Abstract
Transcranial magnetic stimulation (TMS) is a non-invasive technique for focal brain stimulation based on electromagnetic induction where a fluctuating magnetic field induces a small intracranial electric current in the brain. For more than 35 years, TMS has shown promise in the diagnosis and treatment of neurological and psychiatric disorders in adults. In this review, we provide a brief introduction to the TMS technique with a focus on repetitive TMS (rTMS) protocols, particularly theta-burst stimulation (TBS), and relevant rTMS-derived metrics of brain plasticity. We then discuss the TMS-EEG technique, the use of neuronavigation in TMS, the neural substrate of TBS measures of plasticity, the inter- and intraindividual variability of those measures, effects of age and genetic factors on TBS aftereffects, and then summarize alterations of TMS-TBS measures of plasticity in major neurological and psychiatric disorders including autism spectrum disorder, schizophrenia, depression, traumatic brain injury, Alzheimer’s disease, and diabetes. Finally, we discuss the translational studies of TMS-TBS measures of plasticity and their therapeutic implications.
Similar content being viewed by others
Introduction
In this review, we begin by providing an overview of the fundamentals of the transcranial magnetic stimulation (TMS) technique and its various forms, with a focus on TMS protocols that enable measures of brain plasticity, i.e., an intrinsic capacity for use-dependent change that is essential for normal learning and development [1]. We then focus on alterations of TMS-derived plasticity measures in common neuropsychiatric disorders including autism spectrum disorder, schizophrenia, depression, traumatic brain injury, Alzheimer’s disease, and diabetes. Our goal in discussing these disorders and in the dedicated section on therapeutics is not to cover the entire array of therapeutic TMS studies in each disorder but to focus on the implications of TMS measures of plasticity and brain states for diagnostic, predictive, and therapeutic purposes. We anticipate that the reader will appreciate how brain plasticity can be measured by TMS and the potential neural substrates of TMS-derived indices of brain plasticity. We will also review factors that can influence TMS plasticity measures, how TMS can be combined with other techniques such as EEG, and how the corresponding TMS plasticity measures can be obtained in translational (preclinical) studies in rodents.
Foundations of the TMS technique
TMS is a non-invasive brain stimulation technique first proposed by Barker et al. [2] based on the principles of electromagnetic induction discovered by Faraday in the 19th century [3]: a brief and rapidly changing, high-intensity electric current (the TMS pulse) is passed through loops of conducting wire within a protective case and held against a given area of the scalp. The change in the electrical current induces a powerful fluctuating magnetic field that readily penetrates the skull and can induce a second electric current (in the opposite direction of the original current) within the excitable tissues of the brain [4, 5]. When delivered with sufficient intensity over the cortex, the induced current depolarizes cortical neuronal assemblies located directly underneath the coil as well as in nearby and remote brain regions, thereby generating neurophysiological and behavioral effects [6,7,8,9]. To evoke activity in the human brain, the initial current typically needs to be in the order of 4–8 kA with a peak-to-peak rate of change of 100–200 μs, which induces an electric current perpendicular to the coil surface and in the order of 7–15 mA/cm−2 [3, 10]. The intensity of the induced current is proportional to that of the original current and attenuates with distance by bone, air, tissues, subdural and subarachnoid cerebrospinal fluid, and alterations in the cortical structure [11, 12]. TMS is considered safe when applied according to the recommended safety and application guidelines endorsed by the International Federation of Clinical Neurophysiology [13, 14].
Single-pulse and repetitive TMS
Single-pulse TMS (spTMS) protocols consist of discharges of single pulses often separated by 4–8 s intervals. Applying a TMS pulse over the primary motor cortex (M1) can activate the corticospinal tract and associated neural circuits and cause a twitch in the muscle(s) represented by the stimulated brain region [2, 15, 16]. The electrical activity associated with the muscle twitch can be recorded in the form of a motor evoked potential (MEP) via surface electromyography (EMG). Applying a TMS pulse to the primary visual cortex can induce a percept such as a brief flash of light, called phosphenes, in the location of the visual field retinotopically represented by the stimulated visual cortex [17,18,19] or posterior parietal cortex [20]. Higher cognitive functions such as attention, memory, language, etc. can also be probed by applying TMS pulses to higher cortices and associated networks, transiently disrupting physiological processes and/or behavioral activities subserved by the stimulated brain regions [8, 21,22,23,24,25,26]. Importantly, beyond local effects, TMS can have remote neurophysiological or behavioral effects in regions that have structural (white-matter) connectivity [27, 28] or functional connectivity [29,30,31,32,33] with the targeted brain region.
The motor cortex has been the most common targeted region in TMS studies conventionally because of its objective and easily obtainable neurophysiological output. The TMS-induced MEP is often characterized by its amplitude and latency relative to the onset of spTMS, reflecting the functional integrity of the corticospinal tract. The magnitude of the MEP provides a measure of motor cortical excitability, or the balance between cortical excitation and inhibition (E:I), two opposing forces that control the activity in the cortex [34, 35]. The latency of the MEP provides a measure of conduction time along central corticospinal motor pathways.
TMS also has been used in other forms, including paired-pulse (ppTMS) and repetitive TMS (rTMS) at specific intensities, frequencies, and patterns or bursts of stimulation (Fig. 1) to probe, modulate, or restore activity in the brain [8, 14]. rTMS protocols involve combinations of more than two pulses or bursts of stimulation delivered at a fixed frequency of 0.5–20 Hz, with or without interruption by stimulation-free intervals, for durations from several seconds up to 30–40 min. rTMS can modulate cortical excitability beyond the stimulation period and can be used in both motor and non-motor brain regions, with local and remote effects on brain activity [6, 8, 22, 36]. When applied to M1, high-frequency (≥5 Hz) rTMS protocols often increase cortical excitability, as measured by the increased size of induced MEPs within and following the rTMS train [37, 38]. In contrast, low-frequency (≤1 Hz) rTMS often decreases cortical excitability as measured by the post-rTMS decrease in MEP size [38, 39]. However, it is important to emphasize the substantial interindividual variability of such modulatory effects of rTMS [40,41,42]. It is also critical to recognize that the effects vary depending on the state of the cortex at the stimulation time. Such state-dependency effects [43] can in turn be used to increase the specificity of the rTMS modulatory effects [44].
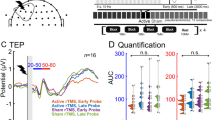
a Resting motor threshold (rMT) for the APB muscle is calculated by identifying the minimum stimulus strength, measured in percent machine output (% MO), that evokes an MEP of a fixed amplitude (typically ≥50 μV) in the APB at rest in a majority of trials. Stimulus strength is indicated in the left panel, with resulting MEPs shown in the right panel, where red arrows indicate the time of stimulation and percent stimulator output is proportional to the arrow length. b ppTMS paradigms where a subthreshold conditioning stimulus (short red vertical line) followed by a suprathreshold test stimulus (longer red vertical line). At short interstimulus-intervals (ISIs) (1–5 ms) short interval intracortical inhibition (SICI) is seen with inhibition of the test MEP by the antecedent conditioning stimulus. At longer ISIs (10–20 ms), test MEP amplitude is enhanced relative to the control MEP, such that ICF is seen. c Still longer ISIs (50–300 ms) are applied with two suprathreshold stimuli in LICI protocols where the MEP resultant from the test stimulus is predictably lower in amplitude than the preceding MEP resulting from the conditioning stimulus. ICF intracortical facilitation, ISI interstimulus interval, LICI long-interval intracortical inhibition, ppTMS paired-pulse transcranial magnetic stimulation, rTMS repetitive transcranial magnetic stimulation, spTMS single-pulse transcranial magnetic stimulation (adapted from ref. [333]).
Paired associative stimulation
Paired associative stimulation (PAS) consists of repeated pairing (e.g., 90–200 pairs) of stimuli, either peripheral-cortical (pcPAS) or cortico-cortical (ccPAS). In pcPAS, a peripheral electrical pulse delivered to a nerve of the hand (commonly the median nerve at the wrist), which activates the primary somatosensory cortex (S1), is followed by delivering a single TMS pulse over the corresponding hand representation in the contralateral M1 [45]. This S1-M1 coupling can modulate corticospinal excitability as reflected by a change in MEP amplitude [45, 46]. The characteristics of pcPAS modulatory effects are similar to those of Hebbian synaptic plasticity [47], including the dependency of the direction of MEP modulation on the interstimulus interval (ISI) between the peripheral stimulation and the cortical TMS pulse. Following a temporally asymmetric Hebbian rule [48], shorter intervals (e.g., ISI = 10 ms or PAS10) typically induce MEP suppression, whereas longer intervals (e.g., ISI = 25 ms or PAS25) induce MEP facilitation [45, 46, 48, 49]. The aftereffects of pcPAS develop rapidly (often within 30 mins), are relatively long-lasting (up to 60 min post-PAS), reversible, and cortically generated [45, 46, 50]. The effects of PAS10 and PAS25 are believed to be mediated by LTD- and LTP-like mechanisms of plasticity, respectively [45, 48, 50]. A variant of PAS known as PASN20 + 2 commonly involves 225 pairs (at a rate of 0.25 Hz) of median-nerve stimulation followed by delivering a single TMS pulse to the hand area of the contralateral M1 after an ISI equal to the N20 latency of the individual somatosensory-evoked cortical potential plus 2 ms [46, 51]. Similar interactions between a peripheral and a cortical stimulus can be induced by timing the interval between the two stimuli so that interaction takes place at the spinal level rather than the somatosensory cortex (spinal associative stimulation or SAS) [52].
In ccPAS, pairs of TMS pulses are delivered to two brain regions at an appropriate ISI (e.g., 200 paired pulses with an ISI of 10 ms), which can modulate inter-regional coupling according to spike-timing dependent plasticity mechanisms, including LTP-like plasticity [53, 54]. ccPAS can also induce behavioral changes in motor and visual systems [55]. Given an appropriate ISI, the plasticity effects of ccPAS follow the principles of Hebbian plasticity [56]. The TMS pulse over the first targeted brain region, e.g., the left dorsolateral prefrontal cortex (DLPFC), causes spikal activity to reach a second, functionally connected brain region, e.g., the left inferior parietal lobule (IPL). If a second TMS pulse is delivered to the left IPL at an appropriate time, the DLPFC-IPL connection is strengthened via an LTP-like mechanism. The repeated pairing of such two TMS pulses reinforces the synaptic efficacy between the two targeted brain regions, giving rise to the ccPAS aftereffects. Beyond modulating inter-regional connectivity, ccPAS can also be used to modulate network-to-network connectivity [57].
Integration of TMS with EEG to assess non-motor cortical regions
Human electroencephalography (EEG) [58, 59] is another non-invasive technique that enables the assessment of neural activity with millisecond temporal resolution by recording electrical fields from the scalp [60]. EEG reflects the activities of cortical neurons aligned perpendicular and radial to the scalp. The spatial and temporal summation of excitatory and inhibitory postsynaptic potentials of those neurons give rise to the EEG signal. When one or more large neuronal populations operate in synchrony, either spontaneously or in response to an event or external stimulation, a relatively strong electric field is generated and can be recorded via scalp EEG [61].
The combination of TMS and EEG techniques (TMS-EEG) is a powerful technique that enables non-invasive, in vivo assessment and modulation of cortical excitability, connectivity, and plasticity across motor and non-motor brain regions and networks [62,63,64,65,66,67,68]. One can also use the EEG oscillations to temporally coordinate the TMS pulse with ongoing or evoked brain activity in an effort to optimize brain states [69,70,71]. Thus, TMS-EEG can provide insights into the functional dynamics of the brain and brain-behavior relationships across the lifespan in healthy and clinical populations [29, 30, 32, 57, 64, 65, 72,73,74,75,76,77,78]. TMS-EEG studies have shown great potential for diagnostic, predictive, prognostic, and therapeutic purposes in neurology and psychiatry [68, 77,78,79,80,81,82,83,84,85]. Before such uses of this technique can become widespread, however, there are important aspects of TMS-EEG that need to be clarified, including to what extent TMS-evoked EEG potentials (TEPs) are peripherally evoked rather than direct cortical responses to the TMS pulse [86, 87].
Neuronavigated TMS for spatial precision
Neuronavigated TMS involves the use of magnetic resonance imaging-(MRI-)based frameless stereotaxy for consistent and reliable intra- and inter-session coil positioning that improves the spatial precision and reliability of TMS delivery [88, 89]. The use of neuronavigation can also improve the robustness of behavioral and neurophysiologic effects of TMS in healthy and clinical populations [90,91,92].
Theta-burst stimulation
A special form of patterned rTMS referred to as theta-burst stimulation (TBS) mimics neural oscillations considered to be associated with a type of non-Hebbian plasticity and typically consists of 50 Hz bursts of triplet pulses repeated at 5 Hz for a total of 600–1800 pulses [93, 94]. As with other forms of rTMS, TBS protocols can be used to induce cortical plasticity in health and disease and to modulate neural activity for research and clinical purposes [95, 96].
The two commonly used variants of the TBS protocol include (1) 190 s of stimulation in a 2 s on, 8 s off pattern called intermittent theta-burst stimulation (iTBS); and (2) 40 s of continuous stimulation called continuous theta-burst stimulation (cTBS). Studies of iTBS and cTBS of the primary motor cortex have found they typically produce lasting increased and decreased cortical plasticity, respectively, that often exceed those obtained with standard rTMS protocols [97,98,99].
TMS-TBS measures of mechanisms of plasticity
Combining plasticity-inducing rTMS protocols such as TBS with pre- and post-rTMS application of single TMS pulses allows for investigating the mechanisms of brain plasticity [1]. Plasticity-inducing protocols can have behavioral effects [6] and may be utilized for therapeutic purposes [100,101,102,103]. When applied to M1, iTBS can induce MEP facilitation by ~35% for up to 60 min, whereas cTBS can induce MEP suppression by ~25% for up to 50 min post-TBS [97].
The facilitation and suppression of MEPs by iTBS and cTBS protocols, respectively, are considered to involve mechanisms similar to long-term potentiation (LTP) and long-term depression (LTD), respectively (Fig. 2) [93, 104, 105]. As such, the return of post-TBS MEP amplitudes to their baseline levels is considered a neurophysiologic index of the efficacy of the mechanisms of cortical plasticity [64, 98, 106,107,108]. However, as discussed below, it is important in this context to consider the substantial inter- and intraindividual variability of the modulatory effects of rTMS and TBS, and their state-dependent effects [99].
Evaluation of corticospinal excitability by comparing MEPs recorded from the peripheral muscle in response to a single pulse to M1. TBS involves applying triplet pulses at 50 Hz repeated at intervals of 200 ms. MEPs are recorded at baseline and following either cTBS or iTBS, providing an index of local cortical plasticity. Following iTBS, MEPs typically show increased amplitude for ~30 min, whereas cTBS induces a suppression MEPs for approximately the same amount of time [93]. Post-TBS enhancement or suppression of cortical activity is considered an index of LTP- or LTD-like mechanism of plasticity in the targeted brain area. Applying paired pulses to M1 at an ISI of either 3 or 100 ms can be used to evaluate intracortical inhibition. cTBS continuous theta-burst stimulation, ISI interstimulus interval, iTB intermittent theta-burst stimulation, LTD long-term depression, LTP long-term potentiation, M1 primary motor cortex, MEP motor evoked potential, TBS theta-burst stimulation (adapted from ref. [106]).
Neural substrates of TMS-TBS measures of plasticity in humans
Three main lines of evidence support the notion that aftereffects of rTMS protocols involve mechanisms of synaptic plasticity. First, pharmacological characteristics of rTMS-induced changes in MEP amplitude are similar to those established for LTD or LTP of glutamatergic synapses in animal studies and drugs that modulate the function of critical receptors/channels for plasticity, e.g., N-methyl-D-aspartate (NMDA) receptors and Ca2+ channels, modify the TMS-induced plasticity effects [109]. Several pharmacological studies have shown that TMS-TBS measures of plasticity involve mechanisms of gamma-aminobutyric acid- (GABA-)ergic and NMDA-type glutamatergic synaptic plasticity [104, 110,111,112,113,114,115,116,117,118].
The second line of evidence comes from invasive epidural recordings of efferent corticospinal neurons in patients with implanted electrodes [119,120,121,122,123] (Fig. 3). A TMS-evoked corticospinal volley is often composed of a D-wave produced by direct activation of cortical layer-V pyramidal tract neurons (PTNs) and/or one or more I waves, which reflect indirect (likely via presynaptic interneurons) and synchronous activation of PTNs [16, 17, 124, 125]. These data have given rise to different theoretical cortical models of TMS–brain interactions [123, 126, 127]. cTBS has been shown to suppress the I1 wave while leaving the late I waves mostly unaffected [121], suggesting that cTBS exerts its effects mainly on the synapse between the inputs responsible for the I1 wave and the PTNs [123]. In contrast, iTBS induces a selective enhancement of late I waves while leaving the I1 wave unaffected [128], suggesting that iTBS selectively modulates the bursting cells of layers 2 and 3 that project upon PTNs and generate the later I waves [123]. The findings that TBS mainly alters I waves rather than the D-wave indicate that TBS effects occur trans-synaptically.
a Epidural volleys recorded at baseline (black trace) and after excitatory (red traces) and inhibitory (green traces) plasticity protocols. Each trace is the average of the responses to 10–25 TMS pulses. 5 Hz rTMS increases the amplitude of the proximal D wave and all I waves. Paired associative stimulation at a 25 ms ISI (PAS25) selectively facilitates the late I waves without changing the I1 wave. cTBS leads to suppression of the amplitude of the I1 wave. 1 Hz rTMS causes a selective suppression of late I waves with no change in the I1 wave. b Corticospinal volleys and MEPs evoked by single TMS pulses in baseline conditions and after iTBS. Each trace is the average of 20 sweeps. Three descending waves can be observed in the epidural recording. iTBS facilitates the amplitude of I2 and I3 waves without affecting the amplitude of the I1 wave. The amplitude of MEP is slightly (but, in this case, nonsignificantly) increased following iTBS. c It is proposed that 5 Hz subthreshold rTMS suppresses the excitability of superficial inhibitory circuits, including superficial (L1) cortical neurons, and that most of the protocols (iTBS, PAS25, PAS10, 1 Hz rTMS, TDCS) modulate bursting neurons of layer 2 and 3 (L2/L3) that project upon PTNs and generate the late I waves. It is proposed that cTBS selectively suppresses the excitability of monosynaptic connections to PTNs. 5 Hz rTMS may produce its excitatory effects by enhancing the excitability of PTNs. cTBS continuous theta-burst stimulation, ISI interstimulus interval, iTBS intermittent theta-burst stimulation, MEP motor evoked potential, PAS paired associative stimulation, PTNs pyramidal tract neurons, rTMS repetitive transcranial magnetic stimulation. TDCS transcranial direct current stimulation (adapted from refs. [123, 128]).
The third line of evidence in support of the role of synaptic plasticity in rTMS aftereffects comes from the observed interaction between the effect of TMS protocols and learning that is compatible with the common rules of synaptic plasticity, including (a) metaplasticity, i.e., modulation of synaptic plasticity by prior synaptic activity [129] and (b) reversal of previously induced synaptic plasticity [130]. Studies using priming stimulation by TMS have found evidence for Bienenstock-Cooper-Munro (BCM) homeostatic metaplasticity [131], i.e., reductions of corticospinal excitability by one TMS protocol causing stronger facilitatory effects by a subsequent protocol, in TBS [132, 133] and between different non-invasive brain stimulation protocols [134, 135]. The findings that rTMS aftereffects interact between protocols and motor- and cognitive learning indicate that rTMS effects are involved in motor and cognitive processes related to plasticity [109].
However, it is worth remembering that LTP and LTD refer to very specific neurophysiologic phenomena at the level of individual synaptic connections, and the changes induced by TMS or TBS are certainly not limited to specific individual synaptic terminals. Rather, the modulatory effects of rTMS or TBS likely involve neurons and glia, along with intracortical and cortico-(sub)cortical loops [123, 136,137,138,139].
Variability of TMS-TBS measures of plasticity
In recent years, there has been an increased focus on the degree of inter- and intraindividual variability in TMS-TBS measures of plasticity and the factors contributing to such variability [94, 99, 140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156].
Some of the factors identified as potential contributors to the interindividual variability in TBS measures of plasticity include differentially activated intracortical networks [144], functional connectivity in the motor system [94, 148], state-dependent factors and genetic polymorphisms [98], stimulation intensity [153], the target muscle, and baseline MEP amplitude [99].
Several factors can potentially influence the intraindividual variability of TBS measures of plasticity including age [156], genetic polymorphisms [151, 153], phase of the menstrual cycle across visits [157, 158], changes in the blood glucose levels and caffeine intake across visits [159,160,161], amount and quality of sleep the night before each visit [162, 163], intensity and duration of exercise before each visit [164, 165], the intervisit interval [151], the time of day [166], the use of neuronavigation [88, 89], changes in baseline MEP amplitude across visits [99, 151], the use of robotic arms such as the TMS-Robot (Axilum Robotics, Schiltigheim, France), which can reduce trial-to-trial variability of MEP amplitude [167], and the use of closed-loop systems that time the delivery of TMS pulses based on EEG-derived indices of brain states [69,70,71].
Recent studies indicate that the modulatory effects of TBS may be overestimated [140, 154, 168]. One possible reason for this lack of reliable effects may be applying TBS at intensities that are insufficient to induce consistent plasticity across individuals. TBS is commonly applied at a subthreshold intensity, i.e., 80% of individual active motor threshold (aMT). There is some evidence suggesting that determining the TBS intensity based on the individual resting motor threshold (rMT), e.g., 70% of rMT, may enhance the consistency of TBS aftereffects [169]. However, in that context, it is important to remember that the modulatory effects of TBS depend on whether or not there is a contraction of the target muscle before the onset of the stimulation [170]. Moreover, recent clinical studies have found robust therapeutic TBS effects at substantially higher stimulation intensities, i.e., at 90–120% of individual RMT to the left DLPFC for treatment of major depressive disorder [171, 172]. Determining the precise role of stimulation intensity and other potential factors influencing the robustness of TBS aftereffects within and across individuals requires further systematic investigations.
Effects of age in TMS-TBS measures
A cross-sectional study of individuals aged 19–81 found that cTBS-induced motor cortical plasticity linearly declined across the lifespan, suggesting that the efficiency of cTBS-induced LTD-like plasticity in the motor cortex is progressively reduced with aging [173]. In contrast, iTBS-induced LTP-like plasticity was generally found to be less impacted by aging [128, 174]. Enhanced priming of iTBS aftereffects with preceding cTBS was observed in younger adults but not older adults, indicating reduced homeostatic metaplasticity with aging [175].
A longitudinal study of the effects of aging on iTBS-induced plasticity as measured by changes in resting-state functional connectivity (rsFC) of the default-mode network (DMN) found younger adults (age ≤ 30 yrs) showed iTBS increased rsFC in distal DMN regions whereas iTBS aftereffects in older adults (age ≥ 60 yrs) were more local and limited to proximal DMN regions [176]. Importantly, older adults with “young-like” functional responses to iTBS had greater structural integrity in the brain (as measured by diffusion tensor imaging), higher education, and superior cognitive performance at baseline as well as less cognitive decline after 3 years [176]. These results suggest that iTBS measures of plasticity as measured by changes in rsFC can provide an index of the brain’s resilience in aging and enable the assessment of network plasticity, which may constitute a neurophysiological substrate of cognitive reserve [177, 178].
A large-scale analysis of individual participant data from several TBS studies found a small, non-significant linear attenuation of iTBS- and cTBS-induced plasticity with age [99]. However, age had a significant non-linear effect, with young participants exhibiting increased iTBS-induced facilitation, and older adults exhibiting a steady drop-off in facilitation [99]. Because the data used in that analysis included very few participants in the 35–50 age group, further systematic investigations of changes in TBS measures of plasticity across the lifespan with adequate sampling from all age groups are warranted.
Effects of genetic variability in TMS-TBS measures
Genetic factors clearly influence the effects of TMS [179]. Brain-derived neurotrophic factor (BDNF) is one of the most important neurotrophins critically involved in the NMDA-type glutamate receptor-dependent LTP [180] and LTD [181]. The common single-nucleotide polymorphism (SNP) Val66Met in the BDNF gene influences the intracellular transport and packaging of the LTD-associated precursor peptide of BDNF (pro-BDNF), while the regulated secretion of the mature (m)BDNF protein is involved in LTP [182, 183].
Several studies have shown the effects of BDNF Val66Met polymorphism on TMS measures of plasticity in humans, including reduced training-dependent facilitation of MEPs [184, 185], reduced cTBS-induced suppression [141, 153], and reduced iTBS-induced facilitation of MEPs [141, 143, 185, 186]. Mori et al. [187] reported that individuals carrying the G allele in the GRIN2B rs1805247 SNP of the NR2B subunit gene of the NMDA receptor showed greater iTBS-induced cortical plasticity.
Other genetic influences on TMS measures of plasticity include reduced efficacy of the mechanism of cortical plasticity due to the presence of the ε4 allele in the apolipoprotein E (ApoE) gene [188,189,190,191] and reduced cTBS-induced suppression of MEPs in healthy older adults with the Val158Met SNP in the catechol-O-methyltransferase (COMT) gene [192]. The results from these small studies need to be replicated in larger studies before they can be considered reliable genetic determinants of TBS-induced plasticity.
TMS plasticity measures in disease
Autism spectrum disorder
Rodent models of autism spectrum disorder (ASD) and studies on genetic syndromes with a high prevalence of ASD symptoms in humans indicate altered mechanisms of synaptic plasticity, including use-dependent changes in synaptic strength [193,194,195,196,197,198] and aberrant LTP and LTD of excitatory synaptic strength [199,200,201,202,203,204,205,206].
Studies in adults with ASD have found greater and longer-lasting TBS-induced changes in MEP amplitude in adults with ASD compared to neurotypical (NT) adults, indicating an exaggerated, hyperplastic response to TBS in ASD [107, 133, 207]. Similarly, children and adolescents with high-functioning ASD (HF-ASD) show abnormally greater facilitatory responses to cTBS than typically developing children [208]. Moreover, children and adolescents with HF-ASD show a maturational trajectory of cTBS measures of plasticity, in which the extent or the maximum amount of cTBS-induced suppression of MEPs increases linearly with age [208, 209]. These findings indicate the utility of cTBS measures of cortical plasticity as diagnostic biomarkers for individuals with ASD across the lifespan [210].
Schizophrenia
A pilot M1 cTBS study on five early-onset, first-episode, antipsychotic-naive schizophrenia patients found the duration of cTBS-induced aftereffects was noticeably shorter in patients with schizophrenia compared with their age- and gender-matched counterparts [211]. This finding suggested that the efficacy of the mechanisms of cortical plasticity is already reduced in the early stages of schizophrenia. A more recent study [212] compared M1 cTBS aftereffects in 10 schizophrenia patients under treatment by antipsychotics with 10 healthy controls and found fewer schizophrenia patients exhibited the expected plasticity response induced by cTBS compared to healthy controls. These results suggest the reduced efficacy of the mechanism of plasticity induced by cTBS among patients with schizophrenia [212].
Depression
TMS studies in patients with depression have found evidence of decreased cortical excitability in the frontal cortex [213,214,215,216,217,218,219], interhemispheric imbalances in prefrontal and motor cortices in the form of reduced excitability in the left hemisphere [213,214,215,216, 219] or increased excitability in the right hemisphere [220]. One study found that 1-Hz rTMS applied to the left M1 expectedly reduced cortical excitability in that hemisphere, but did not modulate the excitability in the right hemisphere, indicating loss of normal interhemispheric modulation in depression [221]. Subsequent studies found applying high-frequency rTMS to the left prefrontal cortex would increase cortical excitability (as indexed by lowered rMT) in the ipsilateral hemisphere [222] or reduce the interhemispheric difference in excitability [216] and was associated with lasting clinical improvements [223].
rTMS studies have found evidence of reduced modulation of cortical plasticity in patients with major depressive disorder (MDD), including reduced post-exercise MEP facilitation [224, 225], and reduced plasticity induced by PAS [226], both in the motor cortex [227] and in the prefrontal cortex [228]. Consistent with these findings, drug-free patients with MDD showed reduced iTBS-induced facilitation of MEPs compared to healthy controls, suggesting that LTP-like mechanisms mediated by the glutamatergic NMDA receptor pathway are impaired in patients with MDD [229].
Interestingly, recent studies have found that the extent of MEP modulation induced by 10-Hz rTMS applied to M1 predicts the clinical response to the standard 10-Hz rTMS treatment applied to the DLPFC in depressed patients [230, 231]. If confirmed in larger multisite studies, these findings can help stratify patients with depression based on their likelihood of positive response to 10-Hz rTMS, support physicians in choosing the best therapeutic approach, and assist TMS clinics in prioritizing and allocating their resources. Accelerated protocols using iTBS instead of 10-Hz rTMS can alleviate some of these concerns, allowing for more eligible patients to be treated within the same time period and using comparable human and equipment resources [171, 172]. For example, the Stanford Accelerated Intelligent Neuromodulation Therapy (SAINT) protocol delivered iTBS at 90% rMT in 10 daily sessions over 5 consecutive days to an individually targeted region in the left DLPFC based on the strongest resting-state functional connectivity (negative correlation) with the subgenual anterior cingulate cortex and found remission in 90% of patients [172].
Traumatic brain injury
Traumatic brain injury (TBI) can cause several neurological symptoms including chronic pain [232], mood and sleep disturbances [233, 234], as well as increased susceptibility to seizures and post-traumatic epilepsy [235, 236], which can be attributed to impaired cortical inhibition. This notion is supported by animal studies that have found a post-TBI shift of cortical E:I ratio toward excess excitation due to loss of GABA-mediated synaptic inhibition and reduction in GABA-synthesizing enzymes in the cortical inhibitory synapses [237,238,239]. Evidence for loss of such cortical inhibitory tone after TBI was obtained in vivo in a rat fluid percussion injury model [240] using a form of ppTMS known as long-interval cortical inhibition (LICI) [241] that is an index of cortical GABA-mediated inhibition [242, 243]. The results showed a reduction in LICI for up to 6 weeks following TBI [241]. Various studies using different ppTMS measures of intracortical inhibition have reported heterogeneous results including facilitation, suppression, and no effect on MEPs following mild traumatic brain injury (mTBI) [244].
Support for altered cortical plasticity after mTBI has been obtained by rTMS measures of plasticity. In one of the first rTMS studies in humans with mTBI, 1-Hz rTMS-induced irregular shape alterations in the MEP waveform at 2 weeks after injury, which was normalized in 9 out of 15 patients when re-tested after 3 months [245]. Bashir et al. [246] used cTBS to assess changes in cortical plasticity following mTBI and found that, unlike control subjects, mTBI patients exhibited paradoxical MEP facilitation at week 2, at 0, 5, 10, and 20 min post-cTBS. The cTBS response in mTBI patients returned to the expected inhibition at week 6 post-injury [246]. Another evidence for abnormal TMS measures of plasticity in mTBI was reported in a proof-of-principle study [108], in which cTBS did not induce a significant suppression of MEPs 2 weeks after injury, but cTBS response was normalized at 6 weeks post-injury when most patients became asymptomatic.
Further evidence for altered mechanisms of cortical plasticity as measured by M1 cTBS was obtained in a TMS-EMG-EEG study [244], where patients with mTBI showed greater suppression of MEPs at 30 min post-cTBS relative to the controls. Moreover, mTBI patients exhibited significant inhibition of TMS-evoked EEG potentials (TEPs) P30 and N45 at 30 min post-cTBS, while the corresponding TEPs in the control subjects were unaffected. These results provide evidence for enhanced cTBS-induced LTD-like plasticity up to ~4 months post-injury among patients with mTBI [244].
Alzheimer’s disease
In Alzheimer’s disease (AD), synaptic degeneration is strongly correlated with cognitive decline and plays a critical role in the development of dementia [247]. The results of preclinical studies have shown that soluble Aβ oligomers can block hippocampal LTP [248], which is an electrophysiological index of learning and memory [249] but enhance LTD [250]. These aberrations in the mechanisms of plasticity can induce conformational changes in the tau proteins, resulting in pathological consequences in synaptic plasticity and cognition [251, 252]. Because iTBS-induced LTP-like plasticity is likely mediated by the strengthening of glutamatergic synapses via NMDA receptor, α-amino-3-hydroxy-5-methyl-4- isoxazolepropionic acid (AMPA) receptor, and calcium channel [110], iTBS measures of M1 plasticity can be used as a tool for investigating alterations in cortical plasticity in early AD [253].
In a large group of newly diagnosed AD patients, Koch et al. found evidence for impaired iTBS-induced LTP-like cortical plasticity—with a paradoxical LTD relative to healthy controls—and impaired short-latency afferent inhibition (SAI), which is considered an index of central cholinergic impairment [254]. Based on the fact that impairment of cortical plasticity was not correlated with age of onset, it was argued that impairment in cortical LTP is a central mechanism of AD pathophysiology that is independent of the age of onset [255]. A study on the reproducibility of iTBS aftereffects in patients with AD [151] found that reproducibility was higher in AD patients than in individuals with healthy aging, which was attributed to the pathological rigidity of neurophysiological systems in AD.
Importantly, paradoxical LTD-like cortical plasticity in response to iTBS has been found to be associated with high tau in the cerebrospinal fluid and more rapid cognitive decline [256]. A subsequent study from the same group found the impairment in LTP-like plasticity was associated with less efficient verbal memory but not with other cognitive functions [257]. The association between reduced LTP-like cortical plasticity and faster cognitive decline in patients with AD was replicated in another study on 60 newly diagnosed patients [258], which found iTBS aftereffects had excellent accuracy for differentiating between AD patients and healthy controls. A separate study on mostly medicated AD patients found the extent of M1 iTBS-induced plasticity at baseline predicted the change in patients’ cognition following treatment with 10-Hz rTMS [259]. These findings collectively support the crucial role of synaptic degeneration in the pathophysiology of dementia [260] and indicate the diagnostic and predictive utility of rTMS measures of plasticity in assessing the AD pathophysiology and predicting clinical response to rTMS treatment [260].
It should be noted, however, that AD-related alterations in LTP-like plasticity as indexed by iTBS are not necessarily uniform across all stages of the disease. For example, Buss et al. [261] used iTBS to assess LTP-like cortical plasticity in patients with amnestic mild cognitive impairment (aMCI), which is considered a prodromal stage of AD [262, 263]. The patients with aMCI showed a lack of LTP-like plasticity unrelated to the quantitative Aβ burden on positron emission tomography. Unexpectedly, greater LTP-like response to iTBS was associated with worse memory function in aMCI patients, underlining the complexity of alterations in neuroplasticity in the prodromal stages of AD [261].
Diabetes
Rodent models of type-2 diabetes mellitus (T2DM) show aberrant synaptic plasticity in the hippocampus resulting in cognitive deficits [264] and less likelihood of post-stroke recovery due to impaired neuroplasticity [265]. Consistent with those findings, individuals with T2DM showed reduced iTBS-induced plasticity, indicating altered efficacy of neuroplastic mechanisms in the cortex [266]. Interestingly, those alterations were associated with cognitive impairment as indexed by lower learning scores on the Rey auditory verbal learning test (RAVLT) and fewer correct trials of the Digit Span Backwards test [266]. Those results were in line with mouse models of T2DM that showed impaired object discrimination, hippocampal LTP, and impaired spatial memory [267,268,269].
Considering that iTBS aftereffects, RAVLT, and working memory performance in humans have all been shown to be NMDA-dependent [104, 270,271,272,273], the association between reduced verbal learning and working memory and reduced LTP-like plasticity in T2DM may be due to a brain-wide reduction in the density or efficacy of NMDA receptors [266]. A subsequent study found that reduced iTBS measures of cortical plasticity in T2DM patients were already present in individuals with prediabetes, and reduced corticomotor plasticity in T2DM, but not in prediabetes, was associated with lower cortical glutamate concentration [274].
Translational studies of TMS-TBS measures—insights and challenges
In each of the sections above on specific disease states, we have cited the key evidence from animal models that form a foundation for investigating pathophysiological changes in plasticity using human rTMS measures. Here, we summarize some of the parallel TMS-TBS studies in animal models that can be translated to human studies using the same protocols (Fig. 4).
a TMS-MMG setup. An unanesthetized rat is restrained on a platform with 4 straps with minimal discomfort. A figure-of-8 TMS coil is centered over the dorsal scalp at the interaural line. The MMG is obtained by two 3-axis accelerometers on each ventral surface of the foot while the rat is under brief isoflurane anesthesia before placement into the restraint. b Representative data during spTMS (left) or ppTMS (right) obtained with ISIs of 50, 100, and 200 ms in rats. ppTMS traces show long-interval intracortical inhibition of the test-evoked MMG. Arrow indicates the onset of the test stimulus. c Examples of MEP changes following sham stimulation, iTBS, and cTBS in anesthetized rats. Representative MEP traces following TBS show no obvious change after sham stimulation, whereas MEP traces show increased amplitude after iTBS and reduced amplitude after cTBS. cTBS continuous theta-burst stimulation, ISI interstimulus interval, iTBS intermittent theta-burst stimulation, MEP motor evoked potential, MMG mechanomyogram, ppTMS paired-pulse transcranial magnetic stimulation, spTMS single-pulse transcranial magnetic stimulation, TMS transcranial magnetic stimulation (adapted from refs. [243, 285]).
LTP as a use-dependent enhancement of excitatory synaptic strength was first identified in the hippocampus of an anesthetized rabbit in 1973 [275] and subsequently replicated in rodent brain slices [276, 277]. LTD as a homeostatic mechanism was first identified in rat hippocampal slices in 1992, is reliably reproducible in vitro in numerous laboratories, and appears to be due to the reversal of the LTP-related molecular mechanisms [278]. Translational research in this field ideally involves in vivo experimental rTMS [279]TBS studies in animals that can help elucidate the mechanisms of TBS-induced plasticity in humans.
Extrapolating from animal LTP/LTD studies to human brain stimulation protocols is not without challenges. For instance, the rate at which synaptic plasticity matures varies in different regions of the rodent brain [280, 281]. Similarly, the capacity for LTP in mice varies by age; it is greater in the visual cortex of adult mice (older than 6 months) than in 4–5-week-old mice [282]. In contrast, younger rats exhibit a lower threshold for LTD induction and a greater magnitude of hippocampal LTD than older rats [282, 283].
An iTBS study in rats found functional modification of cortical GABAergic parvalbumin-positive (PV+) interneurons only after 32 days of life (human age: 4–11 years), which peaked by postnatal day 40 (human age: 12–18 years). This result may be due to a lack of enough synaptic connections in immature PV+ cells that prevents them from receiving the level of excitatory input required to be influenced by iTBS [284].
Another important issue to consider when extrapolating from results of in vitro studies of synaptic plasticity is that most of the results of those studies are obtained by precise electrical stimulation of isolated pathways, whereas in vivo brain stimulation protocols such as TMS have much coarser localization and lower spatial resolution, and activate entire neuronal networks and their supporting glial cells [278].
A major challenge of implementing translational rTMS studies in rodent models is the low focalization of stimulation due to the large size of the coil relative to the rodent’s brain. To overcome this challenge, Hsieh et al. developed a cortical electrical stimulation (CES) technique for iTBS and cTBS protocols in rats [285], which is both less invasive and has higher accuracy and spatial resolution than TMS for targeting a specific area of the motor cortex. The authors used direct CES over the motor cortex using conventional iTBS and cTBS patterns and found CES-TBS protocol induced both LTP- and LTD-like plasticity effects for at least 30 min post-TBS, similar to those obtained in human TBS studies [285]. Because the rats were under anesthesia to suppress major stress, the stimulation intensity was set to 80% of rMT, instead of the lower intensity of 80% of active aMT commonly used in human TBS protocols. The facilitation of MEPs by CES-iTBS and suppression of MEPs by CES-cTBS were replicated in a subsequent study [286]. These results indicate that CES-TBS animal models can be useful for investigating the mechanisms of cortical plasticity and bridging the human and animal studies for establishing new diagnostic and therapeutic applications for neurological disorders [285, 287,288,289,290].
Therapeutic implications of TMS measures of plasticity and brain state
As reviewed above, at a system level, rTMS modulates excitability and plasticity in a targeted cortical region [6, 291,292,293] and exerts broader effects across networks connected to that region [30, 294,295,296,297]. Additionally, the putative mechanism of action of the therapeutic effects of rTMS is thought to be the modulation of dysfunction within and between functional networks [298,299,300,301]. Thus, targeting the rTMS based on structural and/or functional networks has become a prominent focus for optimizing the efficacy of rTMS for therapeutic purposes. That being said, the stimulation location is only one factor influencing the clinical and physiological impact of rTMS. The therapeutic benefit of such neuromodulation is also impacted by how the stimulation is received and processed in the brain. In other words, the impact of applying a pulsed magnetic field to an electrically charged region of the cortex is influenced by both the degree and direction of the magnetic field itself as well as the state of excitability of the targeted cortical region and the broader functional network [43, 179, 302, 303] (Fig. 5). Thus, to optimize the induced changes in plasticity and modulation of the putative mechanism underlying the clinical symptom, it is important to characterize the current brain state in terms of local cortical excitability and network connectivity in order to determine the optimal treatment protocol for a given patient.
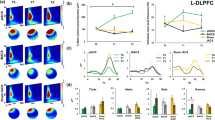
The intrinsic spontaneous activity of the SGC (a) can be compared with that of other regions of the brain to identify regions of strong positively or negatively correlated FC. The dorsolateral prefrontal cortex (DLPFC) (b) includes regions of positive (red) and negative (blue) FC with the SGC. Stronger negative FC with the SGC occurs at more anterolateral sites. c Illustration of negative (anticorrelated) time course between the DLPFC (green) and SGC (red). d, e Greater treatment outcome (% change in clinical score) was associated with more negative SGC FC at the individual DLPFC stimulation site across Boston (d) and Melbourne (e) cohorts. For the Boston cohort, the green and red circles in (d) highlight individual participants with good and poor clinical outcomes, corresponding to circled cortical sites of negative and positive SGC FC, respectively, displayed in (b). BDI Beck Depression Inventory, BOLD blood oxygen–level dependent, HCP Human Connectome Project, MADRS Montgomery–Åsberg Depression Rating Scale (adapted from ref. [334]).
Impact of pharmacology on brain state
For both safety and feasibility, most rTMS treatments have allowed participants to continue their current medications [304]. Safety reviews suggest that rTMS in those receiving stable doses of antidepressant medication does not increase the risk of adverse events [13] but nonetheless increases within-group variability in neurochemical state and decreases statistical power. To control for variability due to medications, investigators could enroll only participants who have been withdrawn from all psychotropic medications; however, withdrawing symptomatic patients from their medications introduces safety concerns of increased suicidal ideation and withdrawal-related side effects, and it demands close medical monitoring. A more feasible approach would be to require participants to maintain a steady medication dose and to apply a within-subject model controlling for baseline severity as the primary outcome measure. While this would not eliminate variability across participants, it reduces the effects of neurochemical brain state on the primary outcome measure. Combining rTMS and pharmacological treatment is another novel multimodal intervention being developed in adults and adolescents with depression [305, 306].
Impact of concurrent behavioral task on brain state
To increase the tolerability of rTMS sessions (especially those that may last several minutes to hours, in the case of long research study visits), clinics and research studies have allowed patients to read, watch TV, or listen to music during rTMS sessions. However, factors such as attention, arousal, and mood state have been shown to affect the modulation of excitability by rTMS [55, 307,308,309]. The difference in behavioral engagement/arousal this causes is another potential source of within-group variability and suboptimal therapeutic efficacy. To control and optimize brain state, some studies have the participant watch or listen to specific stimuli or engage in a behavioral or cognitive task that engages the same brain networks as the rTMS target, thus amplifying the impact of the rTMS on the targeted network [303]. Studies of rTMS for post-traumatic stress disorder (PTSD), smoking cessation, and obsessive-compulsive disorder (OCD) have shown increased treatment response when the participant’s symptoms were provoked (e.g., by asking questions about thoughts, images, or impulses related to their obsessions or compulsions or asking the patient to perform a task related to their symptoms [310] immediately before rTMS stimulation [311,312,313]. Others are beginning to pair rTMS with concurrent behavioral interventions such as cognitive-behavioral therapy (CBT) [314]. This is a burgeoning field with many groups seeking to harness the power of plasticity provided by the rTMS and appropriately guide it toward the ultimate functional network and clinical target (Fig. 6).
The stimulation can be adapted to individual patient needs by achieving specificity in the key elements of dosing (spatial, temporal, and contextual). Spatial precision can be achieved through individual imaging-guided targeting, electric field modeling, and focal or multifocal coils/electrodes. Temporal precision can be achieved by optimizing the pulse waveform, frequency, and train characteristics and by coupling it to endogenous oscillations via closed-loop techniques. Contextual precision can be achieved by controlling brain state during stimulation via online cognitive task performance, synchronizing to endogenous neural oscillations, or combination therapies such as simultaneous brain stimulation and pharmacotherapy (adapted from ref. [335]).
Individualizing rTMS protocols based on brain state
The degree of modulation at the brain network level can be quantified using functional magnetic resonance imaging (fMRI) as well as quantitative EEG. Previous research has shown that high-frequency rTMS (as is often used for therapeutic protocols in depression and other neuropsychiatric disorders) leads to synchronization of EEG activity primarily in alpha and beta frequency bands, both at the targeted site as well as distant areas [63, 315,316,317,318,319,320,321,322,323,324]. It has been proposed that rTMS may act through triggering and inducing long-term entrainment of oscillations to the stimulation frequency. In doing so, rTMS may reset cortical and thalamocortical oscillators and facilitate more stable intrinsic oscillatory activity [315, 317, 318]. Alpha frequency (8–13 Hz), in particular the upper alpha band (10–12 Hz), seems to be well-suited to coordinating activities over distance in the brain both at the cortical and the cortico-subcortical (e.g., thalamic) level [325, 326]. Standard rTMS protocols often apply stimulation at a standard 10-Hz frequency, but there is large variability in response, perhaps partially due to interindividual variability in intrinsic alpha frequency and its proximity to 10 Hz [327]. There is some evidence to support that individualizing the frequency of stimulation to match the individual’s intrinsic alpha frequency results in better clinical efficacy for both schizophrenia and depression [328,329,330,331].
Most recently, in this area of research, “closed-loop EEG-rTMS” devices have been developed to record EEG and process it in real-time such that rTMS pulses can be applied not only at a specific frequency, but also at a specific time-point within the oscillation [70, 71]. Using this device, Zrenner et al. found that TMS applied at the trough (or negative peak) of the alpha rhythm induced greater LTP-like facilitation in the primary motor cortex than pulses applied at the positive peak of the alpha rhythm [70]. They extended these findings to DLPFC and found that closed-loop rTMS applied at the trough of the individual’s alpha rhythm, compared to random alpha phase stimulation, leads to reduced left frontal resting-state alpha power and increased TMS-induced beta oscillations over medial frontocentral EEG channels [71]. The therapeutic potential of closed-loop EEG-rTMS has yet to be established, however, and a clinical trial is ongoing [332].
References
Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401.
Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;325:1106–7.
Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–65.
Barker AT. An introduction to the basic principles of magnetic nerve stimulation. J Clin Neurophysiol. 1991;8:26–37.
Barker AT. Transcranial magnetic stimulation—past, present and future. Brain Stimulation 2017;10:540.
Pascual-Leone A, Rubio B, Pallardó F, Catalá MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–7.
Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–99.
Valero-Cabré A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA. Transcranial magnetic stimulation in basic and clinical neuroscience: a comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev. 2017;83:381–404.
Di Lazzaro V, Dileone M, Pilato F, Capone F, Musumeci G, Ranieri F, et al. Modulation of motor cortex neuronal networks by rTMS: comparison of local and remote effects of six different protocols of stimulation. J Neurophysiol. 2011;105:2150–6.
Pascual-Leone A, Davey N, Rothwell J, Wasserman EM, Puri BK, editors. Handbook of transcranial magnetic stimulation. London: New York, NY: Arnold; Oxford University Press [distributor]; 2002.
Wagner T, Fregni F, Eden U, Ramos-Estebanez C, Grodzinsky A, Zahn M, et al. Transcranial magnetic stimulation and stroke: a computer-based human model study. Neuroimage. 2006;30:857–70.
Wagner T, Rushmore J, Eden U, Valero-Cabre A. Biophysical foundations underlying TMS: setting the stage for an effective use of neurostimulation in the cognitive neurosciences. Cortex. 2009;45:1025–34.
Rossi S, Antal A, Bestmann S, Bikson M, Brewer C, Brockmöller J, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert Guidelines. Clin Neurophysiol. 2021;132:269–306.
Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–107.
Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93.
Amassian VE, Quirk GJ, Stewart M. A comparison of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex. Electroencephalogr Clin Neurophysiol. 1990;77:390–401.
Amassian VE, Cracco RQ, Maccabee PJ. Focal stimulation of human cerebral cortex with the magnetic coil: a comparison with electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989;74:401–16.
Fernandez E, Alfaro A, Tormos JM, Climent R, Martínez M, Vilanova H, et al. Mapping of the human visual cortex using image-guided transcranial magnetic stimulation. Brain Res Brain Res Protoc. 2002;10:115–24.
Elkin-Frankston S, Fried PJ, Pascual-Leone A, Rushmore III R, Valero-Cabré A. A novel approach for documenting phosphenes induced by transcranial magnetic stimulation. J Vis Exp. 2010:e1762.
Fried PJ, Elkin-Frankston S, Rushmore RJ, Hilgetag CC, Valero-Cabre A. Characterization of visual percepts evoked by noninvasive stimulation of the human posterior parietal cortex. PLoS ONE. 2011;6:e27204.
Pascual-Leone A, Gates JR, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology. 1991;41:697–702.
Pascual-Leone A, Gomez-Tortosa E, Grafman J, Alway D, Nichelli P, Hallett M. Induction of visual extinction by rapid-rate transcranial magnetic stimulation of parietal lobe. Neurology. 1994;44:494–8.
Coslett HB, Monsul N. Reading with the right-hemisphere: evidence from transcranial magnetic stimulation. Brain Lang. 1994;46:198–211.
Ganis G, Keenan JP, Kosslyn SM, Pascual-Leone A. Transcranial magnetic stimulation of primary motor cortex affects mental rotation. Cereb Cortex. 2000;10:175–80.
Mottaghy FM, Pascual-Leone A, Kemna LJ, Töpper R, Herzog H, Müller-Gärtner H-W, et al. Modulation of a brain–behavior relationship in verbal working memory by rTMS. Cogn Brain Res. 2003;15:241–9.
Jahanshahi M, Rothwell J. Transcranial magnetic stimulation studies of cognition: an emerging field. Exp Brain Res. 2000;131:1–9.
Quentin R, Chanes L, Migliaccio R, Valabrègue R, Valero-Cabré A. Fronto-tectal white matter connectivity mediates facilitatory effects of non-invasive neurostimulation on visual detection. Neuroimage. 2013;82:344–54.
Quentin R, Chanes L, Vernet M, Valero-Cabré A. Fronto-parietal anatomical connections influence the modulation of conscious visual perception by high-beta frontal oscillatory activity. Cereb Cortex. 2015;25:2095–101.
Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci USA. 2011;108:21229–34.
Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603.
Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci. 2014;111:E4367–75.
Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A. Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci. 2014;34:12049–56.
Brady RO, Gonsalvez I, Lee I, Öngür D, Seidman LJ, Schmahmann JD, et al. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiatry. 2019;176:512–20.
Haider B, McCormick DA. Rapid neocortical dynamics: cellular and network mechanisms. Neuron. 2009;62:171–89.
Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–43.
Rotenberg A, Horvath JC, Pascual-Leone A, editors. Transcranial magnetic stimulation. New York: Humana Press: Springer; 2014.
Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–58.
Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–96.
Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low‐frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–403.
Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–5.
Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS). Clin Neurophysiol. 2002;113:376–82.
Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–30.
Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10.
Silvanto J, Cattaneo Z, Battelli L, Pascual-Leone A. Baseline cortical excitability determines whether TMS disrupts or facilitates behavior. J Neurophysiol. 2008;99:2725–30.
Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–84.
Müller-Dahlhaus JFM, Orekhov Y, Liu Y, Ziemann U. Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Exp Brain Res. 2008;187:467–75.
Hebb DO. The organization of behavior: a neuropsychological theory. New York: John Wiley and Sons, 1949.
Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, et al. A temporally asymmetric hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–45.
Classen J, Wolters A, Stefan K, Wycislo M, Sandbrink F, Schmidt A, et al. Paired associative stimulation. Suppl Clin Neurophysiol. 2004;57:563–9.
Di Lazzaro V, Dileone M, Profice P, Pilato F, Oliviero A, Mazzone P, et al. LTD-like plasticity induced by paired associative stimulation: direct evidence in humans. Exp Brain Res. 2009;194:661–4.
Ziemann U, Ilić TV, Iliać TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–72.
Cortes M, Thickbroom GW, Valls-Sole J, Pascual-Leone A, Edwards DJ. Spinal associative stimulation: a non-invasive stimulation paradigm to modulate spinal excitability. Clin Neurophysiol. 2011;122:2254–9.
Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3:1178–83.
Buch ER, Johnen VM, Nelissen N, O’Shea J, Rushworth MF. Noninvasive associative plasticity induction in a corticocortical pathway of the human brain. J Neurosci. 2011;31:17669–79.
Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, Thut G. Spontaneous fluctuations in posterior—band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex. 2008;18:2010–8.
Koch G, Ponzo V, Di Lorenzo F, Caltagirone C, Veniero D. Hebbian and anti-Hebbian spike-timing-dependent plasticity of human cortico-cortical connections. J Neurosci. 2013;33:9725–33.
Momi D, Ozdemir RA, Tadayon E, Boucher P, Shafi MM, Pascual-Leone A, et al. Network-level macroscale structural connectivity predicts propagation of transcranial magnetic stimulation. Neuroimage. 2021;229:117698.
Berger H. Über das elektroenkephalogramm des menschen. Arch Für Psychiatr Und Nervenkrankheiten. 1929;87:527–70.
Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. Handbook of psychophysiology, 2nd ed., New York, NY, US: Cambridge University Press; 2000. p. 27–52.
Ingber L, Nunez PL. Neocortical dynamics at multiple scales: EEG standing waves, statistical mechanics, and physical analogs. Math Biosci. 2011;229:160–73.
Kirschstein T, Köhling R. What is the source of the EEG? Clin EEG Neurosci. 2009;40:146–9.
Thut G, Pascual-Leone A, Integrating TMS. with EEG: how and what for? Brain Topogr. 2010;22:215–8.
Thut G, Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010;22:219–32.
Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, et al. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr. 2011;24:302–15.
Rogasch NC, Fitzgerald PB. Assessing cortical network properties using TMS-EEG. Hum Brain Mapp. 2013;34:1652–69.
Farzan F, Vernet M, Shafi MMD, Rotenberg A, Daskalakis ZJ, Pascual-Leone A. Characterizing and modulating brain circuitry through transcranial magnetic stimulation combined with electroencephalography. Front Neural Circ. 2016;10:73.
Rogasch NC, Sullivan C, Thomson RH, Rose NS, Bailey NW, Fitzgerald PB, et al. Analysing concurrent transcranial magnetic stimulation and electroencephalographic data: a review and introduction to the open-source TESA software. NeuroImage. 2017;147:934–51.
Tremblay S, Rogasch NC, Premoli I, Blumberger DM, Casarotto S, Chen R, et al. Clinical utility and prospective of TMS–EEG. Clin Neurophysiol. 2019;130:802–44.
Tervo AE, Nieminen JO, Lioumis P, Metsomaa J, Souza VH, Sinisalo H, et al. Closed-loop optimization of transcranial magnetic stimulation with electroencephalography feedback. Brain Stimul. 2022;15:523–31.
Zrenner C, Desideri D, Belardinelli P, Ziemann U. Real-time EEG-defined excitability states determine efficacy of TMS-induced plasticity in human motor cortex. Brain Stimul. 2018;11:374–89.
Zrenner B, Zrenner C, Gordon PC, Belardinelli P, McDermott EJ, Soekadar SR, et al. Brain oscillation-synchronized stimulation of the left dorsolateral prefrontal cortex in depression using real-time EEG-triggered TMS. Brain Stimul. 2020;13:197–205.
Daskalakis ZJ, Farzan F, Radhu N, Fitzgerald PB. Combined transcranial magnetic stimulation and electroencephalography: its past, present and future. Brain Res. 2012;1463:93–107.
Vernet M, Bashir S, Yoo W-K, Perez JM, Najib U, Pascual-Leone A. Insights on the neural basis of motor plasticity induced by theta burst stimulation from TMS-EEG. Eur J Neurosci. 2013;37:598–606.
Canali P, Sarasso S, Rosanova M, Casarotto S, Sferrazza-Papa G, Gosseries O, et al. Shared reduction of oscillatory natural frequencies in bipolar disorder, major depressive disorder and schizophrenia. J Affect Disord. 2015;184:111–5.
Ozdemir RA, Tadayon E, Boucher P, Momi D, Karakhanyan KA, Fox MD, et al. Individualized perturbation of the human connectome reveals reproducible biomarkers of network dynamics relevant to cognition. Proc Natl Acad Sci USA. 2020;117:8115–25.
Ozdemir RA, Tadayon E, Boucher P, Sun H, Momi D, Ganglberger W, et al. Cortical responses to noninvasive perturbations enable individual brain fingerprinting. Brain Stimul. 2021;14:391–403.
Shafi MM, Westover MB, Fox MD, Pascual‐Leone A. Exploration and modulation of brain network interactions with noninvasive brain stimulation in combination with neuroimaging. Eur J Neurosci. 2012;35:805–25.
Shafi MM, Vernet M, Klooster D, Chu CJ, Boric K, Barnard ME, et al. Physiological consequences of abnormal connectivity in a developmental epilepsy. Ann Neurol. 2015;77:487–503.
Ragazzoni A, Pirulli C, Veniero D, Feurra M, Cincotta M, Giovannelli F, et al. Vegetative versus minimally conscious states: a study using TMS-EEG, sensory and event-related potentials. PloS ONE. 2013;8:e57069.
Casali AG, Gosseries O, Rosanova M, Boly M, Sarasso S, Casali KR, et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med. 2013;5:198ra105.
Canali P. A role for TMS/EEG in neuropsychiatric disorders. Neurol Psychiatry Brain Res. 2014;20:37–40.
Bortoletto M, Veniero D, Thut G, Miniussi C. The contribution of TMS–EEG coregistration in the exploration of the human cortical connectome. Neurosci Biobehav Rev. 2015;49:114–24.
Sun Y, Farzan F, Mulsant BH, Rajji TK, Fitzgerald PB, Barr MS, et al. Indicators for remission of suicidal ideation following magnetic seizure therapy in patients with treatment-resistant depression. JAMA Psychiatry. 2016;73:337.
Casarotto S, Comanducci A, Rosanova M, Sarasso S, Fecchio M, Napolitani M, et al. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann Neurol. 2016;80:718–29.
Kimiskidis VK, Tsimpiris A, Ryvlin P, Kalviainen R, Koutroumanidis M, Valentin A, et al. TMS combined with EEG in genetic generalized epilepsy: a phase II diagnostic accuracy study. Clin Neurophysiol. 2017;128:367–81.
Conde V, Tomasevic L, Akopian I, Stanek K, Saturnino GB, Thielscher A, et al. The non-transcranial TMS-evoked potential is an inherent source of ambiguity in TMS-EEG studies. NeuroImage. 2019;185:300–12.
Belardinelli P, Biabani M, Blumberger DM, Bortoletto M, Casarotto S, David O, et al. Reproducibility in TMS–EEG studies: a call for data sharing, standard procedures and effective experimental control. Brain Stimul. 2019;12:787–90.
Julkunen P, Säisänen L, Danner N, Niskanen E, Hukkanen T, Mervaala E, et al. Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. NeuroImage. 2009;44:790–5.
Sack AT, Kadosh RC, Schuhmann T, Moerel M, Walsh V, Goebel R. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J Cogn Neurosci. 2009;21:207–21.
Herwig U, Padberg F, Unger J, Spitzer M, Schönfeldt-Lecuona C. Transcranial magnetic stimulation in therapy studies: examination of the reliability of “standard” coil positioning by neuronavigation. Biol Psychiatry. 2001;50:58–61.
Bashir S, Edwards D, Pascual-Leone A. Neuronavigation increases the physiologic and behavioral effects of low-frequency rTMS of primary motor cortex in healthy subjects. Brain Topogr. 2011;24:54–64.
Modak A, Fitzgerald PB. Personalising transcranial magnetic stimulation for depression using neuroimaging: a systematic review. World J Biol Psychiatry. 2021;22:647–69.
Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005;45:201–6.
Nettekoven C, Volz LJ, Kutscha M, Pool E-M, Rehme AK, Eickhoff SB, et al. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci. 2014;34:6849–59.
Pascual-Leone A, Bartres-Faz D, Keenan JP. Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of ‘virtual lesions’. Philos Trans R Soc Lond B Biol Sci. 1999;354:1229–38.
Chen R, Udupa K. Measurement and modulation of plasticity of the motor system in humans using transcranial magnetic stimulation. Mot Control. 2009;13:442–53.
Wischnewski M, Schutter DJLG. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimul. 2015;8:685–92.
Suppa A, Huang Y-Z, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, et al. Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Brain Stimul. 2016;9:323–35.
Corp DT, Bereznicki HGK, Clark GM, Youssef GJ, Fried PJ, Jannati A, et al. Large-scale analysis of interindividual variability in theta-burst stimulation data: results from the ‘Big TMS Data Collaboration’. Brain Stimul. 2020;13:1476–88.
Buss SS, Fried PJ, Pascual-Leone A. Therapeutic noninvasive brain stimulation in Alzheimer’s disease and related dementias. Curr Opin Neurol. 2019;32:292.
Cole EJ, Enticott PG, Oberman LM, Gwynette MF, Casanova MF, Jackson SLJ, et al. The potential of repetitive transcranial magnetic stimulation for autism spectrum disorder: a consensus statement. Biol Psychiatry. 2019;85:e21–2.
Menardi A, Rossi S, Koch G, Hampel H, Vergallo A, Nitsche MA, et al. Toward noninvasive brain stimulation 2.0 in Alzheimer’s disease. Ageing Res Rev. 2021;75:101555.
Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur J-P, Magistris MR, et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–32.
Huang Y-Z, Chen R-S, Rothwell JC, Wen H-Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–32.
Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J Neuroeng Rehabil. 2009;6:7.
Oberman L, Ifert-Miller F, Najib U, Bashir S, Woollacott I, Gonzalez-Heydrich J, et al. Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile X syndrome and autism spectrum disorder. Front Synaptic Neurosci. 2010;2:26.
Oberman LM, Eldaief M, Fecteau S, Ifert-Miller F, Tormos JM, Pascual-Leone A. Abnormal modulation of corticospinal excitability in adults with Asperger’s syndrome. Eur J Neurosci. 2012;36:2782–8.
Tremblay S, Vernet M, Bashir S, Pascual-Leone A, Théoret H. Theta burst stimulation to characterize changes in brain plasticity following mild traumatic brain injury: a proof-of-principle study. Restor Neurol Neurosci. 2015;33:611–20.
Huang Y-Z, Lu M-K, Antal A, Classen J, Nitsche M, Ziemann U, et al. Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin Neurophysiol. 2017;128:2318–29.
Huang Y-Z, Rothwell JC, Edwards MJ, Chen R-S. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18:563–70.
Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, et al. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009;101:2872–7.
Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A. Theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp Brain Res. 2009;199:411–21.
Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, et al. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci. 2011;31:1193–203.
Vlachos A, Müller-Dahlhaus F, Rosskopp J, Lenz M, Ziemann U, Deller T. Repetitive magnetic stimulation induces functional and structural plasticity of excitatory postsynapses in mouse organotypic hippocampal slice cultures. J Neurosci. 2012;32:17514–23.
Lenz M, Galanis C, Müller-Dahlhaus F, Opitz A, Wierenga CJ, Szabó G, et al. Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nat Commun. 2016;7:1–13.
Lenz M, Vlachos A. Releasing the cortical brake by non-invasive electromagnetic stimulation? rTMS induces LTD of GABAergic neurotransmission. Front Neural Circ. 2016;10:96.
Ziemann U. Pharmaco-transcranial magnetic stimulation studies of motor excitability. Handb Clin Neurol. 2013;116:387–97.
Korchounov A, Ziemann U. Neuromodulatory neurotransmitters influence LTP-like plasticity in human cortex: a pharmaco-TMS study. Neuropsychopharmacology. 2011;36:1894–902.
Di Lazzaro V, Oliviero A, Mazzone P, Insola A, Pilato F, Saturno E, et al. Comparison of descending volleys evoked by monophasic and biphasic magnetic stimulation of the motor cortex in conscious humans. Exp Brain Res. 2001;141:121–7.
Di Lazzaro V, Oliviero A, Pilato F, Mazzone P, Insola A, Ranieri F, et al. Corticospinal volleys evoked by transcranial stimulation of the brain in conscious humans. Neurological Res. 2003;25:143–50.
Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, et al. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex: Theta-burst repetitive transcranial magnetic stimulation. J Physiol. 2005;565:945–50.
Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, et al. I-wave origin and modulation. Brain Stimul. 2012;5:512–25.
Di Lazzaro V, Rothwell JC. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex: Corticospinal activity and the human motor cortex. J Physiol. 2014;592:4115–28.
Thompson P, Day B, Crockard H, Calder I, Murray N, Rothwell J, et al. Intra-operative recording of motor tract potentials at the cervico-medullary junction following scalp electrical and magnetic stimulation of the motor cortex. J Neurol Neurosurg Psychiatry. 1991;54:618–23.
Burke D, Hicks R, Gandevia S, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol. 1993;470:383.
Ziemann U, Rothwell J. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397–405.
Rusu CV, Murakami M, Ziemann U, Triesch J. A model of TMS-induced I-waves in motor cortex. Brain Stimul. 2014;7:401–14.
Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, et al. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex: theta-burst rTMS of the human motor cortex. J Physiol. 2008;586:3871–9.
Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–30.
Zhou Q, Poo M. Reversal and consolidation of activity-induced synaptic modifications. Trends Neurosci. 2004;27:378–83.
Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48.
Murakami T, Müller‐Dahlhaus F, Lu M, Ziemann U. Homeostatic metaplasticity of corticospinal excitatory and intracortical inhibitory neural circuits in human motor cortex. J Physiol. 2012;590:5765–81.
Oberman LM, Ifert-Miller F, Najib U, Bashir S, Gonzalez-Heydrich J, Picker J, et al. Abnormal mechanisms of plasticity and metaplasticity in autism spectrum disorders and fragile X syndrome. J Child Adolesc Psychopharmacol. 2016;26:617–24.
Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–85.
Ni Z, Gunraj C, Kailey P, Cash RF, Chen R. Heterosynaptic modulation of motor cortical plasticity in human. J Neurosci. 2014;34:7314–21.
Fujiki M, Steward O. High frequency transcranial magnetic stimulation mimics the effects of ECS in upregulating astroglial gene expression in the murine CNS. Mol Brain Res. 1997;44:301–8.
Bartrés-Faz D, Tormos J, Junque C, Pascual-Leone A. Transcranial magnetic stimulation: contribution to psychiatry and to the study of brain-behavior relationship. Actas Esp Psiquiatr. 2000;28:130–6.
Cullen CL, Young KM. How does transcranial magnetic stimulation influence glial cells in the central nervous system? Front Neural Circ. 2016;10:26.
Peters JC, Reithler J, Graaf TA, de, Schuhmann T, Goebel R, Sack AT. Concurrent human TMS-EEG-fMRI enables monitoring of oscillatory brain state-dependent gating of cortico-subcortical network activity. Commun Biol. 2020;3:1–11.
Ozdemir RA, Boucher P, Fried PJ, Momi D, Jannati A, Pascual-Leone A, et al. Reproducibility of cortical response modulation induced by intermittent and continuous theta-burst stimulation of the human motor cortex. Brain Stimul. 2021;14:949–64.
Cheeran BJ, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–25.
Cheeran BJ, Ritter C, Rothwell JC, Siebner HR. Mapping genetic influences on the corticospinal motor system in humans. Neuroscience. 2009;164:156–63.
Antal A, Chaieb L, Moliadze V, Monte-Silva K, Poreisz C, Thirugnanasambandam N, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 2010;3:230–7.
Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex. 2013;23:1593–605.
Goldsworthy MR, Müller-Dahlhaus F, Ridding MC, Ziemann U. Inter-subject variability of LTD-like plasticity in human motor cortex: a matter of preceding motor activation. Brain Stimul. 2014;7:864–70.
Goldsworthy MR, Hordacre B, Ridding MC. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience. 2016;320:205–9.
López-Alonso V, Cheeran B, Río-Rodríguez D, Fernández-Del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014;7:372–80.
Nettekoven C, Volz LJ, Leimbach M, Pool E-M, Rehme AK, Eickhoff SB, et al. Inter-individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. NeuroImage. 2015;118:209–18.
Vernet M, Bashir S, Yoo W-K, Oberman LM, Mizrahi I, Ifert-Miller F, et al. Reproducibility of the effects of theta burst stimulation on motor cortical plasticity in healthy participants. Clin Neurophysiol. 2014;125:320–6.
Vallence A-M, Goldsworthy MR, Hodyl NA, Semmler JG, Pitcher JB, Ridding MC. Inter- and intra-subject variability of motor cortex plasticity following continuous theta-burst stimulation. Neuroscience. 2015;304:266–78.
Fried PJ, Jannati A, Davila-Pérez P, Pascual-Leone A. Reproducibility of single-pulse, paired-pulse, and intermittent theta-burst TMS measures in healthy aging, type-2 diabetes, and Alzheimer’s disease. Front Aging Neurosci. 2017;9:263.
Hordacre B, Goldsworthy MR, Vallence A-M, Darvishi S, Moezzi B, Hamada M, et al. Variability in neural excitability and plasticity induction in the human cortex: a brain stimulation study. Brain Stimul. 2017;10:588–95.
Jannati A, Block G, Oberman LM, Rotenberg A, Pascual-Leone A. Interindividual variability in response to continuous theta-burst stimulation in healthy adults. Clin Neurophysiol. 2017;128:2268–78.
Boucher PO, Ozdemir RA, Momi D, Burke MJ, Jannati A, Fried PJ, et al. Sham-derived effects and the minimal reliability of theta burst stimulation. Sci Rep. 2021;11:21170.
Rocchi L, Ibáñez J, Benussi A, Hannah R, Rawji V, Casula E, et al. Variability and predictors of response to continuous theta burst stimulation: a TMS-EEG study. Front Neurosci. 2018;12:400.
Jannati A, Fried PJ, Block G, Oberman LM, Rotenberg A, Pascual-Leone A. Test–retest reliability of the effects of continuous theta-burst stimulation. Front Neurosci. 2019;13:447.
Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DA, et al. Menstrual cycle effects on cortical excitability. Neurology. 1999;53:2069.
Hattemer K, Knake S, Reis J, Rochon J, Oertel WH, Rosenow F, et al. Excitability of the motor cortex during ovulatory and anovulatory cycles: a transcranial magnetic stimulation study. Clin Endocrinol. 2007;66:387–93.
Specterman M, Bhuiya A, Kuppuswamy A, Strutton P, Catley M, Davey N. The effect of an energy drink containing glucose and caffeine on human corticospinal excitability. Physiol Behav. 2005;83:723–8.
Cerqueira V, de Mendonça A, Minez A, Dias AR, de Carvalho M. Does caffeine modify corticomotor excitability? Neurophysiol Clinl. 2006;36:219–26.
Badawy RAB, Vogrin SJ, Lai A, Cook MJ. Cortical excitability changes correlate with fluctuations in glucose levels in patients with epilepsy. Epilepsy Behav. 2013;27:455–60.
Civardi C, Boccagni C, Vicentini R, Bolamperti L, Tarletti R, Varrasi C, et al. Cortical excitability and sleep deprivation: a transcranial magnetic stimulation study. J Neurol Neurosurg Psychiatry. 2001;71:809.
Kreuzer P, Langguth B, Popp R, Raster R, Busch V, Frank E, et al. Reduced intra-cortical inhibition after sleep deprivation: a transcranial magnetic stimulation study. Neurosci Lett. 2011;493:63–6.
Samii A, Wassermann EM, Hallett M. Post-exercise depression of motor evoked potentials as a function of exercise duration. Electroencephalogr Clin Neurophysiol. 1997;105:352–6.
Lentz M, Nielsen JF. Post-exercise facilitation and depression of M wave and motor evoked potentials in healthy subjects. Clin Neurophysiol. 2002;113:1092–8.
Cohen DA, Pascual-Leone A, Press DZ, Robertson EM. Off-line learning of motor skill memory: A double dissociation of goal and movement. Proc Natl Acad Sci. 2005;102:18237–41.
Foucher J, Lorgouilloux K, Turek J, Pham B-T, Elowe J, Bayle B, et al. Robotic assistance in coil positioning improves reliability and comfort. 3rd Annual Conference of the German Society for Brain Stimulation—Modulating Emotions, Berlin, Germany; 2012.
Park JH. Reliability of theta burst stimulation as a neuromodulation tool. J Neurophysiol. 2022;127:1532–4.
Fried P, Jannati A, Morris T, Buss S, Santarnecchi E, Shafi M, et al. Relationship of active to resting motor threshold influences the aftereffects of theta-burst stimulation. Brain Stimul. 2019;12:465.
Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 2008;18:2046–53.
Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–92.
Cole E, Gulser M, Stimpson K, Bentzley B, Hawkins J, Xiao X, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression (SAINT-TRD). Brain Stimul. 2019;12:402.
Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, et al. Changes in cortical plasticity across the lifespan. Front Aging Neurosci. 2011;3:5.
Young-Bernier M, Tanguay AN, Davidson PSR, Tremblay F. Short-latency afferent inhibition is a poor predictor of individual susceptibility to rTMS-induced plasticity in the motor cortex of young and older adults. Front Aging Neurosci. 2014;6:182.
Opie GM, Vosnakis E, Ridding MC, Ziemann U, Semmler JG. Priming theta burst stimulation enhances motor cortex plasticity in young but not old adults. Brain Stimul. 2017;10:298–304.
Abellaneda-Pérez K, Vaqué-Alcázar L, Vidal-Piñeiro D, Jannati A, Solana E, Bargalló N, et al. Age-related differences in default-mode network connectivity in response to intermittent theta-burst stimulation and its relationships with maintained cognition and brain integrity in healthy aging. Neuroimage. 2019;188:794–806.
Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28.
Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–12.
Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118.
Figurov A, Pozzo-Miller LD, Olafsson P. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706.
Woo NH, Teng HK, Siao C-J, Chiaruttini C, Pang PT, Milner TA, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–77.
Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69.
Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125.
Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–7.
Lee M, Kim SE, Kim WS, Lee J, Yoo HK, Park K-D, et al. Interaction of motor training and intermittent theta burst stimulation in modulating motor cortical plasticity: influence of BDNF Val66Met polymorphism. PLoS ONE. 2013;8:e57690.
Di Lazzaro V, Pellegrino G, Di Pino G, Corbetto M, Ranieri F, Brunelli N, et al. Val66Met BDNF gene polymorphism influences human motor cortex plasticity in acute stroke. Brain Stimul. 2015;8:92–6.
Mori F, Ribolsi M, Kusayanagi H, Siracusano A, Mantovani V, Marasco E, et al. Genetic variants of the NMDA receptor influence cortical excitability and plasticity in humans. J Neurophysiol. 2011;106:1637–43.
Mahley RW, Rall SC Jr. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genom Hum Genet. 2000;1:507–37.
White F, Nicoll JAR, Roses AD, Horsburgh K. Impaired neuronal plasticity in transgenic mice expressing human apolipoprotein E4 compared to E3 in a model of entorhinal cortex lesion. Neurobiol Dis. 2001;8:611–25.
Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE ε4 mice. Alzheimers Dement. 2009;5:287–94.
Peña-Gomez C, Solé-Padullés C, Clemente IC, Junqué C, Bargalló N, Bosch B, et al. APOE status modulates the changes in network connectivity induced by brain stimulation in non-demented elders. PLoS ONE. 2012;7:e51833.
Lee NJ, Ahn HJ, Jung K-I, Ohn SH, Hong J, Kim YJ, et al. Reduction of continuous theta burst stimulation-induced motor plasticity in healthy elderly with COMT Val158Met polymorphism. Ann Rehabil Med. 2014;38:658–64.
Bhakar AL, Dölen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses). Annu Rev Neurosci. 2012;35:417–43.
Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–4.
Gipson TT, Johnston MV. Plasticity and mTOR: towards restoration of impaired synaptic plasticity in mTOR-related neurogenetic disorders. Neural Plast. 2012;2012:486402.
Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med. 2011;62:411–29.
Peça J, Ting J, Feng G. SnapShot: autism and the synapse. Cell. 2011;147:706, 706.e1.
Percy AK. Rett syndrome: exploring the autism link. Arch Neurol. 2011;68:985–9.
Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2005;102:12560–5.
Gogolla N, Leblanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1:172–81.
Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA. 2002;99:7746–50.
Narita N, Kato M, Tazoe M, Miyazaki K, Narita M, Okado N. Increased monoamine concentration in the brain and blood of fetal thalidomide- and valproic acid-exposed rat: putative animal models for autism. Pediatr Res. 2002;52:576–9.
Rinaldi T, Kulangara K, Antoniello K, Markram H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc Natl Acad Sci USA. 2007;104:13501–6.
Rinaldi T, Perrodin C, Markram H. Hyper-connectivity and hyper-plasticity in the medial prefrontal cortex in the valproic Acid animal model of autism. Front Neural Circ. 2008;2:4.
Rinaldi T, Silberberg G, Markram H. Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb Cortex. 2008;18:763–70.
Tordjman S, Drapier D, Bonnot O, Graignic R, Fortes S, Cohen D, et al. Animal models relevant to schizophrenia and autism: validity and limitations. Behav Genet. 2007;37:61–78.
Jannati A, Ryan MA, Block G, Kayarian FB, Oberman LM, Rotenberg A, et al. Modulation of motor cortical excitability by continuous theta-burst stimulation in adults with autism spectrum disorder. Clin Neurophysiol. 2021;132:1647–62.
Jannati A, Block G, Ryan MA, Kaye HL, Kayarian FB, Bashir S, et al. Continuous theta-burst stimulation in children with high-functioning autism spectrum disorder and typically developing children. Front Integr Neurosci. 2020;14:13.
Oberman LM, Pascual-Leone A, Rotenberg A. Modulation of corticospinal excitability by transcranial magnetic stimulation in children and adolescents with autism spectrum disorder. Front Hum Neurosci. 2014;8:627.
Jannati A, Ryan MA, Kaye HL, Tsuboyama M, Rotenberg A. Biomarkers obtained by transcranial magnetic stimulation in neurodevelopmental disorders. J Clin Neurophysiol. 2021. https://doi.org/10.1097/WNP.0000000000000784.
McClintock SM, Freitas C, Oberman LM, Lisanby SH, Pascual-Leone A. Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry. 2011;70:19–27.
Hasan A, Brinkmann C, Strube W, Palm U, Malchow B, Rothwell JC, et al. Investigations of motor-cortex cortical plasticity following facilitatory and inhibitory transcranial theta-burst stimulation in schizophrenia: a proof-of-concept study. J Psychiatr Res. 2015;61:196–204.
Maeda F, Keenan JP, Pascual-Leone A. Interhemispheric asymmetry of motor cortical excitability in major depression as measured by transcranial magnetic stimulation. Br J Psychiatry. 2000;177:169–73.
Fitzgerald PB, Brown TL, Marston NA, Daskalakis ZJ, de Castella A, Bradshaw JL, et al. Motor cortical excitability and clinical response to rTMS in depression. J Affect Disord. 2004;82:71–6.
Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ. Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry. 2010;67:458–64.
Concerto C, Lanza G, Cantone M, Pennisi M, Giordano D, Spampinato C, et al. Different patterns of cortical excitability in major depression and vascular depression: a transcranial magnetic stimulation study. BMC Psychiatry. 2013;13:1–10.
Croarkin PE, Nakonezny PA, Husain MM, Melton T, Buyukdura JS, Kennard BD, et al. Evidence for increased glutamatergic cortical facilitation in children and adolescents with major depressive disorder. JAMA Psychiatry. 2013;70:291–9.
Veronezi B, Moffa A, Carvalho A, Galhardoni R, Simis M, Benseñor I, et al. Evidence for increased motor cortical facilitation and decreased inhibition in atypical depression. Acta Psychiatr Scandin. 2016;134:172–82.
Spampinato C, Aguglia E, Concerto C, Pennisi M, Lanza G, Bella R, et al. Transcranial magnetic stimulation in the assessment of motor cortex excitability and treatment of drug-resistant major depression. IEEE Trans Neural Syst Rehabil Eng. 2013;21:391–403.
Bajbouj M, Lisanby SH, Lang UE, Danker-Hopfe H, Heuser I, Neu P. Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol Psychiatry. 2006;59:395–400.
Bajwa S, Bermpohl F, Rigonatti SP, Pascual-Leone A, Boggio PS, Fregni F. Impaired interhemispheric interactions in patients with major depression. J Nerv Ment Dis. 2008;196:671–7.
Triggs WJ, McCoy KJ, Greer R, Rossi F, Bowers D, Kortenkamp S, et al. Effects of left frontal transcranial magnetic stimulation on depressed mood, cognition, and corticomotor threshold. Biol Psychiatry. 1999;45:1440–6.
Concerto C, Lanza G, Cantone M, Ferri R, Pennisi G, Bella R, et al. Repetitive transcranial magnetic stimulation in patients with drug-resistant major depression: a six-month clinical follow-up study. Int J Psychiatry Clin Pract. 2015;19:252–8.
Samii A, Wassermann EM, Ikoma K, Mercuri B, George MS, O’Fallon A, et al. Decreased postexercise facilitation of motor evoked potentials in patients with chronic fatigue syndrome or depression. Neurology. 1996;47:1410–4.
Shajahan PM, Glabus MF, Gooding PA, Shah PJ, Ebmeier KP. Reduced cortical excitability in depression: Impaired post-exercise motor facilitation with transcranial magnetic stimulation. Br J Psychiatry. 1999;174:449–54.
Suppa A, Quartarone A, Siebner H, Chen R, Di Lazzaro V, Del, et al. The associative brain at work: Evidence from paired associative stimulation studies in humans. Clin Neurophysiol. 2017;128:2140–64.
Player MJ, Taylor JL, Weickert CS, Alonzo A, Sachdev P, Martin D, et al. Neuroplasticity in depressed individuals compared with healthy controls. Neuropsychopharmacol. 2013;38:2101–8.
Noda Y, Zomorrodi R, Vila-Rodriguez F, Downar J, Farzan F, Cash RFH, et al. Impaired neuroplasticity in the prefrontal cortex in depression indexed through paired associative stimulation. Depress Anxiety. 2018;35:448–56.
Vignaud P, Damasceno C, Poulet E, Brunelin J. Impaired modulation of corticospinal excitability in drug-free patients with major depressive disorder: a theta-burst stimulation study. Front Hum Neurosci. 2019;13:72.
Oliveira-Maia AJ, Press D, Pascual-Leone A. Modulation of motor cortex excitability predicts antidepressant response to prefrontal cortex repetitive transcranial magnetic stimulation. Brain Stimul. 2017;10:787–94.
Hinchman CA, Fried PJ, Jannati A, Press DZ, Pascual-Leone A, Stern AP. Corticomotor plasticity as a predictor of response to high frequency transcranial magnetic stimulation treatment for major depressive disorder. J Affect Disord. 2022;303:114–22.
Andary MT, Crewe N, Ganzel SK, Haines-Pepi C, Kulkarni MR, Stanton DF, et al. Traumatic brain injury/chronic pain syndrome: a case comparison study. Clin J Pain. 1997;13:244–50.
Jorge RE, Acion L, Starkstein SE, Magnotta V. Hippocampal volume and mood disorders after traumatic brain injury. Biol Psychiatry. 2007;62:332–8.
Hou L, Han X, Sheng P, Tong W, Li Z, Xu D, et al. Risk factors associated with sleep disturbance following traumatic brain injury: clinical findings and questionnaire based study. PLoS ONE. 2013;8:e76087.
Golarai G, Greenwood AC, Feeney DM, Connor JA. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J Neurosci. 2001;21:8523–37.
Lucke-Wold BP, Nguyen L, Turner RC, Logsdon AF, Chen Y-W, Smith KE, et al. Traumatic brain injury and epilepsy: Underlying mechanisms leading to seizure. Seizure. 2015;33:13–23.
Prince DA, Jacobs K. Inhibitory function in two models of chronic epileptogenesis. Epilepsy Res. 1998;32:83–92.
Huusko N, Pitkänen A. Parvalbumin immunoreactivity and expression of GABAA receptor subunits in the thalamus after experimental TBI. Neuroscience. 2014;267:30–45.
Cantu D, Walker K, Andresen L, Taylor-Weiner A, Hampton D, Tesco G, et al. Traumatic brain injury increases cortical glutamate network activity by compromising GABAergic control. Cereb Cortex. 2015;25:2306–20.
Hunt RF, Boychuk JA, Smith BN. Neural circuit mechanisms of post-traumatic epilepsy. Front Cell Neurosci. 2013;7:89.
Hsieh T-H, Lee HHC, Hameed MQ, Pascual-Leone A, Hensch TK, Rotenberg A. Trajectory of parvalbumin cell impairment and loss of cortical inhibition in traumatic brain injury. Cereb Cortex. 2017;27:5509–24.
Vahabzadeh-Hagh AM, Muller PA, Pascual-Leone A, Jensen FE, Rotenberg A. Measures of cortical inhibition by paired-pulse transcranial magnetic stimulation in anesthetized rats. J Neurophysiol. 2011;105:615–24.
Hsieh T-H, Dhamne SC, Chen J-JJ, Pascual-Leone A, Jensen FE, Rotenberg A. A new measure of cortical inhibition by mechanomyography and paired-pulse transcranial magnetic stimulation in unanesthetized rats. J Neurophysiol. 2012;107:966–72.
Opie GM, Foo N, Killington M, Ridding MC, Semmler JG. Transcranial magnetic stimulation-electroencephalography measures of cortical neuroplasticity are altered after mild traumatic brain injury. J Neurotrauma. 2019;36:2774–84.
Chistyakov AV, Soustiel JF, Hafner H, Elron M, Feinsod M. Altered excitability of the motor cortex after minor head injury revealed by transcranial magnetic stimulation. Acta Neurochir. 1998;140:467–72.
Bashir S, Vernet M, Yoo W-K, Mizrahi I, Theoret H, Pascual-Leone A. Changes in cortical plasticity after mild traumatic brain injury. Restor Neurol Neurosci. 2012;30:277–82.
Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, et al. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 2008;28:4231–7.
Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42.
Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–8.
Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801.
Ghoshal N, García-Sierra F, Wuu J, Leurgans S, Bennett DA, Berry RW, et al. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer’s disease. Exp Neurol. 2002;177:475–93.
Boekhoorn K, Terwel D, Biemans B, Borghgraef P, Wiegert O, Ramakers GJA, et al. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci. 2006;26:3514–23.
Battaglia F, Wang H-Y, Ghilardi MF, Gashi E, Quartarone A, Friedman E, et al. Cortical plasticity in Alzheimer’s disease in humans and rodents. Biol Psychiatry. 2007;62:1405–12.
Koch G, Di Lorenzo F, Bonnì S, Ponzo V, Caltagirone C, Martorana A. Impaired LTP-but not LTD-like cortical plasticity in Alzheimer’s disease patients. J Alzheimer’s Dis. 2012;31:593–9.
Di Lorenzo F, Ponzo V, Bonnì S, Motta C, Negrão Serra PC, Bozzali M, et al. Long-term potentiation-like cortical plasticity is disrupted in Alzheimer’s disease patients independently from age of onset. Ann Neurol. 2016;80:202–10.
Koch G, Di Lorenzo F, Del Olmo MF, Bonní S, Ponzo V, Caltagirone C, et al. Reversal of LTP-Like Cortical Plasticity in Alzheimer’s Disease Patients with Tau-Related Faster Clinical Progression. J Alzheimers Dis. 2016;50:605–16.
Di Lorenzo F, Motta C, Bonnì S, Mercuri NB, Caltagirone C, Martorana A, et al. LTP-like cortical plasticity is associated with verbal memory impairment in Alzheimer’s disease patients. Brain Stimul. 2019;12:148–51.
Motta C, Di Lorenzo F, Ponzo V, Pellicciari MC, Bonnì S, Picazio S, et al. Transcranial magnetic stimulation predicts cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2018;89:1237–42.
Brem A-K, Di Iorio R, Fried PJ, Oliveira-Maia AJ, Marra C, Profice P, et al. Corticomotor plasticity predicts clinical efficacy of combined neuromodulation and cognitive training in Alzheimer’s disease. Front Aging Neurosci. 2020;12:200.
Di Lazzaro V, Bella R, Benussi A, Bologna M, Borroni B, Capone F, et al. Diagnostic contribution and therapeutic perspectives of transcranial magnetic stimulation in dementia. Clin Neurophysiol. 2021;132:2568–607.
Buss SS, Press DZ, McDonald K, Kitchener E, O’Connor M, Donohoe K, et al. LTP-like plasticity is impaired in amyloid-positive amnestic MCI but independent of PET-amyloid burden. Neurobiol Aging. 2020;96:109–16.
Vos SJB, Verhey F, Frölich L, Kornhuber J, Wiltfang J, Maier W, et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain. 2015;138:1327–38.
Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15:321–87.
Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–17.
Sweetnam D, Holmes A, Tennant KA, Zamani A, Walle M, Jones P, et al. Diabetes impairs cortical plasticity and functional recovery following ischemic stroke. J Neurosci. 2012;32:5132–43.
Fried PJ, Schilberg L, Brem A-K, Saxena S, Wong B, Cypess AM, et al. Humans with type-2 diabetes show abnormal long-term potentiation-like cortical plasticity associated with verbal learning deficits. J Alzheimers Dis. 2016;55:89–100.
Abbas T, Faivre E, Hölscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behavioural Brain Res. 2009;205:265–71.
Nisticò R, Cavallucci V, Piccinin S, Macrì S, Pignatelli M, Mehdawy B, et al. Insulin receptor β-subunit haploinsufficiency impairs hippocampal late-phase LTP and recognition memory. Neuromolecular Med. 2012;14:262–9.
Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, et al. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes. 2015;64:3927–36.
Parwani A, Weiler MA, Blaxton TA, Warfel D, Hardin M, Frey K, et al. The effects of a subanesthetic dose of ketamine on verbal memory in normal volunteers. Psychopharmacology. 2005;183:265–74.
Rowland LM, Astur RS, Jung RE, Bustillo JR, Lauriello J, Yeo RA. Selective cognitive impairments associated with NMDA receptor blockade in humans. Neuropsychopharmacology. 2005;30:633–9.
Driesen NR, McCarthy G, Bhagwagar Z, Bloch MH, Calhoun VD, D’souza DC, et al. The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology. 2013;38:2613–22.
Levin R, Dor-Abarbanel AE, Edelman S, Durrant AR, Hashimoto K, Javitt DC, et al. Behavioral and cognitive effects of the N-methyl-D-aspartate receptor co-agonist D-serine in healthy humans: initial findings. J Psychiatr Res. 2015;61:188–95.
Fried PJ, Pascual-Leone A, Bolo NR. Diabetes and the link between neuroplasticity and glutamate in the aging human motor cortex. Clin Neurophysiol. 2019;130:1502–10.
Lømo T. The discovery of long-term potentiation. Philos Trans R Soc Lond B. 2003;358:617–20.
Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–99.
Malenka RC, Bear MF. LTP and LTD. Neuron. 2004;44:5–21.
Hameed MQ, Sanchez MJ, Gersner R, Rotenberg A. Insights into pediatric brain stimulation protocols from preclinical research. In: Kirton A, Gilbert DL, editors. Pediatric Brain Stimulation. Ch. 6. Oxford: Academic Press; 2016;117–30.
Muller PA, Dhamne SC, Vahabzadeh-Hagh AM, Pascual-Leone A, Jensen FE, Rotenberg A. Suppression of motor cortical excitability in anesthetized rats by low frequency repetitive transcranial magnetic stimulation. PLoS ONE. 2014;9:e91065.
An S, Yang J-W, Sun H, Kilb W, Luhmann HJ. Long-term potentiation in the neonatal rat barrel cortex in vivo. J Neurosci. 2012;32:9511–6.
Cao G, Harris KM. Developmental regulation of the late phase of long-term potentiation (L-LTP) and metaplasticity in hippocampal area CA1 of the rat. J Neurophysiol. 2012;107:902–12.
Kirkwood A, Silva A, Bear MF. Age-dependent decrease of synaptic plasticity in the neocortex of alphaCaMKII mutant mice. Proc Natl Acad Sci USA. 1997;94:3380–3.
Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–8.
Mix A, Hoppenrath K, Funke K. Reduction in cortical parvalbumin expression due to intermittent theta-burst stimulation correlates with maturation of the perineuronal nets in young rats. Dev Neurobiol. 2015;75:1–11.
Hsieh T-H, Huang Y-Z, Chen J-JJ, Rotenberg A, Chiang Y-H, Chien W-SC, et al. Novel use of theta burst cortical electrical stimulation for modulating motor plasticity in rats. J Med Biol Eng. 2015;35:62–8.
Wu C-W, Chiu W-T, Hsieh T-H, Hsieh C-H, Chen, JJ J-. Modulation of motor excitability by cortical optogenetic theta burst stimulation. PLoS ONE. 2018;13:e0203333.
Li Y-T, Chen S-C, Yang L-Y, Hsieh T-H, Peng C-W. Designing and implementing a novel transcranial electrostimulation system for neuroplastic applications: a preliminary study. IEEE Trans Neural Syst Rehabil Eng. 2019;27:805–13.
Kuo C-W, Chang M-Y, Liu H-H, He X-K, Chan S-Y, Huang Y-Z, et al. Cortical electrical stimulation ameliorates traumatic brain injury-induced sensorimotor and cognitive deficits in rats. Front Neural Circ. 2021;15:693073.
Marufa SA, Hsieh T-H, Liou J-C, Chen H-Y, Peng C-W. Neuromodulatory effects of repetitive transcranial magnetic stimulation on neural plasticity and motor functions in rats with an incomplete spinal cord injury: a preliminary study. PLoS ONE. 2021;16:e0252965.
Kuo C-W, Chang M-Y, Chou M-Y, Pan C-Y, Peng C-W, Tseng H-C, et al. Long-term motor cortical electrical stimulation ameliorates 6-hydroxydopamine-induced motor dysfunctions and exerts neuroprotective effects in a rat model of Parkinson’s disease. Front Aging Neurosci. 2022;14:848380.
Nahas Z, Lomarev M, Roberts DR, Shastri A, Lorberbaum JP, Teneback C, et al. Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biol Psychiatry. 2001;50:712–20.
Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EM, et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry. 1999;46:1603–13.
Fitzgerald PB, Sritharan A, Daskalakis ZJ, de Castella AR, Kulkarni J, Egan G. A functional magnetic resonance imaging study of the effects of low frequency right prefrontal transcranial magnetic stimulation in depression. J Clin Psychopharmacol. 2007;27:488–92.
Beynel L, Powers JP, Appelbaum LG. Effects of repetitive transcranial magnetic stimulation on resting-state connectivity: a systematic review. Neuroimage. 2020;211:116596.
Hawco C, Voineskos AN, Steeves JKE, Dickie EW, Viviano JD, Downar J, et al. Spread of activity following TMS is related to intrinsic resting connectivity to the salience network: a concurrent TMS-fMRI study. Cortex. 2018;108:160–72.
Tang Y, Jiao X, Wang J, Zhu T, Zhou J, Qian Z, et al. Dynamic functional connectivity within the fronto-limbic network induced by intermittent theta-burst stimulation: a Pilot study. Front Neurosci. 2019;13:944.
Alkhasli I, Sakreida K, Mottaghy FM, Binkofski F. Modulation of fronto-striatal functional connectivity using transcranial magnetic stimulation. Front Hum Neurosci. 2019;13:190.
Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–26.
Philip NS, Barredo J, van’t Wout-Frank M, Tyrka AR, Price LH, Carpenter LL. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 2018;83:263–72.
Siddiqi SH, Taylor SF, Cooke D, Pascual-Leone A, George MS, Fox MD. Distinct symptom-specific treatment targets for circuit-based neuromodulation. AJP. 2020;177:435–46.
Cash RFH, Cocchi L, Lv J, Fitzgerald PB, Zalesky A. Functional magnetic resonance imaging–guided personalization of transcranial magnetic stimulation treatment for depression. JAMA Psychiatry. 2021;78:337.
Siebner H, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16.
Schiena G, Maggioni E, Pozzoli S, Brambilla P. Transcranial magnetic stimulation in major depressive disorder: Response modulation and state dependency. J Affect Disord. 2020;266:793–801.
Philip NS, Dunner DL, Dowd SM, Aaronson ST, Brock DG, Carpenter LL, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized Pilot study. Brain Stimulation. 2016;9:251–7.
Best SRD, Pavel DG, Haustrup N. Combination therapy with transcranial magnetic stimulation and ketamine for treatment-resistant depression: a long-term retrospective review of clinical use. Heliyon. 2019;5:e02187.
Zhang T, Zhu J, Xu L, Tang X, Cui H, Wei Y, et al. Add-on rTMS for the acute treatment of depressive symptoms is probably more effective in adolescents than in adults: Evidence from real-world clinical practice. Brain Stimul. 2019;12:103–9.
Facchini S, Muellbacher W, Battaglia F, Boroojerdi B, Hallett M. Focal enhancement of motor cortex excitability during motor imagery: a transcranial magnetic stimulation study: focal enhancement of motor cortex excitability. Acta Neurol Scand. 2002;105:146–51.
Li S, Latash ML, Zatsiorsky VM. Effects of motor imagery on finger force responses to transcranial magnetic stimulation. Cogn Brain Res. 2004;20:273–80.
Scangos KW, Makhoul GS, Sugrue LP, Chang EF, Krystal AD. State-dependent responses to intracranial brain stimulation in a patient with depression. Nat Med. 2021;27:229–31.
Tendler A, Sisko E, Barnea-Ygael N, Zangen A, Storch EA. A method to provoke obsessive compulsive symptoms for basic research and clinical interventions. Front Psychiatry. 2019;10:814.
Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder—a Pilot Study. Brain Stimul. 2013;6:377–83.
Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M, et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry. 2014;76:742–9.
Carmi L, Tendler A, Bystritsky A, Hollander E, Blumberger DM, Daskalakis J, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. AJP. 2019;176:931–8.
Sathappan AV, Luber BM, Lisanby SH. The Dynamic Duo: combining noninvasive brain stimulation with cognitive interventions. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:347–60.
Paus T, Sipila PK, Strafella AP. Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: an EEG study. J Neurophysiol. 2001;86:1983–90.
Klimesch W, Sauseng P, Gerloff C. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency: rTMS and individual alpha frequency. Eur J Neurosci. 2003;17:1129–33.
Fuggetta G, Fiaschi A, Manganotti P. Modulation of cortical oscillatory activities induced by varying single-pulse transcranial magnetic stimulation intensity over the left primary motor area: a combined EEG and TMS study. NeuroImage. 2005;27:896–908.
Fuggetta G, Pavone EF, Fiaschi A, Manganotti P. Acute modulation of cortical oscillatory activities during short trains of high-frequency repetitive transcranial magnetic stimulation of the human motor cortex: a combined EEG and TMS study. Hum Brain Mapp. 2008;29:1–13.
Brignani D, Manganotti P, Rossini PM, Miniussi C. Modulation of cortical oscillatory activity during transcranial magnetic stimulation. Hum Brain Mapp. 2008;29:603–12.
Hamidi M. Repetitive transcranial magnetic stimulation affects behavior by biasing endogenous cortical oscillations. Front Integr Neurosci. 2009;3:14.
Thut G, Miniussi C. New insights into rhythmic brain activity from TMS–EEG studies. Trends Cogn Sci. 2009;13:182–9.
Thut G, Schyns PG, Gross J. Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front Psychol. 2011;2:170.
Johnson JS, Kundu B, Casali AG, Postle BR. Task-dependent changes in cortical excitability and effective connectivity: a combined TMS-EEG study. J Neurophysiol. 2012;107:2383–92.
Fuggetta G, Noh NA. A neurophysiological insight into the potential link between transcranial magnetic stimulation, thalamocortical dysrhythmia and neuropsychiatric disorders. Exp Neurol. 2013;245:87–95.
Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–9.
Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud A-L, Kleinschmidt A. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. J Neurosci. 2010;30:10243–50.
Notbohm A, Kurths J, Herrmann CS. Modification of brain oscillations via rhythmic light stimulation provides evidence for entrainment but not for superposition of event-related responses. Front Hum Neurosci. 2016;10:10.
Jin Y, Potkin SG, Kemp AS, Huerta ST, Alva G, Thai TM, et al. Therapeutic effects of individualized alpha frequency transcranial magnetic stimulation (TMS) on the negative symptoms of schizophrenia. Schizophr Bull. 2005;32:556–61.
Jin Y, Kemp AS, Huang Y, Thai TM, Liu Z, Xu W, et al. Alpha EEG guided TMS in schizophrenia. Brain Stimul. 2012;5:560–8.
Corlier J, Carpenter LL, Wilson AC, Tirrell E, Gobin AP, Kavanaugh B, et al. The relationship between individual alpha peak frequency and clinical outcome with repetitive Transcranial Magnetic Stimulation (rTMS) treatment of Major Depressive Disorder (MDD). Brain Stimul. 2019;12:1572–8.
Roelofs CL, Krepel N, Corlier J, Carpenter LL, Fitzgerald PB, Daskalakis ZJ, et al. Individual alpha frequency proximity associated with repetitive transcranial magnetic stimulation outcome: an independent replication study from the ICON-DB consortium. Clin Neurophysiol. 2021;132:643–9.
EEG Synchronized TMS Trial for Depression. ClinicalTrialsGov. https://clinicaltrials.gov/ct2/show/study/NCT03421808. Accessed 1 May 2021.
Tsuboyama M, Kaye HL, Rotenberg A. Biomarkers obtained by transcranial magnetic stimulation of the motor cortex in epilepsy. Front Integr Neurosci. 2019;13:57.
Cash RFH, Weigand A, Zalesky A, Siddiqi SH, Downar J, Fitzgerald PB, et al. Using brain imaging to improve spatial targeting of transcranial magnetic stimulation for depression. Biol Psychiatry. 2021;90:689–700.
Deng Z-D, Luber B, Balderston NL, Velez Afanador M, Noh MM, Thomas J, et al. Device-based modulation of neurocircuits as a therapeutic for psychiatric disorders. Annu Rev Pharm Toxicol. 2020;60:591–614.
Funding
APL was partly supported by the National Institutes of Health (R24AG06142 and P01 AG031720) and received funding from MagStim, Inc. AR was partly supported by the National Institutes of Health (NIH R01 MH100186, R01 NS088583), and The Boston Children’s Hospital Translational Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University and its affiliated academic health care centers, the National Institutes of Health, or any of the other listed granting agencies.
Author information
Authors and Affiliations
Contributions
APL and AJ contributed to the conception and design of the paper. AJ drafted the paper with contributions from LMO. All authors contributed to the revising and the final approval of the paper and agreed to be accountable for the content of the paper.
Corresponding authors
Ethics declarations
Competing interests
AJ is an employee of Linus Health. APL is a cofounder of Linus Health and TI Solutions AG; serves on the scientific advisory boards for Starlab Neuroscience, Magstim Inc., and MedRhythms; and is listed as an inventor on several issued and pending patents on the real-time integration of non-invasive brain stimulation with electroencephalography and magnetic resonance imaging. AR is a founder and advisor for Neuromotion and PrevEP, serves on the medical advisory board or has consulted for Cavion, Epihunter, Gamify, Neural Dynamics, NeuroRex, Otsuka, Roche, and is listed as an inventor on a patent related to integration of TMS and EEG. The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jannati, A., Oberman, L.M., Rotenberg, A. et al. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacol. 48, 191–208 (2023). https://doi.org/10.1038/s41386-022-01453-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01453-8
This article is cited by
-
Effect of repetitive transcranial magnetic stimulation-assisted training on lower limb motor function in children with hemiplegic cerebral palsy
BMC Pediatrics (2024)
-
The changes in metabolomics profile induced by intermittent theta burst stimulation in major depressive disorder: an exploratory study
BMC Psychiatry (2023)
-
TMS in Schizophrenia: Potential Mechanistic Insights via Resting-State Network Analyses
Current Behavioral Neuroscience Reports (2023)