Hypothalamic oxidative stress and inflammation, and peripheral glucose homeostasis in Sprague-Dawley rat offspring exposed to maternal and postnatal chocolate and soft drink. Nutr & Diabetes 8, 44 (2018). https://doi.org/10.1038/s41387-018-0051-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-018-0051-z