Abstract
Clinical investigations suggest involvement of the metabotropic glutamate receptor 5 (mGluR5) in the pathophysiology of fear learning that underlies trauma-related disorders. Here, we utilized a 4-day fear learning paradigm combined with positron emission tomography (PET) to examine the relationship between mGluR5 availability and differences in the response of rats to repeated footshock exposure (FE). Specifically, on day 1, male (n = 16) and female (n = 12) rats received 15 footshocks and were compared with control rats who did not receive footshocks (n = 7 male; n = 4 female). FE rats were classified as low responders (LR) or high responders (HR) based on freezing to the context the following day (day 2). PET with [18F]FPEB was used to calculate regional mGluR5 binding potential (BPND) at two timepoints: prior to FE (i.e., baseline), and post-behavioral testing. Additionally, we used an unbiased proteomics approach to assess group and sex differences in prefrontal cortex (PFC) protein expression. Post-behavioral testing we observed decreased BPND in LR females, but increased BPND in HR males relative to baseline. Further, individuals displaying the greatest freezing during the FE context memory test had the largest increases in PFC BPND. Males and females displayed unique post-test molecular profiles: in males, the greatest differences were between FE and CON, including upregulation of mGluR5 and related molecular networks in FE, whereas the greatest differences among females were between the LR and HR groups. These findings suggest greater mGluR5 availability increases following footshock exposure may be related to greater contextual fear memory. Results additionally reveal sex differences in the molecular response to footshock, including differential involvement of mGluR5-related molecular networks.
Similar content being viewed by others
Introduction
Most individuals will experience at least one potentially traumatic event in their lifetime [1]. While exposure to acute stress or trauma is a leading precipitating factor for mental health difficulties, development of psychopathology following a traumatic event is the exception, not the rule [2,3,4]. Type, severity, and chronicity of the trauma all contribute significantly to long-term mental health outcomes [5, 6]. These factors alone, however, cannot account for the extreme heterogeneity in individual responses to stress and trauma [7].
While it is expected for traumatic events to trigger an acute physiological and psychological stress response, mounting evidence suggests the magnitude of early responses can help predict individuals at higher risk for developing trauma-related physical and mental health difficulties [8]. For example, one study found that following physical injury, perceived threat to one’s life and early emergence of acute stress response symptoms were associated with a subsequent PTSD diagnosis, but objective measures of the injury severity, such as length of hospital admission, were not [9]. However, due to the limited number of prospective studies, the neurobiological mechanisms giving rise to differences in the processing and acute response to threatening stimuli are not fully understood.
One potential mediator is metabotropic glutamate receptor 5 (mGluR5). This Gq/11 protein-coupled receptor mediates forms of synaptic plasticity underpinning hippocampal-dependent spatial learning and memory, as well as extinction and reversal learning tasks [10,11,12,13]. Preclinical findings suggest that differences in mGluR5 expression and activity could contribute to susceptibility or resilience in rodent models of stress [14, 15]. Notably, these earlier studies primarily focus on repeated mild stressor exposure and depressive behaviors, rather than the immediate response to a single stressful event and used only male subjects, despite growing recognition of sex differences in stress response and its influence on emotional learning [16,17,18]. Importantly, clinical evidence suggests disruption of glutamate systems and mGluR5 in trauma and stress-related disorders [19,20,21]. Using positron emission tomography (PET) to measure mGluR5 availability in vivo, our group has previously demonstrated higher mGluR5 availability among subjects with PTSD that was associated with symptom severity [22, 23], and have additionally found ketamine-induced reductions in mGluR5 to be associated with ketamine’s rapid antidepressant efficacy [24]. However, it remains unclear if the observed differences in mGluR5 availability are (1) a genetic/molecular risk factor predisposing individuals to developing stress and trauma-related disorders; (2) an acute molecular response to stress or trauma; (3) a change or adaptation that develops over the course of illness; or (4) some combination of these scenarios.
Given the difficulties of performing prospective and mechanistic studies in humans and the lack of preclinical research examining changes in mGluR5 changes after a single stress event, the primary aim of the present study was to assess whether baseline mGluR5 availability could predict response to a single stressful, trauma-like event, or if relative changes in mGluR5 was related to individual differences in behavioral responses, using an established fear learning model in both male and female rats. Primary regions of interest were selected based on previous studies implicating them in fear learning and stress-related psychopathology that overlap with regions of mGluR5 expression, both in preclinical and human work [19, 22, 24,25,26,27].
As an exploratory aim, we performed unbiased analyses of prefrontal cortex proteins to identify and characterize differences in expression profiles following trauma exposure.
Methods and materials
Animals
Eight-week-old male and female Long Evans rats (Charles River Laboratories) were single housed in a climate-controlled room and maintained on a 12-h light/dark cycle (lights on at 7:00) with access to water ad libitum. Rats were acclimated to the facility for 5–6 days with daily handling prior to the experiment. An overview of the experimental timeline is provided in Fig. 1A. All experimental procedures were performed as approved by the Institutional Animal Care and Use Committee at Yale University and according to NIH and institutional guidelines and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
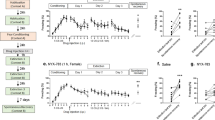
A A schematic of the experimental timeline beginning with the rats arriving to the facility 5–6 days prior to the baseline [18F]FPEB PET session, the 4-day fear learning paradigm, and the post-test [18F]FPEB PET session, after which rats were euthanized and PFC samples collected for subsequent proteomics analysis. B Details and C–F the freezing response during the 4-day fear learning protocol. C On day 1, rats exposed to 15 footshocks (FE; n = 28) displayed greater freezing behavior than rats who received no footshocks (CON; n = 11; main effect of treatment: F = 116.72; p < 0.001), and overall, males (open symbols; n = 23) froze more than females (solid symbols; n = 16; main effect of sex: F = 4.12 p = 0.050). D The test of Context A fear memory on day 2 revealed greater freezing in FE rats relative to CON (main effect of treatment: F = 27.73; p < 0.001). E While no differences were observed between treatment groups prior to the footshock (Fig. S3), FE rats displayed greater freezing in following the single footshock delivered in Context B on day 3 (main effect of treatment: F = 7.04; p = 0.0012). F No significant main effect of group, sex, or interaction was observed on the day 4 test of Context B fear memory. Individual values are displayed with the group mean ± SEM. Bonferroni’s post hoc tests: ns = not significant; *p < 0.05; ***p < 0.001 relative to males within the same group.
Behavioral testing
The fear learning paradigm was conducted in testing chambers individually housed within a sound-attenuating enclosures and equipped with an infrared camera (Med Associates, Inc, Fairfax, VT). The 4-day protocol (Fig. 1B) was adapted from a previously described [28] stress-enhanced fear learning paradigm (SEFL). On day 1, rats were transported to the behavioral suite adjacent to the housing facility and placed into Context A. In Context A, the chamber square walls were left exposed and corncob bedding was placed in the catch-tray below the metal grid floor. Footshock-exposed (FE) rats (n = 16 males, n = 12 females) received 15 footshocks (1.0 mA, pseudorandom ITI) over a 90 min session. Control rats (CON; n = 7 males, n = 4 females) were exposed to Context A for 90 min but received no footshocks. Context A fear memory was assessed 24-h later (day 2) during a 9 min re-exposure to Context A. The following day (day 3), rats were placed in Context B and a single footshock was delivered after 3 min. For Context B, a white curved wall and floor (beneath the grid) insert were used, along with three drops of lemon scent. Context B fear memory was assessed 24-h later (day 4) during a 9 min re-exposure to Context B.
Freezing was quantified using VideoFreeze software (Med Associates, Inc, Fairfax, VT). Percent freezing (%freezing) was calculated as:
Rats were classified as being a “Low Responder” (LR) or “High Responder” (HR) based on %freezing during the day 2 Context A fear memory test. Rats freezing below the within-sex mean were classified as LR and those freezing greater than the within-sex mean classified as HR. As a secondary measure, the number of fecal boli were counted at the end of each session.
[18F]FPEB PET
All rats underwent two scanning sessions with [18F]FPEB. The baseline took place 2 days prior to beginning of the fear learning paradigm. The second scan was acquired 24-h after the day 4 test of Context B fear memory (i.e., post-test). Both scans were performed as detailed in the SI and as described previously [29]. The primary PET outcome measure was non-displaceable binding potential (BPND) in four regions of interest (ROIs): amygdala (AMY), hippocampal formation (HIP, including dorsal and ventral aspects and subiculum), prefrontal cortex (PFC, including orbitofrontal and medial prefrontal regions), and striatum (STR). The percent change in regional mGluR5 availability (ΔBPND) was calculated as follows:
Label-free quantitative LC–MS/MS proteomics
Rats were euthanized immediately after the second PET scan, PFC tissue was isolated (Fig. S1) and stored at −80 °C until further processing. Details of the LC–MS/MS label-free protein identification and quantification pipeline can be found in the SI.
Statistical analyses
Freezing and fecal boli counts were assessed using 2 (treatment: CON, FE) or 3 (group: CON, LR, HR)-by-2 (sex: male, female) analysis of variance (ANOVA). Freezing across time (day 1) or pre vs. post-shock (day 3) was assessed by repeated measures ANOVA. A priori we determined to test for differences between males and females within the same level of the other fixed factor and used Bonferroni’s correction for post hoc comparisons. The primary PET outcome measure of BPND was analyzed using multivariate ANOVA (MANOVA) with ROI (PFC, AMY, HIP, STR) BPND at baseline as dependent measures. We additionally applied a generalized linear mixed-effects model with fixed factors of group (HR, LR) and sex (male, female); and within subject measures of ROI and time (baseline, post-test). All main effects were included in the model along with 2-way (group-by-time, sex-by-time, group-by-sex), 3-way (group-by-sex-by-time), and 4-way (group-by-sex-by-time-by-ROI) interactions. Following significant interaction effects, post hoc tests were performed using Bonferroni’s correction for multiple comparisons. Pearson’s correlations were used to assess relationships between ΔBPND and behaviors. All tests were two-tailed, with alpha at p < 0.05. Statistical analyses were performed in SPSS 28.0.0.0 (IBM Corp., Armonk, NY) and visualized in Prism 9.2 (GraphPad Software, LLC., San Diego, CA).
Independent sample t-test comparing CON vs. FE or LR vs. HR were used to determine differential protein expression with a threshold of p < 0.05 (unadjusted) and log2(fold-change) > ±1.5. Functional analyses of differentially expressed protein lists were performed using Ingenuity Pathway Analysis software (QIAGEN Redwood City, CA, 2021), Qlucore Omics Explorer v 3.7 (Qlucore AB, Lund, Sweden) was used for generating visualizations of the principal component analysis and hierarchical clustering heatmaps.
Results
Males display greater freezing during FE
FE resulted in a rapid induction of freezing in Context A on day 1 (Fig. S2), where we observed a significant main effect of treatment (p < 0.001), as well as a time-by-treatment interaction (p < 0.001). Furthermore, earlier in the session, FE males tended to freeze more than FE-females (time-by-treatment-by-sex: p = 0.035). Assessing average freezing for the total 90 minutes (Fig. 1C), we found male rats froze more than females (sex effect: p = 0.050). FE males displayed greater freezing than their FE-female counterparts (adj. p < 0.001), but no such difference was seen between CON males and CON females. Additionally, FE rats produced more fecal boli relative to CON rats (treatment effect: p < 0.001), with no significant sex effect (Fig. S3A).
On the day 2 test of Context A fear memory, FE rats demonstrated greater freezing than CON rats (treatment effect: p < 0.001) regardless of sex (Fig. 1D). There were no differences between CON males and females on day 2, however, FE males froze more than FE-females (adj. p = 0.012). Similar to day 1, FE rats produced more fecal boli on day 2 (treatment effect: p < 0.001), irrespective of sex (Fig. S3B).
FE augmented responding in context B without enhancing fear memory
No treatment or sex differences were seen in freezing behavior on day 3 in novel Context B during the 3 minutes prior to the footshock (Fig. S4A). However, for the 1 minute following the footshock (Fig. 1E), FE froze significantly more than CON (p = 0.012). The effect of sex (p = 0.165) and treatment-by-sex interaction (p = 0.196) were not significant but the difference between FE males and FE-females is notable (adj. p = 0.013). A repeated measures analysis of freezing (pre- vs. post- shock) indicated FE rats froze more post-shock relative to their own freezing pre-shock, whereas CON rats displayed no such shock-induced change in freezing (time-by-treatment: p = 0.009). Relative CON, FE rats produced a greater number of fecal boli during the total session (p = 0.018; Fig. S3C). The main effect of sex (p = 0.053) and treatment-by-sex interaction (p = 0.053) were approaching significance, with FE males producing a greater number of fecal boli than their female counterparts (adj. p < 0.001).
Despite the enhanced response to footshock in Context B on day 3, we saw no evidence of enhanced Context B fear memory observed in FE rats relative to CON as measured by day 4 freezing. This included no significant effects of treatment (p = 0.262), sex (p = 0.315), or treatment-by-sex interaction (p = 0.388) on freezing behavior (Fig. 1F). Similarly, main effects of treatment (p = 0.178), sex (p = 0.253), and their interaction (p = 0.253) were not significant for fecal boli (Fig. S3D).
Baseline mGluR5 availability is lower in females but not associated with behavioral response to FE
Given that FE rats did not display stress-enhanced fear learning, as illustrated by the lack of difference in the response on the day 4 test of Context B fear relative to CON, we decided to focus our subsequent analyses on factors that could be mediating differences in Context A fear memory as measured on day 2 (Fig. 1D). Leveraging the variability in Context A fear memory among FE rats, we performed a mean split within each sex (Fig. 2A) and classified rats that froze below the within-sex mean as “Low Responders” (LR), and those that froze above the mean as “High Responders” (HR) (Fig. 2B). This approach of using a behavioral metric (such as freezing in fear conditioning, immobility in the forced swim test, etc.) to identify low and high responding subjects has been used in prior research [30,31,32]. In such cases, it is not uncommon for only the animals in the top and bottom 25% to be included in the final analyses; or alternatively, splitting animals into tertiles, resulting in “high”, “moderate” and “low” responder groups. However, here we opted for a mean split in order to incorporate data from all animals in our analysis and ensure sufficient group sizes.
A Day 2 freezing (i.e., Context A fear memory) in male (open symbols, n = 16) and female (closed symbols, n = 12) FE rats. Dashed rectangles represent rats with freezing greater than the within-sex mean who were classified as the FE “High Responders” (HR). Rats who froze less than the mean were classified as “Low Responders” (LR). B Comparing freezing across the three groups (CON, LR, HR), there were main effects of group (F = 82.573; p < 0.001), sex (F = 13.817, p < 0.001), and a group-by-sex interaction (F = 4.825, p = 0.015). Individual values shown with bars representing the group mean (±SEM). Group-wise comparisons (Bonferroni’s p values) are displayed above the brackets for each comparison. Within-group male vs. female comparison: *p < 0.05; ***p = 0.001.
After confirming that there were no differences between males and females, groups, or across days in the injected mass, injected activity, or molar activity of [18F]FPEB injections on either PET day (Table S1), we performed a MANOVA to test for possible differences in baseline BPND that might be associated with future classification of rats a LR or HR. There was not a main effect of group, but there was a significant effect of sex such that females tended to exhibit lower baseline BPND relative to males across ROIs (−2.9%, p = 0.010), with females having lower BPND than males specifically within the AMY (−13.8%; p = 0.012).
Interactions between group and sex are associated with changes in regional BPND
To test whether relative baseline-to-post-test changes in regional BPND were related to group membership and/or sex, we applied a generalized linear mixed-effects model with fixed factors of group (LR, HR) and sex, and within subject measures of ROI and time (baseline, post-test) (Fig. 3A, Table S2). This analysis revealed significant main effects of ROI (p < 0.001) and sex, with females overall displaying lower BPND as compared with males (−5.9%, p = 0.018). There was additionally significant and time-by-group (p = 0.024). Post hoc analyses did not reveal significant differences after correcting for multiple comparisons, however, it is noteworthy that LR rats displayed decreased BPND post-test relative to their baseline (−2.1%, adj. p = 0.241), whereas BPND increased in HR rats (+3.0%, adj. p = 0.099). Additionally, a significant time-by-sex interaction (p = 0.008) indicated BPND increased in male rats post-test relative to baseline (+3.7%, adj. p = 0.022), whereas there was a non-significant decrease in females (−3.0%, adj. p = 0.122). The time-by-group-by-sex three-way interaction was not significant (p = 0.700), but the time-by-ROI-by-sex-by-group four-way interaction was significant (p = 0.045). Post hoc tests revealed, across ROIs, HR males displayed a significant increase in BPND post-test relative to baseline (+6.2%, adj. p = 0.008), whereas LR females had significantly lower BPND post-test as compared with baseline (−5.6%, adj. p = 0.049). None of the ROI-specific pairwise comparisons survived Bonferroni correction.
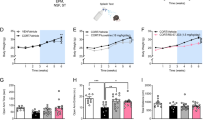
A Graphical results of the generalized linear mixed effect analysis (Table S2) of binding potential, non-displaceable (BPND), a measure of mGluR5 availability, at baseline vs. post-test. The average BPND of the four ROIs is graphed to highlight the significant time-by-group (p = 0.024) and time-by-sex (p = 0.008) interactions. Data are provided for n = 6–8 rats (per group, and sex at each timepoint) and displayed as mean (±SEM). Changes in prefrontal cortex (PFC) BPND post-test relative to baseline (ΔBPND) and freezing behavior in response to footshock exposure on B day 1 and C during the day 2 test of Context A fear memory in male (n = 14) and female (n = 10), RES (n = 12) and VUL (n = 12) rats. Correlation coefficient (r) and significance (p) values are provided. Graphs of individual ROI baseline-to-post-test changes (Fig. S5) and correlations (Figs. S6 and S7) can be found in the SI.
Using Pearson’s correlations, we examine if pre-to-post-test changes in mGluR5 availability (i.e., ΔBPND) were related to fear behaviors among FE rats. We detected significant positive correlations between total freezing during footshock exposure on day 1 and ΔBPND across all 4 ROIs (Figs. 3B, S6; r = 0.42–0.54) such that rats displaying the greatest freezing during day 1 footshock exposure also showed the greatest increases in mGluR5 availability post-test relative to baseline. Positive correlations were also observed between ΔBPND Context A fear memory, as measured by freezing on day 2 (Fig. S7), with this correlation being significant for the PFC (p = 0.0121; Fig. 3C). It is noteworthy that when correlations were conducted in males and females independently, we observed positive correlations of similar magnitude between and freezing during footshock exposure on day 1 (Fig. S6), and during the test of context A fear memory on day 2 (Fig. S7) in each sex, although the results did not achieve statistical significance. We found no significant relationships between ΔBPND and freezing before or after the single footshock in Context B on day 3, nor did we find significant correlations between regional ΔBPND and Context B fear memory as measured by freezing on day 4. Results of correlations between regional ΔBPND and fecal boli can be found in the SI (Fig. S8).
Analysis of PFC proteins reveals sex-dependent molecular responses to FE
Investigating differences between males and females, we found 41 differentially expressed proteins (log2 [Female/Male] with p < 0.05) in the PFC of CON rats (Fig. S9A). In comparison, we observed 379 differentially expressed proteins between FE male and female rats (Fig. S9B). Given the observed sex differences in behavior and PET analyses, we opted to examined treatment (CON vs. FE) and group (LR vs.HR) differences within each sex independently. The principal component analysis (PCA) of FE vs. CON female samples (Fig. S10A) results in the top three principal components (PC) explaining 61%, 23% and 6% of the variance in protein expression, respectively. We found 8 differentially expressed proteins (p < 0.05, FC > 1.5) between FE and CON females (Fig. S10B). The functional analysis of these 8 proteins returned no canonical pathways which overlapped by more than one molecule. The PCA for FE vs. CON male samples (Fig. 4A), resulted in the top 3 PCs explaining 56%, 10% and 9% of the variance, respectively. Thirty-seven proteins were found to be differentially expressed (Fig. 4B), with the majority upregulated in FE males relative to CON males, including mGluR5 (Grm5; p = 0.024, log2(FC) = +1.54). The functional analysis of these 37 proteins revealed top enriched pathways of Synaptic Long-Term Depression (−log(p) = 9.35) and Corticotropin-Releasing Hormone Signaling (−log(p) = 7.03). The top 10 enriched canonical pathways identified by IPA for the CON vs. FE male comparison can be found in Table 1A.
Protein expression in the prefrontal cortex (PFC) was assessed following the post-test FPEB scan. Principal component analysis (PCA) showing separation of A FE (n = 12) from CON (n = 3) males, and C LR (n = 6) from HR (n = 6) females. Axes are labeled with the %variance explained by the first three principal components (PCs). Hierarchical clustering heatmaps depict proteins that were differentially expressed (p < 0.05, log2(FC) > ±1.5) between B male FE and CON samples, and between D female LR and HR samples.
We then probed for differentially expressed proteins that discriminated LR from HR rats within each sex. The PCA for females (Fig. 4C) results in clustering and separation between of LR vs. HR samples, with a total of 85% of the variance being explained by the top 3 PCs. Seventeen proteins were found to be differentially expressed, 12 of which were downregulated in LR relative to HR females (Fig. 4D). This included downregulation of apolipoprotein A4 (Apoa4; p = 0.015, log2(FC) = −2.046), group-specific component, vitamin D binding protein (GC; p = 0.036, log2(FC) = −1.786), and hemopexin (Hpx; p = 0.025, FC = −1.563), which were the 3 proteins overlapping with the top two canonical pathways identified by IPA: LXR/RXR Activation and FXR/RXR Activation (both -log(p) = 2.8) (Table 1B). The analysis of LR vs. HR males identified just one differentially expressed PFC protein: Asparagine synthetase, glutamine dependent (Asns), which was downregulated in LR as compared with HR males (p = 0.032, FC = −1.535).
Discussion
Here we present the results of a study using the novel combination of a fear learning paradigm and in vivo PET imaging with the mGluR5-specific radiotracer, [18F]FPEB. This longitudinal study design provided a unique opportunity to investigate the possibility of mGluR5 as a biomarker of responsivity to footshock stress and the relationship between dynamic changes in mGluR5 availability and individual differences in behavioral responses.
We found that males displayed greater freezing behavior than females during FE (day 1), the test of FE context fear memory (day 2) and after the single footshock in Context B (day 3). Others have observed similar sex differences in freezing during Pavlovian fear conditioning paradigms, and it has been proposed that less freezing in female animals does not reflect reduced sensitivity or fear learning, per se, but rather a difference in fear expression or coping strategy selection between males and females [17, 33, 34]. Greater freezing and fecal boli produced after the delivery of a single footshock in the novel Context B on day 3 was also observed FE rats, especially HR, as compared with CON, whose freezing did not change pre-to-post footshock. These findings are similar to exaggerated responses to subsequent aversive stimuli observed in studies utilizing SEFL models of PTSD [35,36,37], or hyperalgesia induced by chronic or acute stress [38,39,40]. Unlike previous research using SEFL paradigms [28, 41, 42], we did not observe evidence of enhanced contextual fear memory (day 4 behavior) in FE rats overall. Nevertheless, we did observe a wide range of individual responses on days 1 and 2 such that we were able to identify LR and HR animals and examine associations between mGluR5 dynamics and behavioral responses to FE.
We observed no significant baseline differences in mGluR5 availability between LR and HR rats, suggesting that pre-existing mGluR5 availability cannot be used to predict which rats would emerge as LR vs. HR as measured by low or high Context A fear memory, respectively. We did, however, observe overall lower mGluR5 availability in female rats relative to males, particularly in the amygdala at baseline. Although there were no apparent group differences at baseline, using a mixed effect GLM analysis to examine baseline-to-post-test changes in mGluR5 availability again revealed a significant effect of sex, as well as significant time-by-group and time-by-sex interactions. The nature of these interactions indicated that LR females tended to display decreased mGluR5 availability post-test relative to baseline, whereas BPND tended to increase in HR males post-test. It is noteworthy that, while a 2015 evaluation of mGluR5 availability in a healthy human sample revealed no differences between men and women [43], a larger, more recent study by Smart and colleagues found mGluR5 availability to be significantly higher in men (+17% overall) as compared with women [44]. This later finding is in in agreement with our present findings, and it remains a possibility that lower mGluR5 availability contributed to lower freezing behavior in females. To our knowledge, there is no published evidence of sex-by-diagnosis interactions related to mGluR5 expression or its relationship with psychiatric symptoms, but it is certainly a possibility that warrants future examination.
Since relative changes in mGluR5 availability appeared to potentially be an important distinction between LR vs. HR rats, we decided to test for relationships between baseline-to-post-test changes in mGluR5 availability and behavioral measures. The significant positive correlations observed between ΔBPND and total freezing during footshock exposure in Context A, as well as PFC ΔBPND and freezing during the Context A contextual fear memory test, are consistent with clinical findings where higher mGluR5 availability was found to be associated with greater symptom severity among individuals with PTSD [22, 23]. The present findings are also congruent with the study by Yim et al. [45], who demonstrated substantial individual differences in hippocampal mGluR5 upregulation following acute restraint stress in male rats, and rats with the greatest upregulation of mGluR5 displayed significant changes in basal EEG theta spectral power, a form of hippocampal activity that plays a role in associative and affective [46, 47]. Because this study by Yim et al. [45] did not include any behavioral analyses, it is not known whether the rats with greater mGluR5 upregulation would have also displayed altered behavioral responses. However, other preclinical work has demonstrated that pharmacological blockade of mGluR5, specifically preceding the training phase of a Pavlovian conditioning paradigm, can attenuate fear learning [48]. Together with previous clinical evidence and our present findings, these data support the hypothesis that differences in mGluR5 expression or signaling following a traumatic event could be a signature of stress responsivity and potentially mediate the development of stress or trauma-related pathology. It should also be noted that mGluR5 activation facilitates fear extinction and extinction retention [49, 50], as well as enhancing performance in tests of hippocampal-dependent spatial learning [51]. Thus, there is a critical need to ensure agents intended to modulate mGluR5 expression or activity as a strategy for reducing risk or enhancing resilience following a potentially traumatic stress do not induce pathophysiological changes in desirable forms of mGluR5-mediated synaptic plasticity, learning and memory.
Analysis of post-FE PFC protein expression showed that molecular differences between male and female rats seem to be enhanced by footshock stress; that is to say, we observed 379 differences between FE males and females, but only 41 proteins were differentially expressed between CON males and females. Moreover, across sexes, there was little to no overlap between proteins and enriched pathways differentially expressed between groups. Among males, the greatest differences were between CON and FE rats, with FE in males being associated with upregulation of neuroplasticity pathways (e.g., Synaptic LTD and LTP), and mGluR5 itself, as well as neuroendocrine signaling (e.g., Corticotropin-Releasing Hormone Signaling). In contrast, the greatest differences in female rats were between the LR and HR groups, with LR in females being associated with downregulation of LXR/RXR and FXR/RXR nuclear receptor signaling pathways. LXR/RXR and FXR/RXR signaling have been implicated in neuroprotection and inflammation within the context of neurodegenerative disorders [52], as well as preclinical evidence suggesting these pathways may be activated in animal models of depressive and anxiety-like behavior [53]. It is also important to note that the CON vs. FE comparison highlights the exposure to footshock as the important group distinction in males, whereas the LR vs. HR comparison suggests the behavioral response to footshock as the defining group feature among females. This is even more intriguing given the overall lower freezing observed in females, again raising the question of the relevance of freezing as the “hallmark” behavioral response to stress and/or expression of fear for female rodents. Overall, these observations add to the mounting evidence that males and females respond differently to stress at the cellular, molecular, and physiological level [16, 54, 55] that may contribute to the differences at the behavioral level.
Limitations of the present study include that we were not powered to look at the potential influence of estrous cycle in female rats. There is mounting evidence implicating a modulatory role of sex hormone signaling in response to stress [56], PTSD and mood symptoms [57,58,59], pain processing [60], and interactions between estrogen receptor and mGluR5 signaling [61]. Therefore, investigating whether interactions between estrogen and mGluR5 could influence resilience and vulnerability is something to explore in the future. Second, given the relationship between mGluR5, nociception, chronic pain syndromes [62,63,64], and the painful nature of footshocks, it is possible that the observed relationship between increased mGluR5 availability and behavioral response to FE is mediated in part by enhanced pain sensitivity. This potential confound would fit in with clinical observations suggesting overlapping mechanisms mediating chronic pain syndromes and stress-related psychiatric disorders [65,66,67]. Third, freezing on days 1 and 3 are “contaminated” by the immediate locomotor response to the painful footshock(s), thus interpretation of freezing on these days must be done cautiously. Forth, we did not observe enhanced fear learning in FE rats; therefore, further work is required to determine the role of mGluR5 in the SEFL phenotype. Fifth, PFC samples for the proteomics analysis were collected after the post-test PET session while under isoflurane anesthesia, which has short and long-term effects on protein expression [68]. However, scan duration and total time of isoflurane exposure was equivalent for males and females and across groups. Finally, multiple hypotheses were tested using this relatively small dataset. We recognize that this type of multiple hypothesis testing is vulnerable to type 1 error (i.e., false positives). Furthermore, despite baseline-to-post-test changes in mGluR5 availability reported here being statistically significant and greater than the test-retest variability for [18F]FPEB [69], the magnitude of the present findings are still quite modest. Given these realities, replication studies in larger sample sizes will be necessary to validate our results.
In summary, observed associations between greater post-test increases in mGluR5 availability and acute behavioral responses to footshock stress, specifically freezing during the footshcok exposure and the test of contextual fear memory, are consistent with clinical findings implicating mGluR5 in the pathophysiology of psychiatric symptoms [22,23,24]. Our results suggest that increased mGluR5 availability and activity could be related to individual differences in acute stress responsivity and act as a mediator of post-trauma phenotypes. These data additionally highlight potentially important sex differences in responses to acute or traumatic stress, including differential involvement of mGluR5 and related molecular networks. Research aimed towards understanding the contribution of mGluR5 to sex differences in how males and females respond to stress, and differences in factors mediating post-traumatic phenotypes could ultimately translate into the development of preventative strategies or therapeutic targets that leverage individual differences in pathophysiology.
References
Kessler RC, Aguilar-Gaxiola S, Alonso J, Benjet C, Bromet EJ, Cardoso G, et al. Trauma and PTSD in the WHO World Mental Health Surveys. Eur J Psychotraumatol. 2017;8 sup5:1353383.
Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61:4–14.
Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM‐IV and DSM‐5 criteria. J Trauma Stress. 2013;26:537–47.
Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry 1998;55:626–32.
Wamser-Nanney R, Howell KH, Schwartz LE, Hasselle AJ. The moderating role of trauma type on the relationship between event centrality of the traumatic experience and mental health outcomes. Psychol Trauma. 2018;10:499.
Benjet C, Bromet E, Karam E, Kessler R, McLaughlin K, Ruscio A, et al. The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychological Med. 2016;46:327–43.
Galatzer-Levy IR, Bryant RA. 636,120 ways to have posttraumatic stress disorder. Perspect Psychol Sci. 2013;8:651–62.
Garfin DR, Thompson RR, Holman EA. Acute stress and subsequent health outcomes: a systematic review. J Psychosom Res. 2018;112:107–13.
Holbrook TL, Hoyt DB, Stein MB, Sieber WJ. Perceived threat to life predicts posttraumatic stress disorder after major trauma: risk factors and functional outcome. J Trauma Acute Care Surg. 2001;51:287–93.
Hermans E, Challiss RJ. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochemical J. 2001;359:465–84.
Bellone C, Luescher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci. 2008;65:2913–23.
Simonyi A, Schachtman TR, Christoffersen GR. The role of metabotropic glutamate receptor 5 in learning and memory processes. Drug News Perspect. 2005;18:353–61.
Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29:3676–84.
Shin S, Kwon O, Kang JI, Kwon S, Oh S, Choi J, et al. mGluR5 in the nucleus accumbens is critical for promoting resilience to chronic stress. Nat Neurosci. 2015;18:1017–24.
Sun H, Su R, Zhang X, Wen J, Yao D, Gao X, et al. Hippocampal GR-and CB1-mediated mGluR5 differentially produces susceptibility and resilience to acute and chronic mild stress in rats. Neuroscience. 2017;357:295–302.
Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32:709–23.
Jones CE, Monfils MH. Fight, flight, or freeze? The answer may depend on your sex. Trends Neurosci. 2016;39:51–53.
Merz CJ, Wolf OT. Sex differences in stress effects on emotional learning. J Neurosci Res. 2017;95:93–105.
Esterlis I, Holmes SE, Sharma P, Krystal JH, DeLorenzo C. Metabotropic glutamatergic receptor 5 and stress disorders: Knowledge gained from receptor imaging studies. Biol Psychiatry. 2018;84:95–105.
Ferraguti F. Metabotropic glutamate receptors as targets for novel anxiolytics. Curr Opin Pharmacol. 2018;38:37–42.
Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13:22–37.
Holmes SE, Girgenti MJ, Davis MT, Pietrzak RH, DellaGioia N, Nabulsi N, et al. Altered metabotropic glutamate receptor 5 markers in PTSD: In vivo and postmortem evidence. Proc Natl Acad Sci USA. 2017;114:8390–95.
Davis MT, Hillmer A, Holmes SE, Pietrzak RH, DellaGioia N, Nabulsi N, et al. In vivo evidence for dysregulation of mGluR5 as a biomarker of suicidal ideation. Proc Natl Acad Sci USA. 2019;116:11490–95.
Esterlis I, DellaGioia N, Pietrzak RH, Matuskey D, Nabulsi N, Abdallah CG, et al. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [11C] ABP688 and PET imaging study in depression. Mol Psychiatry. 2018;23:824–32.
Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J Neurosci. 2014;34:8462.
Romano C, Sesma MA, McDonald CT, O’malley K, van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–69.
Daggett L, Sacaan A, Akong M, Rao S, Hess S, Liaw C, et al. Molecular and functional characterization of recombinant human metabotropic glutamate receptor subtype 5. Neuropharmacology. 1995;34:871–86.
Rajbhandari AK, Gonzalez ST, Fanselow MS. Stress-enhanced fear learning, a robust rodent model of post-traumatic stress disorder. J Vis Exp: JoVE 2018:58306. https://doi.org/10.3791/58306
Groman SM, Hillmer AT, Liu H, Fowles K, Holden D, Morris ED, et al. Dysregulation of decision making related to metabotropic glutamate 5, but not midbrain D(3), receptor availability following cocaine self-administration in rats. Biol Psychiatry. 2020;88:777–87.
Valencia S, Gonzales EL, Adil KJ, Jeon SJ, Kwon KJ, Cho KS, et al. Comparative behavioral correlation of high and low-performing mice in the forced swim test. Biomol Ther 2019;27:349–56.
Schenberg EE, Ferreira TL, Figueredo LZP, Hipólide DC, Nobrega JN, Oliveira MGM. Fear conditioning performance and NMDA receptor subtypes: NR2A differential expression in the striatum. Brain Res Bull. 2006;69:440–46.
Lehner M, Taracha E, Skórzewska A, Turzyńska D, Sobolewska A, Maciejak P, et al. Expression of c-Fos and CRF in the brains of rats differing in the strength of a fear response. Behav Brain Res. 2008;188:154–67.
Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015;4:e11352.
Russo AS, Parsons RG. Behavioral expression of contextual fear in male and female rats. Front Behav Neurosci. 2021;15671017. https://doi.org/10.3389/fnbeh.2021.671017
Perusini JN, Meyer EM, Long VA, Rau V, Nocera N, Avershal J, et al. Induction and expression of fear sensitization caused by acute traumatic stress. Neuropsychopharmacology. 2016;41:45–57.
Sillivan SE, Joseph NF, Jamieson S, King ML, Chévere-Torres I, Fuentes I, et al. Susceptibility and resilience to posttraumatic stress disorder–like behaviors in inbred mice. Biol Psychiatry. 2017;82:924–33.
Gonzalez, ST. Mechanisms and heterogeneity of stress-enhanced fear learning. Los Angeles: University of California, (2021).
Jennings EM, Okine BN, Roche M, Finn DP. Stress-induced hyperalgesia. Prog Neurobiol. 2014;121:1–18.
Piardi L, Pagliusi M, Bonet I, Brandão AF, Magalhães SF, Zanelatto FB, et al. Social stress as a trigger for depressive-like behavior and persistent hyperalgesia in mice: study of the comorbidity between depression and chronic pain. J Affect Disord. 2020;274:759–67.
Itoga CA, Roltsch Hellard EA, Whitaker AM, Lu Y-L, Schreiber AL, Baynes BB, et al. Traumatic stress promotes hyperalgesia via corticotropin-releasing factor-1 receptor (CRFR1) signaling in central amygdala. Neuropsychopharmacology. 2016;41:2463–72.
Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–23.
Rau V, Fanselow MS. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2009;12:125–33.
DuBois J, Rousset O, Rowley J, Porras-Betancourt M, Reader AJ, Labbe A., et al. Characterization of age/sex and the regional distribution of mGluR5 availability in the healthy human brain measured by high-resolution [(11)C]ABP688 PET. Eur J Nucl Med Mol Imaging.2016;43:152–62.
Smart K, Cox SML, Scala SG, Tippler M, Jaworska N, Boivin M, et al. Sex differences in [(11)C]ABP688 binding: a positron emission tomography study of mGlu5 receptors. Eur J Nucl Med Mol Imaging. 2019;46:1179–83.
Yim YS, Han W, Seo J, Kim CH, Kim DG. Differential mGluR5 expression in response to the same stress causes individually adapted hippocampal network activity. Biochem Biophys Res Commun. 2018;495:1305–11.
Seidenbecher T, Laxmi TR, Stork O, Pape H-C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–50.
Karakaş S. A review of theta oscillation and its functional correlates. Int J Psychophysiol. 2020;157:82–99.
Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci. 2002;22:5219–29.
Sethna F, Wang H. Pharmacological enhancement of mGluR5 facilitates contextual fear memory extinction. Learn Mem. 2014;21:647–50.
Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex. 2011;21:727–35.
Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, et al. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–71.
Willems S, Zaienne D, Merk D. Targeting nuclear receptors in neurodegeneration and neuroinflammation. J Medicinal Chem. 2021;64:9592–638.
Yang C, Zhou C, Li J, Chen Z, Shi H, Yang W, et al. Quantitative proteomic study of the plasma reveals acute phase response and LXR/RXR and FXR/RXR activation in the chronic unpredictable mild stress mouse model of depression. Mol Med Rep. 2018;17:93–102.
Forger NG, Strahan JA, Castillo-Ruiz A. Cellular and molecular mechanisms of sexual differentiation in the mammalian nervous system. Front Neuroendocrinol. 2016;40:67–86.
Wellman CL, Bangasser DA, Bollinger JL, Coutellier L, Logrip ML, Moench KM, et al. Sex differences in risk and resilience: stress effects on the neural substrates of emotion and motivation. J Neurosci. 2018;38:9423–32.
Ney LJ, Gogos A, Hsu C-MK, Felmingham KL. An alternative theory for hormone effects on sex differences in PTSD: The role of heightened sex hormones during trauma. Psychoneuroendocrinology. 2019;109:104416.
Nillni YI, Pineles SL, Patton SC, Rouse MH, Sawyer AT, Rasmusson AM. Menstrual cycle effects on psychological symptoms in women with PTSD. J Trauma Stress. 2015;28:1–7.
Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in womenin transition to menopause. Arch Gen Psychiatry. 2004;61:62–70.
Grigoriadis S, Kennedy SH. Role of estrogen in the treatment of depression. Am J Ther 2002;9:503–09.
Chen Q, Zhang W, Sadana N, Chen X. Estrogen receptors in pain modulation: cellular signaling. Biol Sex Differences. 2021;12:1–10.
Gross KS, Mermelstein PG. Chapter 9 - Estrogen receptor signaling through metabotropic glutamate receptors. In: Litwack G, editor. Vitamins and hormones. Academic Press; 2020;114, p. 211–32. https://doi.org/10.1016/bs.vh.2020.06.003
Niu Y, Zeng X, Zhao L, Zhou Y, Qin G, Zhang D, et al. Metabotropic glutamate receptor 5 regulates synaptic plasticity in a chronic migraine rat model through the PKC/NR2B signal. J Headache Pain. 2020;21:139.
Kolber BJ, Montana MC, Carrasquillo Y, Xu J, Heinemann SF, Muglia LJ, et al. Activation of metabotropic glutamate receptor 5 in the amygdala modulates pain-like behavior. J Neurosci. 2010;30:8203.
Chung G, Kim CY, Yun Y-C, Yoon SH, Kim M-H, Kim YK, et al. Publisher Correction: Upregulation of prefrontal metabotropic glutamate receptor 5 mediates neuropathic pain and negative mood symptoms after spinal nerve injury in rats. Sci Rep. 2018;8:8936.
McKernan LC, Johnson BN, Crofford LJ, Lumley MA, Bruehl S, Cheavens JS. Posttraumatic stress symptoms mediate the effects of trauma exposure on clinical indicators of central sensitization in patients with chronic pain. Clin J Pain. 2019;35:385–93.
Fishbain DA, Pulikal A, Lewis JE, Gao J. Chronic pain types differ in their reported prevalence of Post -Traumatic Stress Disorder (PTSD) and there is consistent evidence that chronic pain is associated with PTSD: An Evidence-Based Structured Systematic Review. Pain Med. 2017;18:711–35.
Humo M, Lu H, Yalcin I. The molecular neurobiology of chronic pain–induced depression. Cell Tissue Res. 2019;377:21–43.
Kalenka A, Gross B, Maurer MH, Thierse H-J, Feldmann RE Jr. Isoflurane anesthesia elicits protein pattern changes in rat hippocampus. J Neurosurg Anesthesiol. 2010;22:144–54.
de Laat B, Leurquin-Sterk G, Celen S, Bormans G, Koole M, Van Laere K, et al. Preclinical evaluation and quantification of 18F-FPEB as a radioligand for PET imaging of the metabotropic glutamate receptor 5. J Nucl Med. 2015;56:1954–59.
Acknowledgements
The authors would like to thank TuKiet Lam, Weiwei Wang, Florine Collin and the Yale Keck Biotechnology Resourse Loboratory for their assistance in the preparation and processing of samples for LC–MS/MS proteiomics.
Funding
This work was funded in part by the United States Department of Veterans Affairs National Center for PTSD. Additional support was provided by the Biological Sciences Training Program (BSTP) in Psychiatry (T32 MH014276) and the State of Connecticut, Department of Mental Health and Addiction Services. This publication does not express the views of the United States Department of Veterans Affairs, Department of Mental Health and Addiction Services, or the State of Connecticut. The views and opinions expressed are those of the authors.
Author information
Authors and Affiliations
Contributions
Experiments were designed by RHA, SMG, and IE. Data collection was performed by RHA, SP, and KF and analyzed by RHA, TT, and RG-M. Additional research support and recourses were provided by RJD. This manuscript was drafted by RHA, with the oversight and mentorship of SMG, RJD, JRT and IE. IE agreed to be held accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asch, R.H., Pothula, S., Toyonaga, T. et al. Examining sex differences in responses to footshock stress and the role of the metabotropic glutamate receptor 5: an [18F]FPEB and positron emission tomography study in rats. Neuropsychopharmacol. 48, 489–497 (2023). https://doi.org/10.1038/s41386-022-01441-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01441-y