Abstract
Previous work indicated that deep brain stimulation (DBS) of the nucleus accumbens shell in male rats attenuated reinstatement of cocaine seeking, an animal model of craving. However, the potential differential impact of DBS on specific populations of neurons to drive the suppression of cocaine seeking is unknown. Medium spiny neurons in the nucleus accumbens are differentiated by expression of dopamine D1 receptors (D1DRs) or D2DRs, activation of which promotes or inhibits cocaine-related behaviors, respectively. The advent of transgenic rat lines expressing Cre recombinase selectively in D1DR-containing or D2DR-containing neurons, when coupled with Cre-dependent virally mediated gene transfer of channelrhodopsin (ChR2), enabled mimicry of DBS in a selective subpopulation of neurons during complex tasks. We tested the hypothesis that high frequency DBS-like optogenetic stimulation of D1DR-containing neurons in the accumbens shell would potentiate, whereas stimulation of D2DR-containing neurons in the accumbens shell would attenuate, cocaine-primed reinstatement of cocaine seeking. Results indicated that high frequency, DBS-like optogenetic stimulation of D2DR-containing neurons attenuated reinstatement of cocaine seeking in male rats, whereas DBS-like stimulation of D1DR-containing neurons did not alter cocaine-primed reinstatement. Surprisingly, DBS-like optogenetic stimulation did not alter reinstatement of cocaine seeking in female rats. In rats which only expressed eYFP, intra-accumbens optogenetic stimulation did not alter cocaine reinstatement, indicating that the effect of DBS-like stimulation to attenuate cocaine reinstatement is mediated specifically by ChR2 rather than by prolonged light delivery. These results suggest that DBS of the accumbens may attenuate cocaine-primed reinstatement in male rats through the selective manipulation of D2DR-containing neurons.
Similar content being viewed by others
Introduction
Following cocaine detoxification, the relapse rate among people with cocaine use disorder is discouragingly high [1]. DBS is now viewed as a legitimate therapeutic option for severe, treatment-resistant substance use disorders [2]. Drug self-administration paradigms in rodents and non-human primates have proven invaluable for the assessment of the neurobiological underpinnings of craving-induced relapse of drug seeking. To date, the nucleus accumbens has received the most attention as a potential target region for examining the impact of DBS on cocaine seeking in preclinical models. DBS of the shell subregion of the nucleus accumbens attenuated cocaine priming-induced reinstatement of drug seeking [3, 4], suppressed cue-induced reinstatement of cocaine seeking [5], and decreased alcohol preference and/or intake in rats [6, 7]; however, DBS actually increased cocaine self-administration following escalation of cocaine taking [8]. Targeting DBS to regions of the brain that prominently innervate the nucleus accumbens shell similarly attenuated cocaine seeking, although only DBS in the infralimbic cortex selectively attenuated cocaine seeking versus sucrose seeking [9]. These preclinical findings directly influenced numerous clinical trials at least some of which indicate that DBS of the nucleus accumbens is an effective therapeutic for refractory substance use disorders [2, 10,11,12].
The biological mechanisms underlying the ability of DBS to attenuate cocaine seeking are not clear. The extent to which distinct populations or subtypes of cells in a region are differentially impacted by DBS, or separately contribute to the behavioral consequences of DBS, is impossible to discern by non-discriminant electrical stimulation. Mimicking DBS with optogenetic activation is a powerful approach to address cell type specificity and untangle the physiological mechanisms underlying the effectiveness of DBS. High frequency optogenetic stimulation was chosen for this study because clinical applications of DBS are delivered at high frequencies [2, 13,14,15,16,17,18] as was our prior work examining the effects of electrical DBS on the reinstatement of cocaine seeking [3,4,5, 9]. Opto-DBS has been used to examine circuits in various animal models of neurological disorders, especially Parkinson’s Disease [19,20,21,22]. For example, high frequency opto-DBS of afferent projections to the subthalamic nucleus reversed motor deficits in a parkinsonian rat model [19]. In terms of drugs of abuse, low frequency (12 Hz) optogenetic stimulation of D1DR-containing neurons blocked cocaine-induced behavioral sensitization in mice [23]. The effect of high frequency opto-DBS in animal models of drug craving has not yet been explored.
Approximately 95% of accumbens neurons are GABAergic medium spiny neurons (MSNs), which fall into two major categories, D1DR- or D2DR-expressing, although a relatively small proportion of these cells express both of these receptors [24]. Canonically, D1DR-containing neurons project exclusively to the ventral tegmental area, whereas D2DR-expressing accumbens efferents extend to the ventral pallidum; however, recent evidence suggests there is some overlap in these pathways, at least for outputs from the nucleus accumbens core [25]. Repeated cocaine produces opposing effects in these two classes of accumbens neurons such that transmission through D1DR-expressing MSNs is favored [26,27,28,29]. Specifically, optogenetic activation of D1DR-containing accumbens MSNs promotes cocaine conditioned reward in mice, whereas selective stimulation of D2DR-expressing MSNs does the opposite [30]. D1DR- and D2DR-containing accumbens projections to the ventral pallidum also oppositely regulate the reinstatement of cue-induced cocaine seeking in mice [31], whereas the D1DR-containing projections to the ventral tegmental area do not appear to govern cue-evoked cocaine seeking [32]. The present study aimed to define the cell type specific physiological effects of accumbens opto-DBS on cocaine reinstatement, a widely accepted model of drug craving, using recently developed and validated D1DR-Cre and D2DR-Cre rats [33,34,35]. We tested the hypothesis that high frequency DBS-like optogenetic stimulation of D1DR-containing neurons in the NAc shell would potentiate, whereas stimulation of D2DR-containing neurons in the NAc shell would attenuate, cocaine-primed reinstatement of cocaine seeking.
Materials and methods
Animals and housing
LE-Tg(Drd1a-iCre)3Ottc (RRRC#:00767; D1DR-Cre) and LE-Tg(Drd2-iCre)1Ottc (RRRC#:00768; D2DR-Cre) male founders were generated by the NIDA Optogenetics and Transgenic Technology Core (now Transgenic Rat Project, NIDA IRP, Bethesda, MA) and obtained from the Rat Resource and Research Center (RRRC, Colombia, MS). Female Long Evans breeders were obtained from Charles River Laboratories (Wilmington, MA) and lines were backcrossed frequently to prevent genetic drift. Adult (PND 60-240) male and female Long Evans transgenic D1DR-Cre or D2DR-Cre rats used in experiments were bred in house and Cre expression in D1DR- or D2DR-containing neurons was confirmed by genotyping with the following primers (5′ to 3′): D1DR forward - CTC CTG ATG GAA CCC TAC CA; D2DR forward - TCA GGG AAC CCT CTT TGA GA; Cre reverse - CAC AGT CAG CAG GTT GGA GA (Sigma Aldrich, St. Louis, MO). Rats were individually housed with food and water available ad libitum. A 12/12 h light/dark cycle was used with the lights on at 6:00 a.m. All experimental procedures were performed during the light cycle. All experimental procedures were consistent with the ethical guidelines of the US National Institutes of Health and were approved by the Institutional Animal Care and Use Committee.
Drugs
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Rockville, MD) and dissolved in bacteriostatic 0.9% saline.
Materials
All experiments used Med-Associates (East Fairfield, VT) instrumentation enclosed within ventilated, sound attenuating chambers. Each operant conditioning chamber was equipped with response levers, stimulus lights, food pellet dispensers and injection pumps for injecting drugs intravenously.
Surgery
Prior to surgery, rats were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine. An indwelling silastic catheter was inserted into the right jugular vein and sutured in place as previously described [36, 37]. The catheter was then threaded subcutaneously over the shoulder blade and was routed to a mesh backmount platform (Strategic Applications Inc., Libertyville, Il) that was sutured below the skin between the shoulder blades. Catheters were sealed with plastic obturators when not in use. Following catheter implantation, rats were mounted in a stereotaxic apparatus (Kopf Instruments, CA). Viral infusions and implantation of fiber optic targeting the nucleus accumbens shell were performed using the coordinates +1.0 mm A/P, ± 3.0 mm M/L, −7.3 mm D/V on a 17° angle, relative to bregma (Paxinos and Watson, 1997). Rats received bilateral intra-accumbens infusions (1 µl/side) of a Cre-dependent adeno-associated viral vector (AAV) expressing eYFP (AAV5-EF1a-DIO-eYFP-WPRE-hGH; Addgene) or a Cre-dependent AAV expressing ChR2 with an eYFP tag (AAV5-EF1a-DIO-hChR2(H134R)-eYFP-WPRE-hGH; Addgene) delivered via Hamilton syringes (Reno, NV). Viral vector delivery was immediately followed by implantation of 200 µm fiber optic (Thor Labs, Newton, NJ) attached to stainless steel ferrules (Fiber Instrument Sales, Oriskany, NY) and cut to length to terminate just above the nucleus accumbens shell. Ferrules were cemented in place by affixing dental acrylic to three stainless steel screws fastened to the skull. Rats recovered for seven days; catheters were flushed daily with 0.2 ml of an antibiotic (Timentin, 0.93 mg/ml) dissolved in heparinized saline during the recovery period and after each daily behavioral session.
Ex vivo slice preparation
Brains from rats expressing ChR2 in D1DR- and D2DR-containing neurons in the nucleus accumbens were collected for whole-cell patch-clamp recordings. Slice preparation was similar to our previous research [38,39,40] using methods designed to increase neuronal survival in adult rodents [41, 42]. Rats were deeply anesthetized with isoflurane, then briefly perfused with 20–30 mL ice-cold cutting solution containing (in mM): 92 N-methyl-d-glucamine (NMDG), 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 10 MgSO4, and 0.5 CaCl2, saturated with carbogen (95% O2/5% CO2), pH adjusted to 7.4 with HCl. Following decapitation, brains were extracted and acute coronal slices of the nucleus accumbens (250 μm thick) were obtained using a VT1000S vibratome (Leica, Weltzar, Germany) in 4 °C cutting solution. Slices were made at 4 °C then transferred to a holding chamber of the same cutting solution, and briefly incubated at 37 °C for 10–12 min. Slices were then placed in a beaker of holding ACSF containing (in mM): 86 NaCl, 2.5 KCl, 1.2 NaH2PO4, 35 NaHCO3, 20 HEPES, 25 glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 1 MgCl2, and 2 CaCl2, saturated with carbogen, pH 7.3–7.4, osmolarity 305–315 mOsm. Slices were kept at room temperature and allowed to recover for at least 45 min before performing recordings.
Ex vivo electrophysiology
Slices were placed on a Nikon (Meliville, NY) Eclipse FN1 upright microscope equipped for Differential Interference Contrast (DIC) infrared optics and continuously perfused with oxygenated recording ACSF containing (in mM): NaCl 119, KCl 2.5, NaHCO3 26, NaH2PO4 1.2, glucose 12.5, HEPES 5, MgSO4 1, CaCl2 2, pH 7.3–7.4, osmolarity 305–315 mOsm. Solution was heated to 32 ± 1 °C using an automatic temperature controller (Warner Instruments, Holliston MA). Target brain regions were identified using a 5X objective and individual neurons were magnified with a 40X water immersion lens. Similar to our previous work [38, 39, 43, 44], MSNs in the nucleus accumbens shell were identified by their morphology and low resting membrane potential (−70 to −85 mV), and D1DR- or D2DR-containing neurons were further identified by fluorescent expression with a GFP filter (Nikon). Recording pipettes were pulled from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL) to a resistance of 4.0–5.3 MΩ when filled with intracellular solution. The intracellular solution contained the following (in mM) potassium gluconate 145, KCl 2.5, NaCl 2.5, BAPTA 0.1, HEPES 10, L-glutathione 1.0, sodium phosphocreatine 7.5, Mg-ATP 2.0, and Tris-GTP 0.25, pH 7.2–7.3 with KOH, osmolarity 285–295 mOsm. All recordings were performed in whole-cell current-clamp mode using a MultiClamp 700B amplifier and Digidata 1440 A Digitizer (Molecular Devices, San Jose, CA). ChR2+ neurons were directly stimulated using blue light (473 nm) from an external 4 channel LED driver (Thor Labs, Newton NJ). The LED was coupled to a fluorescent microscope port, allowing illumination of the slice through the objective, placed immediately above the cell. The LED intensity was adjusted to the minimal amplitude (typically 10-50 mA) necessary to elicit action potential firing with a single 5 ms pulse. Action potential firing capacity was measured across various frequencies of optical stimulation (12–130 Hz, 1 s duration). Action potentials were also elicited with increasing depolarizing steps (25–250 pA, 500 ms) with 500 ms step intervals in the presence or absence of 130 Hz optical stimulation. For all recordings, series resistance was 10–25 MΩ, uncompensated, and monitored continuously during recording. Cells with a change in series resistance beyond 20% were not accepted for data analysis. Synaptic potentials were filtered at 3 kHz, amplified 5 times, and then digitized at 20 kHz.
Cocaine self-administration, extinction, and reinstatement
Following the recovery period, rats were placed in operant conditioning chambers and allowed to press a lever for intravenous cocaine infusions (0.25 mg of cocaine in 59 µL of saline, infused over 5 seconds). Rats initially were trained using a fixed ratio 1 (FR1) schedule of reinforcement. When the animals achieved stable responding with the FR1 schedule (less than 15% variation in active lever presses over three consecutive days), they were switched to an FR5 schedule. A 20 s timeout period during which active, drug-paired lever responses had no scheduled consequences followed each cocaine infusion. Each operant chamber was also equipped with an inactive lever. Responses made on the inactive lever had no scheduled consequences. A maximum of 30 cocaine infusions could be earned per daily two hour self-administration session. After 21 days of cocaine self-administration sessions (encompassing both FR1 and FR5 phases), rats underwent an extinction phase during which cocaine was replaced with 0.9% bacteriostatic saline. Daily two hour extinction sessions were conducted until active lever responding was <20% of the responses averaged over the last three days of cocaine self-administration. Reinstatement of cocaine seeking was promoted by non-contingent administration of cocaine (10 mg/kg, i.p.) immediately prior to the initiation of the reinstatement session, during which satisfaction of the response requirement does not produce any reinforcer. Each reinstatement test day was followed by extinction sessions until responding was again <20% of the responses achieved during self-administration across two consecutive sessions. The present study focused exclusively on cocaine-primed reinstatement because electrical DBS selectively attenuates cocaine-primed vs. sucrose-primed reinstatement [3], whereas electrical DBS non-selectively suppresses cue-evoked reinstatement of both cocaine seeking and sucrose seeking [5].
Each rat underwent two reinstatement sessions, during which 473 nm light stimulation at 130 Hz with a 5 ms pulse width or sham opto-DBS (patch cables attached but 0 mW delivered) was administered in a within-subjects counterbalanced fashion to prevent any effect of test order (none observed). Opto-DBS was administered continuously during the 1-hour reinstatement sessions. Light from a 473 nm laser (OptoEngine, Midvale, UT) was split by a rotary joint (Doric Lenses, Quebec, Canada) delivered bilaterally through 200 µm fiber optic patch cables (Thor Labs) connected to the implanted ferrules. A Master 8 pulse generator (AMPI, Jerusalem, Israel) was used to modulate frequency, and laser output was tuned to deliver 1 mW of light to the accumbens to prevent any effect of heat.
Verification of AAV expression and fiber optic placement
After the completion of all experiments, rats were given an overdose of pentobarbital (100 mg/kg) and perfused intracardially with 0.9% saline followed by 4% paraformaldehyde. The brains were removed and coronal sections (40 µm) were collected after sectioning with a vibratome (Technical Products International; St. Louis, MO) for visualization on a confocal microscope (Leica Biosystems, Buffalo Grove, IL). Alternately, rats were decapitated and viral placement was visualized using NIGHTSEA™ DFP™ Dual Fluorescent Protein Excitation Flashlight with NIGHTSEA™ Barrier Filter Glasses (Electron Microscopy Services, Hatfield, PA). Animals with no Cre-dependent eYFP fluorescence or with fluorescence or fiber optic placement outside of the areas of interest were excluded from subsequent data analysis.
Statistics
Statistical analysis was performed in Prism 9.0 with alpha set at p < 0.05. Self-administration data were analyzed with two-way ANOVA with vector (ChR2 vs. eYFP) and strain (D1DR-Cre vs. D2DR-Cre) as factors. Only rats that maintained catheter patency throughout the duration of cocaine self-administration and had proper placement for AAV infusions and fiber optic were included in the analyses. All reinstatement experiments were analyzed with two-way mixed model ANOVAs with vector (ChR2 vs. eYFP control) as the between-subjects factor and stimulation frequency (sham vs. 130 Hz light stimulation) as the repeated measures and within-subjects factor. Pairwise analyses were made with Bonferroni post-tests (p < 0.05).
Results
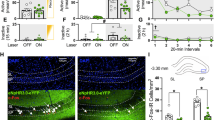
To demonstrate and validate the effect of 130 Hz optogenetic stimulation on firing of nucleus accumbens MSNs, ex vivo patch clamp electrophysiology was performed in slices through the nucleus accumbens from experimentally naïve rats expressing Cre-dependent ChR2 in D1DR- or D2DR-containing neurons. Optogenetic stimulation was applied in 5 ms pulses of light at increasing frequencies and action potential firing was recorded. As shown in the sample traces in Fig. 1A, action potentials are time-locked to light stimulation at 12 Hz, which is expected given that ChR2 can open with high fidelity up to ~40 Hz [45]. Accumbens MSNs also fire in response to light delivery at higher frequencies, including 50 Hz and 130 Hz, although fidelity is diminished at higher frequencies (Fig. 1A). Combining 130 Hz stimulation with depolarizing steps increased the number of action potentials generated relative to action potentials evoked by current steps alone, as depicted in example traces in Fig. 1B. Application of 130 Hz stimulation generated action potentials in the absence of current steps and increased action potential firing evoked by submaximal current steps, but did not enhance action potential firing rate above an individual neuron’s maximum rate elicited by current steps alone (Fig. 1B). Similar effects of high frequency optogenetic stimulation on neuronal firing were observed in D1DR-containing MSNs (example traces) and D2DR-containing MSNs (not shown). Although action potentials are not time-locked at 130 Hz, these data clearly show that high frequency optogenetic stimulation (opto-DBS) enhances neuronal activity.
A Example traces showing action potential firing in response to 25 Hz (left), 50 Hz (center), and 130 Hz (right) optogenetic stimulation. Although fidelity is reduced at higher frequencies, particularly 130 Hz, neurons are activated and fire action potentials. B Sample traces showing action potentials induced by increasing (left to right) current steps alone (top, black) and in combination with 130 Hz optogenetic stimulation (bottom, red). Neuronal firing is increased by co-administration of 130 Hz optogenetic stimulation at baseline (left) and submaximal current injection (center) but does not increase firing above the maximum elicited by current injection (right). C Line graph shows mean (±SEM) daily cocaine infusions earned (left Y-axis) and bar graph shows total cocaine infusions earned (right Y-axis) across 21 days for D1DR-Cre and D2DR-Cre male rats expressing eYFP or ChR2. D Line graph shows mean (±SEM) daily cocaine infusions earned (left Y-axis) and bar graph shows total cocaine infusions earned (right Y-axis) across 21 days for D1DR-Cre and D2DR-Cre female rats expressing eYFP or ChR2.
Rats were allowed to self-administer cocaine for 21 days on an FR1-5 schedule. Figure 1C shows the mean daily cocaine infusions earned (left) and total infusions earned (right) for male D1DR-Cre and D2DR-Cre rats expressing eYFP or ChR2 in the nucleus accumbens shell. In terms of cocaine infusions earned in male rats, there was no main effect of strain [F(1,30) = 0.66, p = 0.4219), no main effect of vector [F(1,30) = 2.60, p = 0.1175], and no strain x vector interaction [F(1,30) = 0.0020, p = 0.9647). Similarly, as shown in Fig. 1D, for female D1DR-Cre and D2DR-Cre rats expressing eYFP or ChR2 in the nucleus accumbens shell there was no main effect of strain [F(1,29) = 1.01, p = 0.3235), no main effect of vector [F(1,29) = 0.014, p = 0.9068], and no strain x vector interaction [F(1,29) = 0.079, p = 0.7812). Cocaine self-administration levels were similar across groups and not associated with performance in subsequent cocaine seeking tests. There was no correlation between total infusions earned and reinstatement in the sham test (r = 0.063, p = 0.6419) or 130 Hz stim test (r = 0.096, p = 0.4370). There is also no correlation between average infusions earned over the last 3 days of cocaine self-administration, when intake is highly stable, and reinstatement in the sham test (r = 0.011, p = 0.9285) or 130 Hz stim test (r = −0.017, p = 0.8896).
Following cocaine self-administration training and extinction of lever pressing, cocaine seeking was assessed in two cocaine-primed reinstatement sessions during which rats received sham and 130 Hz optogenetic stimulation in a within-subjects counterbalanced design. Delivery of optogenetic stimulation was initiated concurrently with the start of the session and administered throughout the entire reinstatement test. High frequency opto-DBS stimulation of nucleus accumbens shell D1DR-containing cells did not alter cocaine priming-induced reinstatement of drug seeking in male rats expressing eYFP (Fig. 2A) or ChR2 (Fig. 2B). In male D1DR-Cre rats, there was no main effect of vector [F(1,14) = 0.00097, p = 0.9756], no main effect of stimulation [F(1,14) = 1.19, p = 0.2931], and no vector by stimulation interaction [F(1,14) = 0.093, p = 0.7646] on active lever presses during the reinstatement sessions (n = 8/group). There was no main effect of vector [F(1,14) = 0.20, p = 0.6422], no main effect of stimulation [F(1,14) = 1.65, p = 0.2196], and a trend to vector by stimulation interaction [F(1,14) = 4.59, p = 0.0503] on inactive lever presses during the reinstatement sessions. Inactive lever presses were slightly lower in the ChR2-Sham group than the ChR2-Stim group, eYFP-Sham, and eYFP-Stim groups, indicating that this trend was likely not driven by a non-specific effect of stimulation. DBS-like optogenetic stimulation of D2DR-containing neurons did not alter reinstatement of cocaine seeking in control rats expressing eYFP (Fig. 2C), but significantly attenuated the reinstatement of cocaine-seeking in rats expressing ChR2 (Fig. 2D). In male D2DR-Cre rats, there was no main effect of vector [F(1,16) = 0.21, p = 0.6527], a main effect of stimulation [F(1,16) = 8.412, p = 0.0104], and a trend to a vector by stimulation interaction [F(1,16) = 3.70, p = 0.0725; n = 8 or 10/group]. Bonferroni post-hoc analysis indicated that DBS-like optogenetic stimulation of D2DR-containing neurons that expressed ChR2 significantly attenuated the reinstatement of cocaine-seeking (p = 0.0104, Fig. 2D). The time course shows that responding on the active lever is lower at the start of the reinstatement session and continues to be attenuated throughout the session. There was no main effect of vector [F(1,16) = 0.26, p = 0.6195], no main effect of stimulation [F(1,16) = 1.02, p = 0.3266], and no vector by stimulation interaction [F(1,16) = 0.070, p = 0.7950] on inactive lever presses during the reinstatement sessions. Figure 2E shows a diagram of the nucleus shell region targeted by viral vector infusion and fiber optic implantation. Figure 2F shows a representative image of eYFP expression and fiber optic track in a D2DR-Cre male rat. Taken together, these data indicate that high frequency optogenetic stimulation of D2DR-containing neurons attenuates cocaine priming-induced reinstatement of drug seeking, similar to that of electric DBS stimulation [3, 4]. These effects are not due to off target effects of prolonged high frequency light stimulation since rats expressing only eYFP did not display any difference in cocaine seeking.
Time courses show cumulative active lever presses during 1 h reinstatement sessions. Bars show total responding overlaid with active lever presses (circles) and inactive lever presses (triangles) for individual rats, with each rat receiving sham and 130 Hz stimulation in a within-subjects design. A In male rats that expressed eYFP in D1DR-containing neurons in the nucleus accumbens shell (n = 8), cocaine seeking did not differ when rats received sham stimulation or 130 Hz opto-DBS stimulation throughout the cocaine-primed reinstatement session. B In male rats that expressed ChR2 in D1DR-containing neurons in the nucleus accumbens shell (n = 8), cocaine seeking did not differ when rats received sham stimulation or 130 Hz opto-DBS stimulation throughout the cocaine-primed reinstatement session C In male rats that expressed eYFP in D2DR-containing neurons in the nucleus accumbens shell (n = 10), cocaine seeking did not differ when rats received sham stimulation or 130 Hz opto-DBS stimulation throughout the cocaine-primed reinstatement session. D In male rats that expressed ChR2 in D2DR-containing neurons in the nucleus accumbens shell (n = 8), cocaine seeking was significantly attenuated by 130 Hz opto-DBS stimulation, relative to sham stimulation in the same rats (*p < 0.05). E Diagram of the target region of the nucleus accumbens shell for viral vector infusion and fiber optic implantation. F Representative image showing eYFP expression in the nucleus accumbens shell and track from the implanted fiber optic.
The effect of high frequency optogenetic stimulation selectively in D1DR-containing or D2DR-containing neurons in the nucleus accumbens shell was assessed in female rats under identical experimental conditions as those used in male rats. In female D1DR-Cre rats expressing eYFP (Fig. 3A) or ChR2 (Fig. 3B), high frequency opto-DBS did not alter cocaine-primed reinstatement of cocaine seeking. There was no main effect of vector [F(1,11) = 0.63, p = 0.4431], no main effect of stimulation [F(1,11) = 0.59, p = 0.4588], and no vector by stimulation interaction [F(1,11) = 0.14, p = 0.7149; n = 6 or 8/group]. There was no main effect of vector [F(1,11) = 1.97, p = 0.1878], no main effect of stimulation [F(1,11) = 1.77, p = 0.2107], and no vector by stimulation interaction [F(1,11) = 0.14, p = 0.7203] on inactive lever presses during the reinstatement sessions. In female D2DR-Cre rats expressing eYFP (Fig. 3C) or ChR2 (Fig. 3D), high frequency opto-DBS did not alter cocaine-primed reinstatement of cocaine seeking. There was no main effect of vector [F(1,18) = 0.57, p = 0.4600], no main effect of stimulation [F(1,18) = 0.67, p = 0.4223], and no vector by stimulation interaction [F(1,18) = 0.056, p = 0.8164; n = 10/group]. There was no main effect of vector [F(1,18) = 0.56, p = 0.4629], no main effect of stimulation [F(1,18) = 0.78, p = 0.3889], and no vector by stimulation interaction [F(1,18) = 0.29, p = 0.5939] on inactive lever presses during the reinstatement sessions. Collectively, these data indicate that high frequency optogenetic stimulation of D1DR-containing or D2DR-containing does not alter cocaine-primed reinstatement of cocaine seeking in female rats.
Time courses show cumulative active lever presses during 1 h reinstatement sessions. Bars show total responding overlaid with active lever presses (circles) and inactive lever presses (triangles) for individual rats, with each rat receiving sham and 130 Hz stimulation in a within-subjects design. In female rats that expressed (A) eYFP (n = 6) or (B) ChR2 (n = 8) in D1DR-containing neurons in the nucleus accumbens shell, cocaine seeking did not differ when rats received sham stimulation or 130 Hz opto-DBS stimulation throughout the cocaine-primed reinstatement session. In female rats that expressed (C) eYFP (n = 10) or (D) ChR2 (n = 10) in D2DR-containing neurons in the nucleus accumbens shell, cocaine seeking did not differ when rats received sham stimulation or 130 Hz opto-DBS stimulation throughout the cocaine-primed reinstatement session. Inactive lever presses for one rat expressing ChR2 in D2DR-containing neurons were 273 in the sham test session; this data point was included in the analyses but omitted from the figure for scale.
Discussion
The present findings indicate that mimicking high frequency DBS with optogenetic stimulation selectively in nucleus accumbens shell D2DR-containing neurons, but not D1DR-containing neurons, attenuates cocaine seeking in male rats. In contrast, high frequency optogenetic stimulation of D1DR-containing or D2DR-containing neurons had no influence on reinstatement of cocaine-seeking behavior in female rats. However, the effect of electrical DBS on cocaine seeking in female rats has not previously been explored, so it is unknown whether our results recapitulate a potential sex difference in the ability of DBS to modulate drug seeking. Control experiments showed that there was no effect of optogenetic stimulation on cocaine seeking in rats that only expressed eYFP in the accumbens shell, indicating that the behavioral consequences of opto-DBS are not merely due to unintended non-specific consequences of prolonged light delivery [46]. The current results demonstrate that high frequency opto-DBS of accumbens D2DR-containing neurons suppresses drug seeking in male rats, which suggests that future research examining cell type-specific mechanisms underlying the effects electrical DBS in the accumbens might focus on D2DR-MSNs.
The specific biological mechanisms by which nucleus accumbens DBS modulates behavior, including the differential influence of DBS on specific cell types, are not well-understood. For instance, some evidence indicates that DBS increases neuronal activity within the stimulated nucleus [47, 48]. In contrast, other results suggest that DBS produces inhibition either through depolarization block or activation of inhibitory neurons [49,50,51]. DBS also is known to preferentially stimulate axon terminals and axons of passage relative to cell bodies [52], which results in broader, circuit-wide influences [19, 53,54,55]. DBS of the nucleus accumbens was shown to antidromically stimulate interneurons in the prefrontal cortex, which in turn inhibited glutamatergic projection neurons from the prefrontal cortex to the nucleus accumbens [55]. Consistent with these findings, DBS of the accumbens shell increased cFos expression in the infralimbic region of the prefrontal cortex, and pharmacological inactivation of the infralimbic cortex attenuated cocaine seeking [4]. Less is known about the local effects of DBS on neurons within the nucleus accumbens shell. Delivery of the GABA receptor agonists, baclofen and muscimol, or the local anesthetic lidocaine into the nucleus accumbens shell did not mimic the effects seen with DBS [4], suggesting local inactivation of medium spiny neurons is not driving the DBS-mediated decrease in cocaine seeking. The present results add a new layer of information by demonstrating that the reduction of cocaine seeking by opto-DBS is mediated through D2DR-containing, but not D1DR-containing neurons, and argue that cellular subtype should be considered in efforts to delineate the mechanisms by which electrical DBS modulates behavior. Subsequent studies which block the effect(s) of electrical DBS specifically in D2DR-containing MSNS and thereby obviate the attenuation of cocaine seeking would demonstrate a causal role for D2DR-containing MSNs in mediating the effect of accumbens DBS.
In animal models, accumbens shell DBS attenuates cocaine seeking [3, 4] and alleviates symptoms of acute cocaine withdrawal [8], whereas DBS actually enhances cocaine-evoked hyperlocomotion and escalation of cocaine self-administration in an extended access paradigm [8]. Refinement of electrical DBS, including through stimulation of defined pathways or cell types, may be necessary to isolate the therapeutically desirable effects of DBS. Mimicking DBS using optogenetics is a tenable approach toward understanding which mechanisms of DBS modify specific aspects of drug-related behavior. Early optogenetic studies addressing medium spiny neuron subtypes showed that activation of D1DR-containing neurons produced a place preference (CPP), whereas activation of D2DR-containing neurons induced a place aversion [30]. The first study mimicking low frequency (12 Hz) DBS optogenetic stimulation of D1DR-containing neurons replicated the attenuated initiation of cocaine-induced behavioral sensitization and induction of long-term depression achieved by accumbens 12 Hz electrical DBS and systemic co-administration of the D1DR antagonist SCH-23390 [23]. Cocaine occludes neuroplasticity in the nucleus accumbens [56,57,58] as well as within specific afferent and efferent projections [59,60,61]. Specifically, cocaine prevents induction of long-term potentiation in D1DR-containing neurons [62] and occludes long term depression in D2DR-containing neurons [31]. High frequency opto-DBS may differentially reverse of cocaine-mediated plasticity at D1DR- vs. D2DR-expressing neurons, or even within distinct projections of these neurons, which may explain the selectivity of high frequency opto-DBS to reduce cocaine seeking only when delivered to D2DR-containing neurons.
A caveat of the D2DR-Cre transgenic rat line used in the present study is that it does not distinguish between nucleus accumbens medium spiny neurons and cholinergic interneurons, which also express D2DRs. Although cholinergic neurons represent a very small population of nucleus accumbens neurons and we hypothesize that the effect of opto-DBS to attenuate cocaine seeking is mediated by D2DR-containing medium spiny neurons, we cannot rule out that D2DR-expressing cholinergic interneurons may be responsible for the attenuation of cocaine seeking by opto-DBS. Future experiments could use opto-DBS to define the contribution of cholinergic neurons and further probe the cell-type specific effect of DBS on cocaine seeking.
All previous studies examining the effect of DBS on cocaine seeking, including ours, have been performed exclusively in males; this study is the first to evaluate the impact of opto-DBS on drug seeking in female rats. A limitation of this approach is that the effect of electrical DBS of the nucleus accumbens shell on cocaine seeking in female rats is unknown. Thus, it is difficult to conclude whether DBS is broadly ineffective at modulating cocaine seeking among female rats, whether D1DR- or D2DR-containing neurons do not play a role in mediating the response to electrical DBS in this region among female rats, or whether factors like gonadal hormone fluctuations across the estrous cycle may influence the response to DBS on cocaine seeking in female rats. For example, reinstatement of cocaine seeking is higher in female vs. male rats [63], and is particularly heightened when female rats are in estrus [64]; perhaps electrical DBS and/or opto-DBS are differentially effective during specific phases of the estrous cycle. Sex differences have been repeatedly described in rodent models of cocaine self-administration and reinstatement, but whether a sex difference is observed can depend on various specific parameters of the experimental design as well as gonadal hormones [65,66,67]. The experiments in this study were performed identically in male and female rats; future studies which consider estrous phase as a variable may elucidate circumstances under which DBS and opto-DBS are optimally suited to attenuated drug seeking in females.
Understanding the mechanisms by which opto-DBS attenuates cocaine seeking at a cellular and network level is an essential aspect of this line of investigation. A distinct advantage of opto-DBS is that it enables selective stimulation of a sub-population of neurons. The 130 Hz frequency of optogenetic stimulation used here and in prior studies [19] is behaviorally relevant but exceeds the kinetic capacity of ChR2. The present electrophysiology results show that despite this limitation, light delivered at 130 Hz evokes action potentials, though in a temporally unsynchronized manner. High frequency optogenetic stimulation also enhances neuronal firing induced by current steps, suggesting that opto-DBS may also enhance submaximal firing of accumbens MSNs. Thus, at 130 Hz, it is possible that some population of neurons is activated with each pulse of light, but no cell is capable of firing action potentials in response to each light pulse. This is undoubtedly true for electrical DBS as well; the maximum firing rate for MSNs is around 40–60 Hz, so high frequency electrical DBS is similarly unlikely to suppress cocaine seeking by entraining accumbens MSNs to fire at that frequency but may enhance neuronal activity [47, 48]. Determining how high frequency stimulation impacts neuronal firing in cocaine-experienced rats will be critical for further uncovering the mechanisms of opto-DBS to attenuate drug seeking. Optogenetic technology can also be leveraged to study the effect of subtype specific opto-DBS on efferent pathways, including the direct and indirect pathways from the nucleus accumbens [25, 61, 68]. Future studies could interrogate the downstream consequences of opto-DBS in downstream regions, such as the ventral pallidum [25, 31, 32, 61].
Clinical experiments are beginning to validate the safety and efficacy of DBS of the nucleus accumbens as a treatment for multiple substance use disorders [15, 16, 18]. Recent efforts, including the present study, have used opto-DBS to better understand the specific neurons and circuits through which DBS modulates behavior [23, 61, 69,70,71], which can ultimately be leveraged to optimize therapeutic strategies. Identifying the specific circuits through which DBS influences cocaine seeking will provide critical new information directly relevant for the treatment of cocaine use disorder.
References
Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51:989–97.
Muller UJ, Voges J, Steiner J, Galazky I, Heinze HJ, Moller M, et al. Deep brain stimulation of the nucleus accumbens for the treatment of addiction. Ann N. Y Acad Sci. 2013;1282:119–28.
Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci. 2008;28:8735–9.
Vassoler FM, White SL, Hopkins TJ, Guercio LA, Espallergues J, Berton O, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation. J Neurosci. 2013;33:14446–54.
Guercio LA, Schmidt HD, Pierce RC. Deep brain stimulation of the nucleus accumbens shell attenuates cue-induced reinstatement of both cocaine and sucrose seeking in rats. Behav Brain Res. 2015;281:125–30.
Knapp CM, Tozier L, Pak A, Ciraulo DA, Kornetsky C. Deep brain stimulation of the nucleus accumbens reduces ethanol consumption in rats. Pharm Biochem Behav. 2009;92:474–9.
Henderson MB, Green AI, Bradford PS, Chau DT, Roberts DW, Leiter JC. Deep brain stimulation of the nucleus accumbens reduces alcohol intake in alcohol-preferring rats. Neurosurg Focus. 2010;29:E12.
Kallupi M, Kononoff J, Melas PA, Qvist JS, de Guglielmo G, Kandel ER, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine withdrawal but increases cocaine self-administration, cocaine-induced locomotor activity, and GluR1/GluA1 in the central nucleus of the amygdala in male cocaine-dependent rats. Brain Stimul. 2021;15:13–22.
Guercio LA, Wimmer ME, Schmidt HD, Swinford-Jackson SE, Pierce RC, Vassoler FM. Deep brain stimulation of the infralimbic cortex attenuates cocaine priming-induced reinstatement of drug seeking. Brain Res. 2020;1746:147011.
Pierce RC, Vassoler FM. Deep brain stimulation for the treatment of addiction: basic and clinical studies and potential mechanisms of action. Psychopharmacol (Berl). 2013;229:487–91.
Luigjes J, van den Brink W, Feenstra M, van den Munckhof P, Schuurman PR, Schippers R, et al. Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry. 2012;17:572–83.
Wang TR, Moosa S, Dallapiazza RF, Elias WJ, Lynch WJ. Deep brain stimulation for the treatment of drug addiction. Neurosurg Focus. 2018;45:E11.
Luigjes J, Segrave R, de Joode N, Figee M, Denys D. Efficacy of invasive and non-invasive brain modulation interventions for addiction. Neuropsychol Rev. 2019;29:116–38.
Kuhn J, Bauer R, Pohl S, Lenartz D, Huff W, Kim EH, et al. Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens. Eur Addict Res. 2009;15:196–201.
Kuhn J, Grundler TO, Bauer R, Huff W, Fischer AG, Lenartz D, et al. Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict Biol. 2011;16:620–3.
Zhou H, Xu J, Jiang J. Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: a case report. Biol Psychiatry. 2011;69:e41–2.
Valencia-Alfonso CE, Luigjes J, Smolders R, Cohen MX, Levar N, Mazaheri A, et al. Effective deep brain stimulation in heroin addiction: a case report with complementary intracranial electroencephalogram. Biol Psychiatry. 2012;71:e35–7.
Mantione M, van de Brink W, Schuurman PR, Denys D. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report. Neurosurgery. 2010;66:E218.
Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–9.
Delbeke J, Hoffman L, Mols K, Braeken D, Prodanov D. And then there was light: perspectives of optogenetics for deep brain stimulation and neuromodulation. Front Neurosci. 2017;11:663.
Magno LAV, Tenza-Ferrer H, Collodetti M, Aguiar MFG, Rodrigues APC, da Silva RS, et al. Optogenetic stimulation of the M2 cortex reverts motor dysfunction in a mouse model of Parkinson’s Disease. J Neurosci. 2019;39:3234–48.
Yu C, Cassar IR, Sambangi J, Grill WM. Frequency-specific optogenetic deep brain stimulation of subthalamic nucleus improves Parkinsonian motor behaviors. J Neurosci. 2020;40:4323–34.
Creed M, Pascoli VJ, Luscher C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347:659–64.
Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr Opin Neurobiol. 2013;23:546–52.
Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–2.
Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41.
Ortinski PI, Briand LA, Pierce RC, Schmidt HD. Cocaine-seeking is associated with PKC-dependent reduction of excitatory signaling in accumbens shell D2 dopamine receptor-expressing neurons. Neuropharmacology. 2015;92C:80–89.
Pierce RC, Wolf ME. Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. In: Pierce RC, Kenny PJ, editors. Addiction. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2013. p. 121–34.
Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–4.
Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–90.
Heinsbroek JA, Neuhofer DN, Griffin WC 3rd, Siegel GS, Bobadilla AC, Kupchik YM, et al. Loss of plasticity in the D2-accumbens pallidal pathway promotes cocaine seeking. J Neurosci. 2017;37:757–67.
Pardo-Garcia TR, Garcia-Keller C, Penaloza T, Richie CT, Pickel J, Hope BT, et al. Ventral pallidum is the primary target for accumbens D1 projections driving cocaine seeking. J Neurosci. 2019;39:2041–51.
Strong CE, Hagarty DP, Brea Guerrero A, Schoepfer KJ, Cajuste SM, Kabbaj M. Chemogenetic selective manipulation of nucleus accumbens medium spiny neurons bidirectionally controls alcohol intake in male and female rats. Sci Rep. 2020;10:19178.
Job MO, Chojnacki MR, Daiwile AP, Cadet JL. Chemogenetic inhibition of dopamine D1-expressing neurons in the dorsal striatum does not alter methamphetamine intake in either male or female long evans rats. Neurosci Lett. 2020;729:134987.
Garcia-Keller C, Scofield MD, Neuhofer D, Varanasi S, Reeves MT, Hughes B, et al. Relapse-associated transient synaptic potentiation requires integrin-mediated activation of focal adhesion kinase and cofilin in D1-expressing neurons. J Neurosci. 2020;40:8463–77.
Schmidt HD, Schassburger RL, Guercio LA, Pierce RC. Stimulation of mGluR5 in the accumbens shell promotes cocaine seeking by activating PKC Gamma. J Neurosci. 2013;33:14160–69.
White SL, Vassoler FM, Schmidt HD, Pierce RC, Wimmer ME. Enhanced anxiety in the male offspring of sires that self-administered cocaine. Addict Biol. 2015.
Ortinski PI, Briand LA, Pierce RC, Schmidt HD. Cocaine-seeking is associated with PKC-dependent reduction of excitatory signaling in accumbens shell D2 dopamine receptor-expressing neurons. Neuropharmacology. 2015;92:80–9.
Ortinski PI, Vassoler FM, Carlson GC, Pierce RC. Temporally dependent changes in cocaine-induced synaptic plasticity in the nucleus accumbens shell are reversed by D1-like dopamine receptor stimulation. Neuropsychopharmacology. 2012;37:1671–82.
Briand LA, Kimmey BA, Ortinski PI, Huganir RL, Pierce RC. Disruption of glutamate receptor-interacting protein in nucleus accumbens enhances vulnerability to cocaine relapse. Neuropsychopharmacology. 2014;39:759–69.
Ting JT, Daigle TL, Chen Q, Feng G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol. 2014;1183:221–42.
Rich MT, Huang YH, Torregrossa MM. Plasticity at Thalamo-amygdala Synapses Regulates Cocaine-Cue Memory Formation and Extinction. Cell Rep. 2019;26:1010–20.e5.
White SL, Ortinski PI, Friedman SH, Zhang L, Neve RL, Kalb RG, et al. A Critical Role for the GluA1 Accessory Protein, SAP97, in Cocaine Seeking. Neuropsychopharmacology. 2016;41:736–50.
Ortinski PI, Turner JR, Pierce RC. Extrasynaptic targeting of NMDA receptors following D1 dopamine receptor activation and cocaine self-administration. J Neurosci. 2013;33:9451–61.
Swinford-Jackson SE, Huffman PJ, Knouse MC, Thomas AS, Mankame S, Worobey SJ, et al. High frequency DBS-like optogenetic stimulation of nucleus accumbens dopamine D2 receptor-containing neurons attenuates cocaine reinstatement in male rats. bioRxiv. 2022; https://doi.org/10.1101/2022.05.26.493617.
Owen SF, Liu MH, Kreitzer AC. Thermal constraints on in vivo optogenetic manipulations. Nat Neurosci. 2019;22:1061–65.
McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–48.
Montgomery EB Jr, Gale JT. Mechanisms of action of deep brain stimulation(DBS). Neurosci Biobehav Rev. 2008;32:388–407.
Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology. 2000;55:S13–6.
Boraud T, Bezard E, Bioulac B, Gross C. High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci Lett. 1996;215:17–20.
Kiss ZH, Mooney DM, Renaud L, Hu B. Neuronal response to local electrical stimulation in rat thalamus: physiological implications for mechanisms of deep brain stimulation. Neuroscience. 2002;113:137–43.
Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. II. Evidence from selective inactivation of cell bodies and axon initial segments. Exp Brain Res. 1998;118:489–500.
Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002;17:S69–72.
Windels F, Bruet N, Poupard A, Urbain N, Chouvet G, Feuerstein C, et al. Effects of high frequency stimulation of subthalamic nucleus on extracellular glutamate and GABA in substantia nigra and globus pallidus in the normal rat. Eur J Neurosci. 2000;12:4141–6.
McCracken CB, Grace AA. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci. 2007;27:12601–10.
Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–9.
Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH, et al. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci. 2011;31:4194–203.
Huang CC, Liang YC, Lee CC, Hsu KS. Cocaine withdrawal impairs mGluR5-dependent long-term depression in nucleus accumbens shell neurons of both direct and indirect pathways. Mol Neurobiol. 2015;52:1223–33.
Zinsmaier AK, Dong Y, Huang YH. Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens. Mol Psychiatry. 2022;27:669–86.
Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Luscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–64.
Creed M, Ntamati NR, Chandra R, Lobo MK, Luscher C. Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum. Neuron. 2016;92:214–26.
Pascoli V, Turiault M, Luscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2011;481:71–5.
Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacol (Berl). 2000;148:196–200.
Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, et al. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacol (Berl). 2005;182:245–52.
Becker JB. Sex differences in addiction. Dialogues Clin Neurosci. 2016;18:395–402.
Knouse MC, Briand LA. Behavioral sex differences in cocaine and opioid use disorders: The role of gonadal hormones. Neurosci Biobehav Rev. 2021;128:358–66.
Nicolas C, Zlebnik NE, Farokhnia M, Leggio L, Ikemoto S, Shaham Y. Sex differences in opioid and psychostimulant craving and relapse: a critical review. Pharm Rev. 2022;74:119–40.
Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34.
Gittis AH, Yttri EA. Translating insights from optogenetics to therapies for Parkinson’s disease. Curr Opin Biomed Eng. 2018;8:14–9.
Spix TA, Nanivadekar S, Toong N, Kaplow IM, Isett BR, Goksen Y, et al. Population-specific neuromodulation prolongs therapeutic benefits of deep brain stimulation. Science. 2021;374:201–06.
Valverde S, Vandecasteele M, Piette C, Derousseaux W, Gangarossa G, Aristieta Arbelaiz A, et al. Deep brain stimulation-guided optogenetic rescue of parkinsonian symptoms. Nat Commun. 2020;11:2388.
Acknowledgements
The authors thank Riley Merkel and Tyler Sacko for their technical contributions.
Funding
This work was supported by the following grants from the National Institutes of Health: R01 DA015214 (RCP), T32 DA028874 (SESJ) and F32 DA052993 (MTR).
Author information
Authors and Affiliations
Contributions
SESJ and RCP designed the study; SESJ, PJH, MCK, AST, MTR, SM, SJW, MS, and AC performed experiments; SESJ, MTR, and RCP analyzed data; SESJ and RCP wrote the manuscript; all authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
COMPETING INTERESTS
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Swinford-Jackson, S.E., Huffman, P.J., Knouse, M.C. et al. High frequency DBS-like optogenetic stimulation of nucleus accumbens dopamine D2 receptor-containing neurons attenuates cocaine reinstatement in male rats. Neuropsychopharmacol. 48, 459–467 (2023). https://doi.org/10.1038/s41386-022-01495-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01495-y
This article is cited by
-
Deep brain stimulation for psychostimulant use disorders
Journal of Neural Transmission (2023)