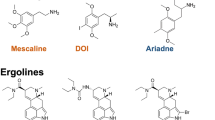
Abstract
Recently, psychedelics have emerged as promising therapeutics for numerous neuropsychiatric disorders. While their potential in the clinic has yet to be fully elucidated, understanding their molecular and biological mechanisms is imperative as these compounds are becoming widely used both in therapeutic and recreational contexts. This review examines the current understanding of basic biology, pharmacology, and structural biology in an attempt to reveal both the knowns and unknowns within the field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nichols DE. Psychedelics. Pharm Rev. 2016;68:264–355. https://doi.org/10.1124/pr.115.011478.
McClure-Begley TD, Roth BL. The promises and perils of psychedelic pharmacology for psychiatry. Nat Rev Drug Discov. 2022;21:463–73. https://doi.org/10.1038/s41573-022-00421-7.
Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30:1181–97. https://doi.org/10.1177/0269881116675513.
Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30:1165–80. https://doi.org/10.1177/0269881116675512.
Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, et al. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2021;78:481–9. https://doi.org/10.1001/jamapsychiatry.2020.3285.
Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. 2021;384:1402–11. https://doi.org/10.1056/NEJMoa2032994.
Holze F, Gasser P, Muller F, Dolder PC, Liechti ME. Lysergic acid diethylamide-assisted therapy in patients with anxiety with and without a life-threatening illness: a randomized, double-blind, placebo-controlled Phase II study. Biol Psychiatry. 2023;93:215–23. https://doi.org/10.1016/j.biopsych.2022.08.025.
Goodwin GM, Aaronson ST, Alvarez O, Arden PC, Baker A, Bennett JC, et al. Single-dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med. 2022;387:1637–48. https://doi.org/10.1056/NEJMoa2206443.
Wooley DW, Shaw E. A biochemical and pharmacological suggestion about certain mental disorders. Proc Natl Acad Sci USA. 1954;40:228–31.
Gaddum JH, Hameed KA. Drugs which antagonize 5-hydroxytryptamine. Br J Pharm. 1954;9:240–8.
Gaddum JH, Khan A, Hathway DE, Stephens FF. Quantitative studies of antagonists for 5-hydroxytryptamine. Q J Exp Physiol. 1955;40:49–74.
Aghjanian GK, Foote WE, Sheard MH. Lysergic acid diethylamide: sensitive neuronal units in the midbrain raphe. Science. 1968;161:706–8.
Kelly PH, Iversen LL. LSD as an agonist at mesolimbic dopamine receptors. Psychopharmacologia. 1975;45:221–4.
Geyer MA, Gordon J, Adams LM. Behavioral effects of xylamine-induced depletions of brain norepinephrine: interaction with LSD. Pharm Biochem Behav. 1985;23:619–25.
Peroutka SJ, Snyder SH. Multiple serotonin receptors: differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol Pharm. 1979;16:687–99.
Glennon RA, Seggel MR, Soine WH, Herrick-Davis K, Lyon RA, Titeler M. 125I-2,5-dimethoxy-4-iodophenyl-2-aminopropane (DOI): an iodinated radioligand that specifically labels the agonist high affinity state of the 5HT2 serotonin receptor. J Med Chem. 1988;31:5–7.
Johnson MP, Hoffman AJ, Nichols DE, Mathis CA. Binding to the serotonin 5-HT2 receptor by the enantiomers of 125I-DOI. Neuropharmacology. 1987;26:1803–6. https://doi.org/10.1016/0028-3908(87)90138-9.
Kroeze WK, Sassano MF, Huang XP, Lansu K, McCorvy JD, Giguère PM, et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol. 2015;22:362–9. https://doi.org/10.1038/nsmb.3014.
Corne SJ, Pickering RW, Warner BT. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br J Pharm Chemother. 1963;20:106–20. https://doi.org/10.1111/j.1476-5381.1963.tb01302.x.
Malick JB, Doren E, Barnett A. Quipazine-induced head-twitch in mice. Pharm Biochem Behav. 1977;6:325–9.
Rodriguez R, Pardo EG. Quipazine, a new type of antidepressant agent. Psychopharmacologia. 1971;21:89–100. https://doi.org/10.1007/bf00404000.
Glennon RA, Titler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–11.
Halberstadt AL, Chatha M, Klein AK, Wallach J, Brandt SD. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology. 2020;167:107933. https://doi.org/10.1016/j.neuropharm.2019.107933.
Sanders-Bush E, Burris KD, Knoth K. Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis. J Pharmacol Exp Ther. 1988;246:924–8.
Sard H, Kumaran G, Morency C, Roth BL, Toth BA, He P, et al. SAR of psilocybin analogs: discovery of a selective 5-HT(2C) agonist. Bioorg Med Chem Lett. 2005;15:4555–9.
Leysen JE, Niemegeers CJE, Van Nueten JM, Laduron PM. [3H]-ketanserin (R 41 468) a selective 3H-ligand for serotonin2 receptor binding sites. Mol Pharmacol. 1982;21:301–14.
Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–902.
Quednow BB, Kometer M, Geyer MA, Vollenweider FX. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology. 2012;37:630–40. https://doi.org/10.1038/npp.2011.228.
Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry. 2012;72:898–906. https://doi.org/10.1016/j.biopsych.2012.04.005.
Kometer M, Schmidt A, Jancke L, Vollenweider FX. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on alpha oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci. 2013;33:10544–51. https://doi.org/10.1523/JNEUROSCI.3007-12.2013.
Bernasconi F, Schmidt A, Pokorny T, Kometer M, Seifritz E, Vollenweider FX. Spatiotemporal brain dynamics of emotional face processing modulations induced by the serotonin 1A/2A receptor agonist psilocybin. Cereb Cortex. 2014;24:3221–31. https://doi.org/10.1093/cercor/bht178.
Pokorny T, Preller KH, Kraehenmann R, Vollenweider FX. Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur Neuropsychopharmacol. 2016;26:756–66. https://doi.org/10.1016/j.euroneuro.2016.01.005.
Barrett FS, Preller KH, Herdener M, Janata P, Vollenweider FX. Serotonin 2A receptor signaling underlies LSD-induced alteration of the neural response to dynamic changes in music. Cereb Cortex. 2018;28:3939–50. https://doi.org/10.1093/cercor/bhx257.
Preller KH, Burt JB, Ji JL, Schleifer CH, Adkinson BD, Stämpfli P, et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife. 2018;7e35082. https://doi.org/10.7554/eLife.35082.
Abbas A, Roth B. Pimavanserin tartrate: a 5-HT2A inverse agonist with potential for treating various neuropsychiatric disorders. Expert Opin Pharmacother. 2008;9:3251–9. https://doi.org/10.1517/14656560802532707.
Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, et al. Characterization of the 5-HT2 antagonist MDL 100907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. J Pharmacol Exp Ther. 1993;266:684–91.
Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, et al. Crystal structure of an LSD-bound human serotonin receptor. Cell. 2017;168:377–89.e312. https://doi.org/10.1016/j.cell.2016.12.033.
Kim K, Che T, Panova O, DiBerto JF, Lyu J, Krumm BE. et al. Structure of a hallucinogen activated gq-coupled 5-HT2A serotonin receptor. Cell. 2020;182:1574–88.e1519. https://doi.org/10.1016/j.cell.2020.08.024.
Kaplan AL, Confair DN, Kim K, Barros-Álvarez X, Rodriguiz RM, Yang Y, et al. Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity. Nature. 2022;610:582–91. https://doi.org/10.1038/s41586-022-05258-z.
Cao D, Yu J, Wang H, Luo Z, Liu X, He L, et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science. 2022;375:403–11. https://doi.org/10.1126/science.abl8615.
Cao C, Barros-Álvarez X, Zhang S, Kim K, Dämgen MA, Panova O, et al. Signaling snapshots of a serotonin receptor activated by the prototypical psychedelic LSD. Neuron. 2022;110:3154–67.e3157. https://doi.org/10.1016/j.neuron.2022.08.006.
Gumpper RH, Fay JF, Roth BL. Molecular insights into the regulation of constitutive activity by RNA editing of 5HT(2C) serotonin receptors. Cell Rep. 2022;40:111211. https://doi.org/10.1016/j.celrep.2022.111211.
Roth BL, Nakaki T, Chuang DM, Costa E. Aortic recognition sites for serotonin (5HT) are coupled to phospholipase C and modulate phosphatidylinositol turnover. Neuropharmacology. 1984;23:1223–5.
Conn PJ, Sanders-Bush E. Selective 5-HT2 antagonists inhibit serotonin-stimulated phosphatidylinositol metabolism in cerebral cortex. Neuropharmacology. 1984;23:993–6.
Leysen JE, De Chaffoy De Courcelles D, De Clerck F, Niemegeers CJE, Van Nueten JM. Serotonin-S2 receptor binding sites and functional correlates. Neuropharmacology. 1984;23:1493–501.
Roth BL, Nakaki T, Chuang DM, Costa E. 5-Hydroxytryptamine2 receptors coupled to phospholipase C in rat aorta: modulation of phosphoinositide turnover by phorbol ester. J Pharm Exp Ther. 1986;238:480–5.
Nakaki T, Roth BL, Chuang DM, Costa E. Phasic and tonic components in 5-HT2 receptor-mediated rat aorta contraction: participation of Ca++ channels and phospholipase C. J Pharm Exp Ther. 1985;234:442–6.
Gray JA, Compton-Toth BA, Roth BL. Identification of two serine residues essential for agonist-induced 5-HT2A receptor desensitization. Biochemistry. 2003;42:10853–62.
Gelber EI, Kroeze WK, Willins DL, Gray JA, Sinar CA, Hyde EG, et al. Structure and function of the third intracellular loop of the 5-hydroxytryptamine(2A) receptor: the third intracellular loop is alpha-helical and binds purified arrestins. J Neurochem. 1999;72:2206–14.
Gray J, Bhatnagar A, Gurevich V, Roth B. The interaction of a constitutively active arrestin with the arrestin-insensitive 5-HT2A receptor induces agonist-independent internalization. Mol Pharmacol. 2003;63:961–72.
Strachan RT, Sheffler DJ, Willard B, Kinter M, Kiselar JG, Roth BL. Ribosomal S6 kinase 2 directly phosphorylates the 5-HT2A serotonin receptor thereby modulating 5-HT2A signaling. J Biol Chem. 2009;284:5557–73. https://doi.org/10.1074/jbc.M805705200.
Sheffler DJ, Kroeze WK, Garcia BG, Deutch AY, Hufeisen SJ, Leahy P, et al. p90 ribosomal S6 kinase 2 exerts a tonic brake on G protein-coupled receptor signaling. Proc Natl Acad Sci USA. 2006;103:4717–22.
Strachan RT, Allen JA, Sheffler DJ, Roth BL. p90 Ribosomal S6 kinase 2, a novel GPCR kinase, is required for growth factor-mediated attenuation of GPCR signaling. Biochemistry. 2010;49:2657–71. https://doi.org/10.1021/bi901921k.
Strachan RT, Sciaky N, Cronan MR, Kroeze WK, Roth BL. Genetic deletion of p90 ribosomal S6 kinase 2 alters patterns of 5-hydroxytryptamine 2A serotonin receptor functional selectivity. Mol Pharm. 2010;77:327–38. https://doi.org/10.1124/mol.109.061440.
Garcia EE, Smith RL, Sanders-Bush E. Role of G(q) protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology. 2007;52:1671–7. https://doi.org/10.1016/j.neuropharm.2007.03.013.
Rodriguiz RM, Nadkarni V, Means CR, Pogorelov VM, Chiu YT, Roth BL, et al. LSD-stimulated behaviors in mice require beta-arrestin 2 but not beta-arrestin 1. Sci Rep. 2021;11:17690. https://doi.org/10.1038/s41598-021-96736-3.
Lucaites VL, Nelson DL, Wainscott DB, Baez M. Receptor subtype and density determine the coupling repertoire of the 5-HT2 receptor subfamily. Life Sci. 1996;59:1081–95. https://doi.org/10.1016/0024-3205(96)00423-7.
Zhang JY, Ashby CR Jr, Wang RY. Effect of pertussis toxin on the response of rat medial prefrontal cortex cells to the iontophoresis of serotonin receptor agonists. J Neural Transm Gen Sect. 1994;95:165–72. https://doi.org/10.1007/BF01271563.
Schmitz GP, Chiu YT, König GM, Kostenis E, Roth BL, Herman MA. Psychedelic compounds directly excite 5-HT2A layer 5 pyramidal neurons in the prefrontal cortex through a 5-HT2A Gq-mediated activation mechanism. bioRxiv [Preprint]. 2022 2022.2011.2015.516655. https://doi.org/10.1101/2022.11.15.516655.
Garnovskaya MN, Nebigil CG, Arthur JM, Spurney RF, Raymond JR. 5-Hydroxytryptamine2A receptors expressed in rat renal mesangial cells inhibit cyclic AMP accumulation. Mol Pharm. 1995;48:230–7.
González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–52.
Cornea-Hébert V, Watkins KC, Roth BL, Kroeze WK, Gaudreau P, Leclerc N, et al. Similar ultrastructural distribution of the 5-HT2A serotonin receptor and microtubule-associated protein MAP1A in cortical dendrites of adult rat. Neuroscience. 2002;113:23–35.
Xia Z, Gray J, Compton-Toth B, Roth B. A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem. 2003;278:21901–8. https://doi.org/10.1074/jbc.M301905200.
Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, et al. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–36. https://doi.org/10.1523/JNEUROSCI.1090-09.2009.
Jones KA, Srivastava DP, Allen JA, Strachan RT, Roth BL, Penzes P. Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci USA. 2009;106:19575–80. https://doi.org/10.1073/pnas.0905884106.
Bhatnagar A, Sheffler D, Kroeze W, Compton-Toth B, Roth B. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected G alpha(q)-coupled protein receptors. J Biol Chem. 2004;279:34614–23. https://doi.org/10.1074/jbc.M404673200.
Allen JA, Yadav PN, Setola V, Farrell M, Roth BL. Schizophrenia risk gene CAV1 is both pro-psychotic and required for atypical antipsychotic drug actions in vivo. Transl Psych. 2011;1:e33 https://doi.org/10.1038/tp.2011.35.
Jones KA, Srivastava DP, Allen JA, Strachan RT, Roth BL, Penzes P. Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci USA. 2009;106:19575–80. https://doi.org/10.1073/pnas.0905884106.
Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170–82. https://doi.org/10.1016/j.celrep.2018.05.022.
Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron. 2021;109:2535–44.e2534. https://doi.org/10.1016/j.neuron.2021.06.008.
Johnson MP, Loncharich RJ, Baez M, Nelson DL. Species variations in transmembrane region V of the 5-hydroxytryptamine type 2A receptor alter the structure-activity relationship of certain ergolines and tryptamines. Mol Pharm. 1994;45:277–86.
Johnson MP, Baez M, Kursar JD, Nelson DL. Species differences in 5-HT2A receptors: cloned pig and rhesus monkey 5-HT2A receptors reveal conserved transmembrane homology to the human rather than rat sequence. Biochim Biophys Acta. 1995;1236:201–6.
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–43. https://doi.org/10.1038/s41586-020-2308-7.
Davies MA, Setola V, Strachan RT, Sheffler DJ, Salay E, Hufeisen SJ, et al. Pharmacologic analysis of non-synonymous coding h5-HT2A SNPs reveals alterations in atypical antipsychotic and agonist efficacies. Pharmacogenomics J. 2006;6:42–51. https://doi.org/10.1038/sj.tpj.6500342.
Schmitz GP, Jain MK, Slocum ST, Roth BL. 5-HT(2A) SNPs alter the pharmacological signaling of potentially therapeutic psychedelics. ACS Chem Neurosci. 2022;13:2386–98. https://doi.org/10.1021/acschemneuro.1c00815.
Kroeze WK, Sassano MF, Huang XP, Lansu K, McCorvy JD, Giguère PM, et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol. 2015;22:362–9. https://doi.org/10.1038/nsmb.3014.
Ray TS. Psychedelics and the human receptorome. PLoS One. 2010;5:e9019. https://doi.org/10.1371/journal.pone.0009019.
Cameron LP, Patel SD, Vargas MV, Barragan EV, Saeger HN, Warren HT, et al. 5-HT2ARs mediate therapeutic behavioral effects of psychedelic tryptamines. ACS Chem Neurosci. 2023;14:351–8. https://doi.org/10.1021/acschemneuro.2c00718.
de la Fuente Revenga M, Zhu B, Guevara CA, Naler LB, Saunders JM, Zhou Z, et al. Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep. 2021;37:109836. https://doi.org/10.1016/j.celrep.2021.109836.
Hesselgrave N, Troppoli TA, Wulff AB, Cole AB, Thompson SM. Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci USA. 2021;118:e2022489118. https://doi.org/10.1073/pnas.2022489118.
Sard H, Kumaran G, Morency C, Roth BL, Toth BA, He P, et al. SAR of psilocybin analogs: discovery of a selective 5-HT 2C agonist. Bioorg Med Chem Lett. 2005;15:4555–9.
Huang XP, Setola V, Yadav PN, Allen JA, Rogan SC, Hanson BJ, et al. Parallel functional activity profiling reveals valvulopathogens are potent 5-hydroxytryptamine(2B) receptor agonists: implications for drug safety assessment. Mol Pharm. 2009;76:710–22. https://doi.org/10.1124/mol.109.058057.
Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, et al. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–41.
Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, et al. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharm. 2000;57:75–81.
Roth BL. Drugs and valvular heart disease. N Engl J Med. 2007;356:6–9.
Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, et al. 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharm. 2003;63:1223–9.
Connolly HM, McGoon MD. Obesity drugs and the heart. Curr Probl Cardiol. 1999;24:745–92.
Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med. 2007;356:39–46.
Montastruc F, Montastruc G, Vigreux P, Bruneval P, Guilbeau-Frugier C, Cron C, et al. Valvular heart disease in a patient taking 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’). Br J Clin Pharm. 2012;74:547–8. https://doi.org/10.1111/j.1365-2125.2012.04252.x.
Droogmans S, Cosyns B, D’haenen H, Creeten E, Weytjens C, Franken PR, et al. Possible association between 3,4-methylenedioxymethamphetamine abuse and valvular heart disease. Am J Cardiol. 2007;100:1442–5. https://doi.org/10.1016/j.amjcard.2007.06.045.
Cavero I, Guillon JM. Safety pharmacology assessment of drugs with biased 5-HT(2B) receptor agonism mediating cardiac valvulopathy. J Pharm Toxicol Methods. 2014;69:150–61. https://doi.org/10.1016/j.vascn.2013.12.004.
Newman-Tancredi A, Cussac D, Quentric Y, Touzard M, Verrièle L, Carpentier N, et al. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. III. Agonist and antagonist properties at serotonin, 5-HT(1) and 5-HT(2), receptor subtypes. J Pharm Exp Ther. 2002;303:815–22.
Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharm Exp Ther. 1997;282:699–706.
Griffith JD, Nutt JG, Jasinski DR. A comparison of fenfluramine and amphetamine in man. Clin Pharm Ther. 1975;18:563–70. https://doi.org/10.1002/cpt1975185part1563.
Koreen AR, Lieberman JA, Alvir J, Chakos M. The behavioral effect of m-chlorophenylpiperazine (mCPP) and methylphenidate in first-episode schizophrenia and normal controls. Neuropsychopharmacology. 1997;16:61–68. https://doi.org/10.1016/S0893-133X(96)00160-1.
Parkes JD, Marsden CD, Donaldson I, Galea-Debono A, Walters J, Kennedy G, et al. Bromocriptine treatment in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1976;39:184–93. https://doi.org/10.1136/jnnp.39.2.184.
Knoll J, Vizi ES. Cross-tolerance between para-bromo-methamphetamine (V-111) and LSD-25. Pharmacology. 1970;4:278–86.
Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature. 2021;589:474–9. https://doi.org/10.1038/s41586-020-3008-z.
Levit Kaplan A, Strachan RT, Braz JM, Craik V, Slocum S, Mangano T, et al. Structure-based design of a chemical probe set for the 5-HT5A serotonin receptor. J Med Chem. 2022;65:4201–17. https://doi.org/10.1021/acs.jmedchem.1c02031.
Kwan AC, Olson DE, Preller KH, Roth BL. The neural basis of psychedelic action. Nat Neurosci. 2022;25:1407–19. https://doi.org/10.1038/s41593-022-01177-4.
Casey AB, Cui M, Booth RG, Canal CE. “Selective” serotonin 5-HT(2A) receptor antagonists. Biochem Pharm. 2022;200:115028 https://doi.org/10.1016/j.bcp.2022.115028.
Leysen JE, Gommeren W, Van Gompel P, Wynants J, Janssen PAJ. Non-serotonergic [3H]-ketanserin binding sites in striatal membranes are associated with a dopac release system on dopaminergic nerve endings. Eur J Pharm. 1987;134:373–5.
Roth BL, McLean S, Zhu XZ, Chuang DM. Characterization of two [3H]ketanserin recognition sites in rat striatum. J Neurochem. 1987;49:1833–8.
Felder CC, Kanterman RY, Ma AL, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci USA. 1990;87:2187–91.
Berg KA, Maayani S, Goldfarb J, Clarke WP. Pleiotropic behavior of 5-HT2A and 5-HT2C receptor agonists. Ann N Y Acad Sci. 1998;861:104–10.
Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharm. 1998;54:94–104.
Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharm Exp Ther. 2003;304:229–37.
González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–7. https://doi.org/10.1038/nature06612.
Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011;493:76–9. https://doi.org/10.1016/j.neulet.2011.01.046.
Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuéllar F, et al. Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A.mGlu2) receptor heteromerization and its psychoactive behavioral function. J Biol Chem. 2012;287:44301–19. https://doi.org/10.1074/jbc.M112.413161.
Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M, et al. Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology. 2012;62:2184–91. https://doi.org/10.1016/j.neuropharm.2012.01.010.
Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–8.
Nagai Y, Miyakawa N, Takuwa H, Hori Y, Oyama K, Ji B, et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat Neurosci. 2020;23:1157–67. https://doi.org/10.1038/s41593-020-0661-3.
Zhang S, Gumpper RH, Huang XP, Liu Y, Krumm BE, Cao C, et al. Molecular basis for selective activation of DREADD-based chemogenetics. Nature. 2022;612:354–62. https://doi.org/10.1038/s41586-022-05489-0.
Yu B, Becnel J, Zerfaoui M, Rohatgi R, Boulares AH, Nichols CD. Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency. J Pharm Exp Ther. 2008;327:316–23. https://doi.org/10.1124/jpet.108.143461.
Grailhe R, Waeber C, Dulawa SC, Hornung JP, Zhuang X, Brunner D, et al. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron. 1999;22:581–91.
Marona-Lewicka D, Thisted RA, Nichols DE. Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology (Berl). 2005;180:427–35. https://doi.org/10.1007/s00213-005-2183-9.
Zhang S, Chen H, Zhang C, Yang Y, Popov P, Liu J, et al. Inactive and active state structures template selective tools for the human 5-HT5A receptor. Nat Struct Mol Biol. 2022;29:677–87. https://doi.org/10.1038/s41594-022-00796-6.
Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116:107–17.
Xia Z, Hufeisen SJ, Gray JA, Roth BL. The PDZ-binding domain is essential for the dendritic targeting of 5-HT(2A) serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience. 2003;122:907–20.
Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364:eaat8078. https://doi.org/10.1126/science.aat8078.
Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci. 2005;21:1299–303. https://doi.org/10.1111/j.1460-9568.2005.03968.x.
Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38:157–60.
Duman RS. Neuropharmacology in the next millennium: promise for breakthrough discoveries [editorial]. Neuropsychopharmacology. 1999;20:97–8.
Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71:996–1005. https://doi.org/10.1016/j.biopsych.2011.09.030.
Gewirtz JC, Chen AC, Terwilliger R, Duman RC, Marek GJ. Modulation of DOI-induced increases in cortical BDNF expression by group II mGlu receptors. Pharm Biochem Behav. 2002;73:317–26.
Cunningham MJ, Bock HA, Serrano IC, Bechand B, Vidyadhara DJ, Bonniwell EM, et al. Pharmacological mechanism of the non-hallucinogenic 5-HT(2A) agonist ariadne and analogs. ACS Chem Neurosci. 2022;14:119–35. https://doi.org/10.1021/acschemneuro.2c00597.
Karst M, Halpern JH, Bernateck M, Passie T. The non-hallucinogen 2-bromo-lysergic acid diethylamide as preventative treatment for cluster headache: an open, non-randomized case series. Cephalalgia. 2010;30:1140–4. https://doi.org/10.1177/0333102410363490.
Hougaku H, Matsumoto M, Hata R, Handa N, Imaizumi M, Sugitani Y. et al. [Therapeutic effect of lisuride maleate on post-stroke depression]. Nihon Ronen Igakkai Zasshi. 1994;31:52–9. https://doi.org/10.3143/geriatrics.31.52.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9. https://doi.org/10.1038/s41586-021-03819-2.
Funding
Work in the Roth lab is supported by grants from the NIH and DARPA to BLR as well as the Michael Hooker Distinguished Professorship.
Author information
Authors and Affiliations
Contributions
BLR and RHG jointly wrote the paper and created the figures.
Corresponding author
Ethics declarations
Competing interests
BLR is a member of the Scientific Advisory Boards of Septerna Pharmaceuticals, Escient Pharmaceuticals and Onsero, Inc. As well, BLR is a scientific co-founder of Onsero and is listed as an inventor on patents related to the research in this review article. RHG declares no conflicts.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gumpper, R.H., Roth, B.L. Psychedelics: preclinical insights provide directions for future research. Neuropsychopharmacol. 49, 119–127 (2024). https://doi.org/10.1038/s41386-023-01567-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01567-7
This article is cited by
-
Therapeutic mechanisms of psychedelics and entactogens
Neuropsychopharmacology (2024)
-
Effects of congeners of amphetamine on the human heart
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)