Abstract
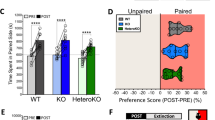
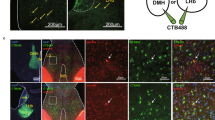
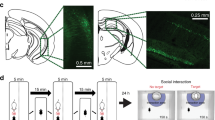
α2a-adrenergic receptor (α2a-AR) agonists are candidate substance use disorder therapeutics due to their ability to recruit noradrenergic autoreceptors to dampen stress system engagement. However, we recently found that postsynaptic α2a-ARs are required for stress-induced reinstatement of cocaine-conditioned behavior. Understanding the ensembles recruited by these postsynaptic receptors (heteroceptors) is necessary to understand noradrenergic circuit control. We utilized a variety of approaches in FosTRAP (Targeted Recombination in Active Populations) mice to define an ensemble of cells activated by the α2a-AR partial agonist guanfacine (“Guansembles”) in the bed nucleus of the stria terminalis (BST/BNST), a region key to stress-induced reinstatement of drug seeking. We define BNST “Guansembles” and show they differ from restraint stress-activated cells. Guanfacine produced inhibition of cAMP-dependent signaling in Guansembles, while chronic restraint stress increased cAMP-dependent signaling. Guanfacine both excited and inhibited aspects of Guansemble neuronal activity. Further, while some stressors produced overall reductions in Guansemble activity, active coping events during restraint stress and exposure to unexpected shocks were both associated with Guansemble recruitment. Using viral tracing, we define a BNST Guansemble afferent network that includes regions involved in the interplay of stress and homeostatic functions. Finally, we show that activation of Guansembles produces alterations in behavior on the elevated plus maze consistent with task-specific anxiety-like behavior. Overall, we define a population of BNST neurons recruited by α2a-AR signaling that opposes the behavioral action of canonical autoreceptor α2a-AR populations and which are differentially recruited by distinct stressors. Moreover, we demonstrate stressor-specific physiological responses in a specific neuronal population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Buffalari DM, Baldwin CK, See RE. Treatment of cocaine withdrawal anxiety with guanfacine: relationships to cocaine intake and reinstatement of cocaine seeking in rats. Psychopharmacol (Berl). 2012;223:179–90.
Gowing L, Farrell M, Ali R & White JM. Alpha2 adrenergic agonists for the management of opioid withdrawal. in Cochrane Database of Systematic Reviews (ed. The Cochrane Collaboration) CD002024.pub2 (John Wiley & Sons, Ltd, 2004). https://doi.org/10.1002/14651858.CD002024.pub2.
Simpson TL, et al. A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res 2015;39:808–17.
Simpson TL, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175:1216–24.
Pergolizzi JV, Annabi H, Gharibo C, LeQuang JA. The role of lofexidine in management of opioid withdrawal. Pain Ther. 2019;8:67–78.
Milivojevic V, Angarita GA, Hermes G, Sinha R, Fox HC. Effects of prazosin on provoked alcohol craving and autonomic and neuroendocrine response to stress in alcohol use disorder. Alcohol Clin Exp Res. 2020;44:1488–96.
Harmon RJ, Riggs PD. Clonidine for posttraumatic stress disorder in preschool children. J Am Acad Child Adolesc Psychiatry. 1996;35:1247–9.
Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9:388–95.
Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacol (Berl). 2001;158:343–59.
Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N. Y Acad Sci. 2008;1141:105–30.
Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35–RC35.
Mantsch JR, et al. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for β-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–78.
Fox H & Sinha R. The role of guanfacine as a therapeutic agent to address stress-related pathophysiology in cocaine-dependent individuals. in Advances in Pharmacology vol. 69 217–65 (Elsevier, 2014).
Fox HC, Morgan PT, Sinha R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology. 2014;39:1527–37.
Fox HC, et al. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol (Oxf). 2012;26:958–72.
Krupitsky E, et al. Naltrexone with or without guanfacine for preventing relapse to opiate addiction in St.-Petersburg, Russia. Drug Alcohol Depend. 2013;132:674–80.
Horrigan JP. Guanfacine for PTSD nightmares. J Am Acad Child Adolesc Psychiatry. 1996;35:975–6.
Anderson J, Wang C, Zaidi A, Rice T, Coffey BJ. Guanfacine as a treatment for posttraumatic stress disorder in an adolescent female. J Child Adolesc Psychopharmacol 2020;30:398–401.
Davis LL, et al. A placebo-controlled trial of guanfacine for the treatment of posttraumatic stress disorder in veterans. Psychopharmacol Bull 2008;41:8–18.
Connor DF et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6–12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 1 (2010) https://doi.org/10.2165/11537790-000000000-00000.
Perez RE, et al. α2A-adrenergic heteroreceptors are required for stress-induced reinstatement of cocaine conditioned place preference. Neuropsychopharmacology. (2020) https://doi.org/10.1038/s41386-020-0641-z.
Harris NA, et al. Dorsal BNST α2A-adrenergic receptors produce HCN-dependent excitatory actions that initiate anxiogenic behaviors. J. Neurosci. 0963–18 (2018) https://doi.org/10.1523/JNEUROSCI.0963-18.2018.
McElligott ZA, Winder DG. Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1329–35.
Wenzel JM, et al. Noradrenergic β-Receptor antagonism within the central nucleus of the amygdala or bed nucleus of the stria terminalis attenuates the negative/anxiogenic effects of cocaine. J Neurosci. 2014;34:3467–74.
Shields AD, Wang Q, Winder DG. α2A-adrenergic receptors heterosynaptically regulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuroscience. 2009;163:339–51.
Vranjkovic O, Pina M, Kash TL, Winder DG. The bed nucleus of the stria terminalis in drug-associated behavior and affect: A circuit-based perspective. Neuropharmacology. 2017;122:100–6.
Pina MM, Young EA, Ryabinin AE, Cunningham CL. The bed nucleus of the stria terminalis regulates ethanol-seeking behavior in mice. Neuropharmacology. 2015;99:627–38.
Guenthner CJ, Miyamichi K, Yang HH, Heller HC, Luo L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron. 2013;78:773–84.
DeNardo LA, et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat Neurosci. 2019;22:460–9.
Luchsinger JR, et al. Delineation of an insula-BNST circuit engaged by struggling behavior that regulates avoidance in mice. Nat Commun. 2021;12:3561.
Fetterly TL, et al. α2A -Adrenergic receptor activation decreases parabrachial nucleus excitatory drive onto BNST CRF neurons and reduces their activity. Vivo J Neurosci 2019;39:472–84.
Jaramillo AA, Williford KM, Marshall C, Winder DG, Centanni SW. BNST transient activity associates with approach behavior in a stressful environment and is modulated by the parabrachial nucleus. Neurobiol Stress. 2020;13:100247.
Salimando GJ, Hyun M, Boyt KM, Winder DG. BNST GluN2D-containing NMDA receptors influence anxiety- and depressive-like behaviors and modulatecell-specific excitatory/inhibitory synaptic balance. J Neurosci. 2020;40:3949–68.
Park Y-G, et al. Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat Biotechnol. 2019;37:73–83.
Hedde PN, Gratton E. Selective plane illumination microscopy with a light sheet of uniform thickness formed by an electrically tunable lens. Microsc Res Tech. 2018;81:924–8.
Dean KM, Roudot P, Welf ES, Danuser G, Fiolka R. Deconvolution-free subcellular imaging with axially swept light sheet microscopy. Biophys J. 2015;108:2807–15.
Ch’ng S, Fu J, Brown RM, McDougall SJ, Lawrence AJ. The intersection of stress and reward: BNST modulation of aversive and appetitive states. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87:108–25.
Giardino WJ, Pomrenze MB. Extended amygdala neuropeptide circuitry of emotional arousal: waking up on the wrong side of the bed nuclei of stria terminalis. Front Behav Neurosci. 2021;15:613025.
Palmiter RD. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 2018;41:280–93.
Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD. Elucidating an affective pain circuit that creates a threat memory. Cell. 2015;162:363–74.
Girasole AE, et al. A subpopulation of striatal neurons mediates levodopa-induced dyskinesia. Neuron. 2018;97:787–795.e6.
Xing B, et al. A subpopulation of prefrontal cortical neurons is required for social memory. Biol Psychiatry. 2021;89:521–31.
Chen Y, Saulnier JL, Yellen G & Sabatini BL. A PKA activity sensor for quantitative analysis of endogenous GPCR signaling via 2-photon FRET-FLIM imaging. Front. Pharmacol. 2014;5:56.
Lee SJ, Chen Y, Lodder B, Sabatini BL. Monitoring behaviorally induced biochemical changes using fluorescence lifetime photometry. Front Neurosci. 2019;13:766.
Lemberger T, et al. Expression of Cre recombinase in dopaminoceptive neurons. BMC Neurosci. 2007;8:4.
Joffe ME, Turner BD, Delpire E, Grueter BA. Genetic loss of GluN2B in D1-expressing cell types enhances long-term cocaine reward and potentiation of thalamo-accumbens synapses. Neuropsychopharmacol Publ Am Coll Neuropsychopharmacol 2018;43:2383–9.
Gong S, et al. Targeting cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–23.
Schmidt, KT et al. Stress-induced alterations of norepinephrine release in the bed nucleus of the stria terminalis of mice. http://biorxiv.org/lookup/doi/10.1101/335653 (2018) https://doi.org/10.1101/335653.
Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43:1139–47.
Joffe, ME et al. Acute restraint stress redirects prefrontal cortex circuit function through mGlu5 receptor plasticity on somatostatin-expressing interneurons. Neuron. S0896627321010448 (2022) https://doi.org/10.1016/j.neuron.2021.12.027.
Hu P, et al. Chronic stress induces maladaptive behaviors by activating corticotropin-releasing hormone signaling in the mouse oval bed nucleus of the stria terminalis. J Neurosci. 2020;40:2519–37.
Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Mol Brain Res. 2005;138:45–57.
Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacol (Berl). 2000;150:337–46.
Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacol (Berl). 1995;119:334–41.
Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacol (Berl). 1996;128:408–12.
Lu L, Liu D, Ceng X. Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. Eur J Pharmacol 2001;415:203–8.
Lu L, Zhang B, Liu Z, Zhang Z. Reactivation of cocaine conditioned place preference induced by stress is reversed by cholecystokinin-B receptors antagonist in rats. Brain Res. 2002;954:132–40.
Zhou P, et al. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. eLife. 2018;7:e28728.
Pnevmatikakis EA, Giovannucci A. NoRMCorre: An online algorithm for piecewise rigid motion correction of calcium imaging data. J Neurosci Methods. 2017;291:83–94.
Krawczyk M, et al. Double-dissociation of the catecholaminergic modulation of synaptic transmission in the oval bed nucleus of the stria terminalis. J Neurophysiol 2011;105:145–53.
Arnsten AFT, Ramos BP, Birnbaum SG, Taylor JR. Protein kinase A as a therapeutic target for memory disorders: rationale and challenges. Trends Mol Med 2005;11:121–8.
Bernabeu R, et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci. 1997;94:7041–6.
Dwivedi Y, Pandey GN. Elucidating biological risk factors in suicide: Role of protein kinase A. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:831–41.
Wang M, et al. α2A-adrenoceptors strengthen working memory networks by Inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410.
Armario A, Vallès A, Dal-Zotto S, Márquez C, Belda X. A single exposure to severe stressors causes long-term desensitisation of the physiological response to the homotypic stressor. Stress. 2004;7:157–72.
Kim S-Y, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–23.
Ahrens S, et al. A central extended amygdala circuit that modulates anxiety. J Neurosci 2018;38:5567–83.
Kash TL, et al. Neuropeptide regulation of signaling and behavior in the BNST. Mol Cells. 2015;38:1–13.
Flavin SA, Matthews RT, Wang Q, Muly EC, Winder DG. 2A-adrenergic receptors filter parabrachial inputs to the bed nucleus of the stria terminalis. J Neurosci 2014;34:9319–31.
Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016;21:450–63.
Holland PC. Different roles for amygdala central nucleus and substantia innominata in the surprise-induced enhancement of learning. J Neurosci 2006;26:3791–7.
Han J-S, Holland PC, Gallagher M. Disconnection of the amygdala central nucleus and substantia innominata/nucleus basalis disrupts increments in conditioned stimulus processing in rats. Behav Neurosci. 1999;113:143–51.
Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73.
Wilson DA, Kadohisa M, Fletcher ML. Cortical contributions to olfaction: Plasticity and perception. Semin Cell Dev Biol. 2006;17:462–70.
Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319:1842–5.
Kadohisa M, Wilson DA. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc Natl Acad Sci. 2006;103:15206–11.
Báez-Mendoza R & Schultz W. The role of the striatum in social behavior. Front Neurosci. 2013;7:233.
Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19:198–205.
Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9.
Groenewegen HJ, Berendse HW. The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends Neurosci. 1994;17:52–7.
Kirouac GJ. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev. 2015;56:315–29.
Matzeu A, Weiss F, Martin-Fardon R. Transient inactivation of the posterior paraventricular nucleus of the thalamus blocks cocaine-seeking behavior. Neurosci Lett 2015;608:34–39.
Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29:802–12.
James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV. Propensity to ‘relapse’ following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience. 2011;199:235–42.
Hua R, et al. Calretinin neurons in the midline thalamus modulate starvation-induced arousal. Curr Biol. 2018;28:3948–3959.e4.
Levine OB, et al. The paraventricular thalamus provides a polysynaptic brake on limbic CRF neurons to sex-dependently blunt binge alcohol drinking and avoidance behavior in mice. Nat Commun. 2021;12:5080.
Centanni SW, et al. Endocannabinoid control of the insular-bed nucleus of the stria terminalis circuit regulates negative affective behavior associated with alcohol abstinence. Neuropsychopharmacology. 2019;44:526–37.
Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34.
Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–80.
Flavin SA, Winder DG. Noradrenergic control of the bed nucleus of the stria terminalis in stress and reward. Neuropharmacology. 2013;70:324–30.
Silberman Y & Winder DG. Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Front Psychiatry. 2013;4:42.
Acknowledgements
We would like to thank Dr. Bernardo L. Sabatini, Harvard University, for the FLEX-FLIM-AKAR virus. Microscopy was performed in part through the use of the Vanderbilt Cell Imaging Shared Resource and the assistance of Dr. Robert Matthews. We also would like to thank Elana Milano, Bridget Morris, Laith Kayat, and Megan Altemus for assistance with mouse colony maintenance and genotyping.
Funding
This work was supported by the following funding sources: Howard Hughes Medical Institute Gilliam Fellowship (Grant No. GT10823 [JAB]), National Institute on Drug Abuse (Grant No. R01-DA042475-06A1[DGW]).
Author information
Authors and Affiliations
Contributions
JAB and DGW designed the project and experiments, and DGW supervised the project. JAB ran all in vivo experiments, including GCaMP fiber photometry, PKA sensor fluorescence lifetime imaging microscopy, and optogenetics. JAB analyzed and graphed all data. JAB ran all in vivo optogenetic experiments using equipment from SP. SWC ran preliminary PKA sensor experiments on D1-Cre and FosTRAP animals. NP ran, analyzed, and graphed all ex vivo calcium imaging. JAB and AYJ ran and analyzed all immunohistochemistry experiments. HJY and SAC ran all shock experiments, supervised by ESC. JRL imaged initial FosTRAP light sheet microscopy with equipment from RBS. MNB assisted with light sheet imaging and analysis, supervised by RBS. JAB and DGW wrote first draft of manuscript with editing and reviewing by NP, SWC, JRL, MNB, RBS, and ESC. Funding was acquired by JAB and DGW.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brown, J.A., Petersen, N., Centanni, S.W. et al. An ensemble recruited by α2a-adrenergic receptors is engaged in a stressor-specific manner in mice. Neuropsychopharmacol. 48, 1133–1143 (2023). https://doi.org/10.1038/s41386-022-01442-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01442-x