Abstract
Brain accumulation rate and magnitude are critical for the acute reinforcing effects of nicotine. Despite electronic cigarettes’ (E-cigs) appeal as substitutes for traditional combustible cigarettes (C-cigs), brain nicotine accumulation (BNA) from E-cigs has not been compared with that from C-cigs using a within-subjects design. BNA was directly assessed with 16 adult dual users (10 females) of E-cigs (e-liquid pH 9.4) and C-cigs, using 11C-nicotine and positron emission tomography (PET). Participants went through two 15-min head scanning sessions during which they inhaled a single puff of E-cig vapor or C-cig smoke containing 11C-nicotine in a randomized order. A full-body scan was also conducted at each session to measure total absorbed dose of 11C-nicotine. Mean maximum concentration (Cmax) and area under curve of BNA were 22.1% and 22.7% lower, respectively, following E-cig compared with C-cig inhalation. Meanwhile, T1/2 was 2.7 times longer following inhalation of E-cig vapor relative to C-cig smoke (all ps < 0.005). Whole-body imaging indicated greater nicotine retention in the respiratory tract from vapor versus smoke inhalation (p < 0.0001). Following vapor inhalation, nicotine retention in the respiratory tract was correlated with Cmax values of BNA (rs = −0.59, p < 0.02). Our results confirm that E-cigs with alkaline pH e-liquid can deliver nicotine rapidly to the brain, albeit less efficiently than C-cigs partly due to greater airway retention of nicotine. Since brain nicotine uptake mediates reinforcement, these results help elucidate actions of E-cigs in terms of abuse liability and effectiveness in substituting for combustible cigarettes.
Similar content being viewed by others
Introduction
In the past decade, there has been rapid growth around the world in the popularity of electronic cigarettes (E-cigs) as safer alternatives or substitutes for highly hazardous combustible cigarettes [1]. While E-cigs are likely less harmful than traditional combustible cigarettes (C-cigs), a major concern is that long-term use of these products can lead to the development and maintenance of nicotine dependence, which may eventually lead to tobacco smoking [2, 3]. It is also recognized that for E-cigs to have the potential to successfully compete with and replace smoking at a population level, it is necessary that these products be capable of delivering nicotine with a similar profile to cigarettes insofar as they can be accepted and adopted for sustained use among smokers by providing adequate satisfaction and behavioral reinforcement [2]. Therefore, E-cig use may necessarily maintain some degree of abuse or dependence liability. Similar to the action of other drugs of abuse, nicotine’s brain accumulation rate and magnitude are key determinants of its acute reinforcing effects and dependence liability [4,5,6]. Several studies comparing venous blood nicotine kinetics following E-cig and C-cig use have reported mixed results with the former resulting in slower [7, 8] or comparable [9,10,11] rises of nicotine concentration in systemic circulation. Unfortunately, after the inhalational route of nicotine administration, venous blood nicotine kinetics do not precisely reflect arterial blood nicotine concentration [12] and therefore the dynamics of rapid brain nicotine accumulation (BNA). Published data directly assessing brain nicotine uptake from E-cigs relative to C-cigs are scarce.
In our preliminary study [12], parameters of BNA following inhalation of a single puff of E-cig vapor were assessed using 11C-nicotine and PET in E-cig users and compared with those from smoking of C-cigs in a group comprised of exclusively C-cig smokers. Mean maximum concentration (Cmax) values, which were normalized to the total absorbed dose (TAD) of radioactivity, were found to be lower following E-cig use compared to C-cig use in both males and females (24% and 32%, respectively). Brain nicotine concentration rose quickly following vapor inhalation with a mean T1/2 (i.e., time to reach 50% of Cmax) of 27 s, comparable to that following smoking (23 s). These results suggest that E-cigs can deliver nicotine rapidly, but less efficiently than C-cigs, to the brain. The study also reported some preliminary evidence that nicotine retention in the upper respiratory tract (RT) may be greater following E-cig relative to C-cig use which could explain the lower BNA from E-cigs given that less nicotine would reach the alveoli where rapid absorption occurs. It is noteworthy that the Cmax values after E-cig use found in this study (3.2% and 4.3% TAD/kg tissue for men and women, respectively) are close to those reported in Wall et al. [13]. In both studies, considerable nicotine deposition in the upper RT was also observed, especially with e-liquid containing freebase nicotine. However, the strength of association between airway nicotine retention and BNA after E-cig use was not evaluated in these two studies.
A major limitation of these two comparative studies is that, even with the efforts to make the samples match closely at a group level in some attributes (e.g., age and years of smoking), they cannot rule out possible confounding factors associated with subject differences between the two cohorts. For instance, sex has been found to be a source of individual variations in BNA after inhalation from E-cigs [12] or C-cigs [14]. Smoking dependence is also shown to be related to kinetics of BNA such that dependent smokers have slower rates of BNA than non-dependent smokers because the former have slower nicotine washout from the lungs, presumably due to heavy smoke-exposure-induced alterations in lung function [15]. To the extent that switching to E-cig use may lead to improvement in lung function among smokers [16], history of E-cig consumption is also likely to be a factor that contributes to individual differences in BNA after inhalation from E-cigs or C-cigs. To control for the confounding effects of these and other unknown subject factors, a rigorous comparison of the kinetics of BNA between the use of E-cigs and C-cigs needs to be conducted with the same group of subjects representing individuals with diverse demographic backgrounds and histories of smoking and E-cig use.
This study was aimed to directly determine the rates of BNA from E-cig use and to compare them with those from smoking regular cigarettes using a within-subjects design. It was hypothesized that BNA is smaller and possibly slower after a single puff inhalation of E-cig vapor relative to cigarette smoke. In addition, we sought to test a prediction that compared with smoking, E-cig use leads to greater nicotine retention in the airways.
Participants and methods
Participants
Participants were recruited through IRB-approved social media advertisements and flyers. Inclusion criteria for eligible participants were being 18–65 years of age, generally healthy, and smoking at least 10 cigarettes per month for the past year and using E-cigs at least 4 times per month for the past 3 months. Exclusion criteria included cardiac or respiratory disorders, brain abnormality or other neurological disorders, psychiatric illness/social situations that would limit compliance with study requirements, self-reported current substance use disorder other than nicotine, and presence of contraindications for PET/CT scan (e.g., pregnancy, lactation, or claustrophobia). The Institutional Review Boards of the Duke University Health System and the Wake Forest University Health Sciences approved this study, and all subjects provided written informed consent and received monetary compensation for their participation. Sixteen subjects completed the study protocol and were included for the current analysis.
Procedure
Cigarette smoking and E-cig use history, including years and frequencies of use of each type of products, were collected using a general questionnaire at the screening visit.
At a lab visit prior to a PET scan visit, participants were instructed to vape from a V2 E-cig with V2 Red e-liquid (nicotine concentration 12 mg/mL, the same as was used in the PET scan sessions; V2 E-cig products currently available at migvapor.com) for up to 7.5 min (15 puffs). Puff parameters (volume: 60 mL; puff duration: 4 s; interpuff interval 30 s; typical for E-cigs use [17]) were controlled with a vapor delivery device identical to that to be used during the PET sessions [12]. Participants could choose to stop vaping at any time when they felt satiated or discomfort. Participants also practiced taking and inhaling 3 to 5 puffs of smoke from a Capri Magenta cigarette (R.J. Reynolds, USA) outdoors to see if they were comfortable with this type of cigarette to be used as research cigarettes in the subsequent PET scan sessions. If they experienced discomfort (e.g., excessive coughing) from vaping from the research E-cig or smoking the cigarette, they could opt to discontinue participation in the study. During this visit, participants also completed the Fagerström Test of Nicotine Dependence (FTND) [18] and the Penn State Electronic Cigarette Dependence Index (PSECDI) [19], which assessed their dependence on traditional tobacco cigarettes and E-cigs.
PET scanning procedure
The PET scans were conducted using a GE Discovery MI DRPET/CT scanner (GE Healthcare, Waukesha, WI, USA). Each participant proceeded in a randomized order through two PET scanning sessions on the same day during which the head was scanned after he/she inhaled a single puff of vapor (55 mL over 4 s) or smoke (35 mL over 2 s) containing 11C-nicotine. The onset of each of the sessions was separated by 2 h to allow for near complete decay of the radioactivity from the preceding session (residual radioactivity 1.7%). Participants were asked to abstain from smoking and vaping for 2 h before the first PET/CT scan session of the day and not to smoke their own cigarettes or vape from their own E-cigs before the last scan session was complete. Due to technical issues, two participants received the second PET scan on a different day. Each standardized puff of vapor was produced from 15 µL V2 Red e-liquid (1.2% nicotine, 20/80 VG/PG) mixed with 11C-nicotine via a V2 EX Blanks refillable cartomizer, coupled with a programmable air syringe pump [12]. The smoke was generated from a shortened Capri Magenta cigarette through a customized smoke delivery device [20] after 11C-nicotine was applied. After a participant was placed in the scanner and shortly before the scanning, each participant took 3 puffs (30 s interval) of vapor or smoke from a non-radioactive product of the same type to ensure she or he was well prepared for inhalation of the crucial puff containing 11C-nicotine. The subject’s head was then scanned over 15 min in a sequence of 249 frames of 1–10 s each (voxel size, mm: 2.73 × 2.73 × 3.27, matrix size: 128 × 128 × 47). The PET scanning was initiated immediately prior to the onset of puffing of vapor or smoke containing 11C-nicotine followed by inhalation of air of the participant’s usual volume. Subsequently, a whole-body scan was performed to measure total absorbed dose of 11C-nicotine (TAD), which was used to normalize the 11C-nicotine uptake values between subjects and between conditions. The whole-body image also allowed assessment of nicotine retention in the RT following inhalation of vapor or smoke during each session.
11C-nicotine was synthesized following an established protocol [12, 14, 15, 21]. Approximately 740 MBq 11C-nicotine, dissolved in 10 μL ethanol, was applied to the tip of the tobacco rod of the study cigarette. Both the tobacco rod and filter were shortened (to 10 mm and 5 mm, respectively) to ensure efficient 11C-nicotine delivery. After evaporation of the ethanol, the cigarette was placed in the combustion chamber of the smoke delivery device and ready for use (for more details, see [20]). For producing radioactively-labeled E-cig vapor, approximately 555 MBq (at time of inhalation) of 11C-nicotine in 15 μL e-liquid was applied to the surface of a shortened wick of a refillable V2 E-cig cartridge.
PET image processing
PET image processing was conducted using PMOD (Version 3.17, PMOD Technologies Ltd., Adliswil, Switzerland). During the head scan, the field of view covered roughly the inferior half of the brain and the oral cavity. Therefore, to obtain measurements of BNA, only the inferior part of the brain in lieu of the whole brain was analyzed. To validate this approach (scanning only the inferior part of the brain instead of the entire brain), we previously analyzed the dynamic PET data of the entire brain scans obtained from a separate study performed with 31 participants who inhaled cigarette smoke containing 11C-nicotine. For each participant, two volumes of interest (VOIs) were placed: one for the entire brain and a second for the inferior part of the brain (48 ± 6% of the entire brain volume). The time activity curves (TACs) and parameters of the brain 11C-nicotine accumulation for both VOIs for each subject were nearly identical. There were strong correlations between the parameters of BNA obtained from the inferior part of the brain and the entire brain with r2 equal to 0.999, 0.999, 0.987, and 0.999 for maximum concentration (Cmax), area under the curve (AUC), time to reach Cmax (Tmax), and time to reach 50% of Cmax values (T1/2), respectively. The respective average percent differences [(Valueinferior brain − Valuewhole brain)/Valuewhole brain, Mean ± SD] were 1.7 ± 1.5%, 1.9 ± 1.4%, −0.4 ± 3.3%, and −1.9 ± 3.5%. The head CT image from the second scan session was co-registered to that from the first session and then these transformation parameters were used for co-registration of the brain dynamic PET images. Individual brain VOI was drawn on the average of time-averaged images from the two sessions and then applied to dynamic images. A cylinder-shaped VOI was generated to cover the entire body image of each subject. After decay correction to the brain scan start time, the radioactivity within the VOI was taken as the TAD/kg tissue. Brain 11C-nicotine radioactivity over time was calculated as a percentage of the TAD/kg tissue. The resulting individual TACs were subject to three-exponential curve fitting as described previously [14]. Values of Cmax, AUC (over 15 min starting from the time of inhalation), Tmax, and T1/2, were also extracted from the fitted TACs. To assess nicotine retention in the RT, a VOI was drawn on the two coregistered whole-body images from each of the two sessions for each participant to include the mouth, pharynx, larynx, trachea, and first secondary and tertiary bronchi. For this purpose, two iso-contour VOIs were applied for the contouring of RT at both sessions in such a way that the volume of each VOI was about 300 mL. The two VOIs were then combined to form a new VOI which was applied to extract the fractional amount of radioactivity as nicotine retention in the RT, expressed as a percentage of the TAD, for each session.
Statistical analysis
Paired two-tailed t-tests were conducted to compare group means of each of the four kinetics parameters of BNA between E-cigs and C-cigs, with Holm–Bonferroni correction applied to control for Type I error with multiple comparisons. A paired t-test was also performed to evaluate the mean difference in nicotine retention in the RT after inhalation between these two products. Spearman rank correlations were calculated to assess the associations of the airway nicotine retention with kinetics parameters of BNA for each product type. The threshold for statistical significance was set at p < 0.05. Group mean values (±SEM) are reported unless otherwise specified.
Results
Sample characteristics
The subjects (n = 16) consisted of adult dual users of both sexes (mean age = 35.8 (SD = 11.0); 62.5% women). Besides whites (56.3%), the sample also included participants of other races (3 American Indians or Alaska Natives, 1 Black, 1 Asian, and 2 of more than one race). They smoked a mean of 15.8 cigarettes (SD = 3.3) per day (CPD) with a mean of 20.1 years (SD = 10.7) of smoking history and a mean score of 5.8 (SD = 3.1) on the FTND, indicating moderate dependence. With a mean of 2.6 years (SD = 1.5) of E-cig use history, they on average vaped 9.0 episodes (SD = 10.6) per day and scored 10.1 (SD = 5.4) on the PSECDI.
PET results
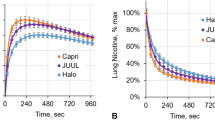
The average brain nicotine accumulation curves (±SE) after inhalation of a single puff of vapor from an E-cig and single puff of smoke from a C-cig are shown in Fig. 1. Results of separate paired t-tests showed significant differences in Cmax, AUC, Tmax, and T1/2 of BNA between inhalation from E-cigs and C-cigs (all ps < 0.005 with Holm–Bonferroni correction). Mean Cmax values, normalized to the total absorbed 11C-nicotine dose (TAD), were 22.1% lower following inhalation of E-cig vapor relative to C-cig smoke (3.7 ± 0.2% TAD/kg tissue vs. 4.7 ± 0.3% TAD/kg tissue; Fig. 2A). The average values of AUC from zero to 15 min were 22.7% lower from E-cigs as compared with C-cigs (48.0 ± 2.7% vs. 62.1 ± 3.1% TAD × min; Fig. 2B). Mean Tmax for E-cig use was approximately twice as long as that for C-cig smoking (9.02 ± 0.77 vs. 4.38 ± 0.46 min; Fig. 2C). The average T1/2 was 2.7 times longer following inhalation of E-cig vapor relative to C-cig smoke (1.03 ± 0.13 vs. 0.38 ± 0.06 min; Fig. 2D).
A The mean maximal nicotine concentration (Cmax); B The mean area under the time activity curve (AUC) from 0 to 15 min; C The mean time to reach the maximal nicotine concentration (Tmax); and D The mean time to reach one-half of the maximal nicotine concentration (T1/2). Brain nicotine accumulation per kg of tissue mass was expressed as a percentage of the total absorbed dose of 11C-nicotine (TAD). **p < 0.005 and ***p < 0.001 after correction for multiple comparisons.
The representative images of the oropharyngeal and tracheobronchial deposition of nicotine after use of C-cig and E-cig are shown in Fig. 3. A paired t-test indicated greater nicotine retention in the RT from E-cigs versus C-cigs as measured at 16–24 min following puff inhalation (10.9 ± 1.3% vs. 4.6 ± 0.8% TAD, p < 0.0001; Fig. 4A). The amounts of nicotine retention in the airways following vapor inhalation were negatively correlated with Cmax (rs = −0.59; Fig. 4B, Table 1) and AUC values (rs = −0.65; Table 1) whereas they were positively correlated with Tmax and T1/2 of BNA (rs = 0.56, rs = 0.61, respectively; Table 1). All p values were < 0.05 after Holm–Bonferroni correction for multiple testing. With inhalation from C-cigs, however, there were no significant correlations between airway nicotine retention and the parameters of BNA (Table 1).
Images from a representative participant show the sum of coronal slices of 3-dimensional radioactivity distribution assessed at 25 min after inhalation of a single puff from the respective 11C-nicotine–containing product and expressed as percentage of total absorbed dose. Maximum value of pseudo color scale is 0.25% total absorbed dose/cm2. Right panel shows combined respiratory tract volumes of interest (RT VOI) obtained from images acquired in E-cig and C-cig scan session. 1 = mouth cavity; 2 = larynx; 3 = trachea; 4 = esophagus; 5 = bronchi.
RT retention of nicotine after use of C-cig and E-cig (A) was assessed at 25 min after inhalation of a single puff from respective 11C-nicotine–containing product and expressed as percentage of total absorbed dose (TAD). Right panel (B) shows the association of brain nicotine accumulation (Cmax) with RT retention after using E-cig (rs = −0.59, p < 0.05 after correction for multiple testing). For other correlations, see Table 1.
Discussion
Using a within-subject design and E-cigs with alkaline pH e-liquid, this study has revealed four important findings: (i) E-cigs can deliver nicotine rapidly to the brain; (ii) the rate and magnitude of BNA from E-cigs are smaller than those from C-cigs; (iii) respiratory tract nicotine retention from E-cigs is higher than that from C-cigs; and (iv) following E-cig vapor inhalation, BNA parameters are significantly correlated with nicotine retention in the respiratory tract.
The present study, controlling for individual user differences (i.e., demographic variables, history of E-cig use and smoking) by using the within-subject design, has contributed new evidence that E-cigs are capable, albeit with less efficiency than traditional combustible cigarettes, of fast brain nicotine delivery. These results are consistent with our previous findings from a comparison of BNA between participants in separate E-cig and C-cig user groups [12]. The rapid BNA from E-cigs we have observed are also in agreement with a recent report from another team [13].
It should be noted that, in the present study, we observed a much bigger mean difference in T1/2 of BNA after inhalation from E-cig relative to C-cig than in our previous study [12] (2.7 vs. 1.2 times). One of the possibilities for such difference is that as compared with the between-subjects design used in the previous study, the present within-subjects design has a greater sensitivity to detect such differences. Indeed, all participants in the current study were dual users while only 8 of 17 participants in the E-cig user group in the previous study were dual users [12].
While BNA is less efficient following E-cig vapor versus C-cig smoke inhalation, it is noteworthy that BNA after E-cig use is most likely faster than that from most nicotine/tobacco products other than cigarettes. Although direct assessment of BNA has not been assessed from use of many of these other non-cigarette nicotine/tobacco products, profiles of venous blood concentrations following use of these products [22] suggest that E-cigs are capable of faster brain nicotine delivery than most of them.
Nicotine retention in the RT was shown to be significantly greater (ca. 2.5 times, Fig. 4A) following E-cig vapor inhalation relative to C-cig smoke inhalation. Wall et al. also recently reported substantial retention of nicotine from E-cigs with e-liquid of pH = 9.98 [13]. In our study, the RT retention of nicotine assessed at 16–24 min after inhalation was ca. 11% of TAD. Assuming that the T1/2 of the nicotine washout from the RT is ca. 20 min [13, 23], the initial deposition of nicotine in RT from E-cigs can be calculated to be as much as 22%. Since our RT VOIs cover only major branches of the tracheobronchial tree (up to tertiary bronchi), the initial deposition of nicotine in the entire RT from E-cigs may be even higher. Such higher deposition of nicotine in RT can diminish nicotine delivery to the alveoli, where rapid nicotine absorption occurs, thereby reducing arterial blood nicotine concentration, and ultimately decreasing brain nicotine accumulation following E-cig use as compared with C-cig smoking. This interpretation is supported by our observation of significant negative correlations between RT nicotine retention and magnitude of BNA (Fig. 4B, Table 1), and positive correlations between RT nicotine retention and Tmax and T1/2 of BNA after using E-cigs. Still, individual differences in RT nicotine retention can explain only 30–40% of variation in BNA kinetic parameters, suggesting other factors also affect BNA. This could be one of the potential explanations for the absence of similar correlations after using C-cigs where the RT nicotine retention was much smaller.
The observed high nicotine deposition in the upper RT may also have some health consequences itself. Many types of cells in the upper RT as well as some white blood cells express neuronal type nicotinic receptors with high sensitivity to nicotine [24,25,26,27]. Therefore, high nicotine deposition in the upper RT could affect the function of these cells. We believe that quantitative PET imaging of nicotine deposition in and its washout from the RT in humans would provide essential information for future investigation of the potential health consequences of nicotine deposition in the RT.
A possible reason for the observed high RT nicotine deposition is the high pH of E-cig liquid used in the current study (9.4 ± 0.1). This pH is very close to that was previously reported for the same brand E-liquid (pH 9.41) [28]. The same report found that pH values of E-liquids from several popular brands varied from 4.78 to 9.60 while over 50% of the nicotine-containing E-liquids had a pH greater than 9. Alkaline pH enhances evaporation of nicotine base from vapor droplets, thereby enhancing its retention in the upper RT. This notion may be supported by several observations: (1) Vapor from a nicotine inhaler resulted in near complete deposition in the upper RT with little nicotine reaching the lungs [23, 29]; (2) nicotine can evaporate from E-cig vapor droplets [30]; and (3) decreasing e-liquid pH from 9.98 to 3.98 significantly decreases nicotine retention in the RT and increases BNA [13].
It should be noted that the reduction of RT nicotine retention from e-liquids with lower pH may affect BNA not only by increasing the fraction of inhaled nicotine reaching lung alveoli but also by promoting a more aggressive vaping topography through minimizing aversive RT nicotine sensation [31]. By reducing RT retention, lower pH E-liquids are expected to induce lower RT nicotine sensation and may thus contribute to increased nicotine exposure among E-cig users. Nicotine sensation affects nicotine product tolerability and “sensory reward” [32, 33]. Users of low pH E-cigs may use higher nicotine concentration products, taking larger puff volumes, and/or directly inhaling vapor into the lungs. These behaviors ultimately lead to increased nicotine exposure and absolute BNA values, and therefore may increase nicotine-related pharmacological effects that give rise to greater behavioral reinforcement and abuse liability. These phenomena may be even more crucial during nicotine use initiation in youths.
That E-cigs have a fast brain nicotine delivery profile close to that of smoking suggests that they can effectively provide users with subjective satisfaction and thus more effectively substitute for combustible cigarettes as compared to most other nicotine products. In addition, to the extent nicotine concentration in e-liquid can be easily controlled from a high dose to zero, the rates of brain nicotine intake can also be gradually decreased which would allow E-cigs to be a feasible tool for dose tapering in a nicotine replacement therapy regimen. Thus, E-cigs may hold substantial promise as an aid in smoking cessation treatment.
Despite using a strong within-subjects design for assessment of BNA and RT nicotine retention from both E-cigs and C-cigs in each participant, the current study has several limitations. Some of them are: (1) Only one type of E-cigarette has been studied; (2) The RT nicotine retention was assessed only at 16–24 min after puff initiation; (3) RT VOIs covered only major branches of the tracheobronchial tree (up to tertiary bronchi); (4) RT VOIs included part of the esophagus; and (5) the modest sample size may limit the generalizability of the results. Future investigations should attempt to address these shortcomings.
In conclusion, the present results suggest that the respiratory tract retention of nicotine from E-cigs with alkaline pH e-liquid reduces brain nicotine accumulation. Nonetheless even these E-cigs can deliver nicotine rapidly to the brain. Therefore, while E-cigs may lead to the development and maintenance of nicotine dependence, they are also promising substitutes for combustible cigarettes and thereby may promote smoking cessation and harm reduction.
References
Jerzyński T, Stimson GV, Shapiro H, Król G. Estimation of the global number of e-cigarette users in 2020. Harm Reduct J. 2021;18:109.
Abrams DB, Glasser AM, Pearson JL, Villanti AC, Collins LK, Niaura RS. Harm minimization and tobacco control: reframing societal views of nicotine use to rapidly save lives. Annu Rev Pubic Health. 2018;39:193–213.
Farsalinos K. Electronic cigarettes: an aid in smoking cessation, or a new health hazard? Ther Adv Respir Dis. 2018;12:1753465817744960–60.
Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharm Ther. 2008;83:531–41.
Carter LP, Stitzer ML, Henningfield JE, O’Connor RJ, Cummings KM, Hatsukami DK. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol, Biomark Prev. 2009;18:3241–62.
Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61:743–50.
Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Henningfield JE. Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers. Drug Alcohol Depend. 2020;217:108395.
Molander L, Lunell E, Andersson SB, Kuylenstierna F. Dose released and absolute bioavailability of nicotine from a nicotine vapor inhaler. Clin Pharm Ther. 1996;59:394–400.
Hajek P, Pittaccio K, Pesola F, Myers Smith K, Phillips-Waller A, Przulj D. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e-cigarette products. Addiction. 2020;115:1141–48.
St Helen G, Havel C, Dempsey DA, Jacob P 3rd, Benowitz NL. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 2016;111:535–44.
Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15:267–70.
Solingapuram Sai KK, Zuo Y, Rose JE, Garg PK, Garg S, Nazih R, et al. Rapid brain nicotine uptake from electronic cigarettes. J Nucl Med. 2020;61:928–30.
Wall A, Roslin S, Borg B, McDermott S, Walele T, Nahde T, et al. E-cigarette aerosol deposition and disposition of [(11)C]nicotine using positron emission tomography: a comparison of nicotine uptake in lungs and brain using two different nicotine formulations. Pharmaceuticals (Basel). 2022;15:367.
Zuo Y, Mukhin AG, Garg S, Nazih R, Behm FM, Garg PK, et al. Sex-specific effects of cigarette mentholation on brain nicotine accumulation and smoking behavior. Neuropsychopharmacology. 2015;40:884–92.
Rose JE, Mukhin AG, Lokitz SJ, Turkington TG, Herskovic J, Behm FM, et al. Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarettes containing 11C-nicotine. Proc Natl Acad Sci USA. 2010;107:5190–5.
Polosa R, Morjaria JB, Caponnetto P, Caruso M, Campagna D, Amaradio MD, et al. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Disco Med. 2016;21:99–108.
Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10:2500–14.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. Br J Addict. 1991;86:1119–27.
Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob Res. 2015;17:186–92.
Zuo Y, Garg PK, Nazih R, Garg S, Rose JE, Murugesan T, et al. A programmable smoke delivery device for PET imaging with cigarettes containing (11)C-nicotine. J Neurosci Methods. 2017;283:55–61.
Halldin C, Någren K, Swahn CG, Långström B, Nybäck H. (S)- and (R)-[11C]nicotine and the metabolite (R/S)-[11C]cotinine. Preparation, metabolite studies and in vivo distribution in the human brain using PET. Int J Rad Appl Instrum B. 1992;19:871–80.
Benowitz NL, Hukkanen J, Jacob P, 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60.
Bergström M, Nordberg A, Lunell E, Antoni G, Långström B. Regional deposition of inhaled 11C-nicotine vapor in the human airway as visualized by positron emission tomography. Clin Pharm Ther. 1995;57:309–17.
Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharm Exp Ther. 1998;287:435–9.
Gotts JE, Jordt SE, McConnell R, Tarran R. What are the respiratory effects of e-cigarettes? BMJ. 2019;366:l5275.
Arredondo J, Chernyavsky AI, Marubio LM, Beaudet AL, Jolkovsky DL, Pinkerton KE, et al. Receptor-mediated tobacco toxicity: regulation of gene expression through alpha3beta2 nicotinic receptor in oral epithelial cells. Am J Pathol. 2005;166:597–613.
Báez-Pagán CA, Delgado-Vélez M, Lasalde-Dominicci JA. Activation of the macrophage α7 nicotinic acetylcholine receptor and control of inflammation. J Neuroimmune Pharm. 2015;10:468–76.
Stepanov I, Fujioka N. Bringing attention to e-cigarette pH as an important element for research and regulation. Tob Control. 2015;24:413–4.
Lunell E, Bergström M, Antoni G, Långström B, Nordberg A. Nicotine deposition and body distribution from a nicotine inhaler and a cigarette studied with positron emission tomography. Clin Pharm Ther. 1996;59:593–4.
David G, Parmentier EA, Taurino I, Signorell R. Tracing the composition of single e-cigarette aerosol droplets in situ by laser-trapping and Raman scattering. Sci Rep. 2020;10:7929.
Leventhal AM, Madden DR, Peraza N, Schiff SJ, Lebovitz L, Whitted L, et al. Effect of exposure to e-cigarettes with salt vs free-base nicotine on the appeal and sensory experience of vaping: a randomized clinical trial. JAMA Netw Open. 2021;4:e2032757.
Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharm Biochem Behav. 2000;67:71–81.
Rose JE, Tashkin DP, Ertle A, Zinser MC, Lafer R. Sensory blockade of smoking satisfaction. Pharm Biochem Behav. 1985;23:289–93.
Acknowledgements
We thank Sandra Norona, Allison Fulp, and Jonathan Richardson for assistance in data acquisition.
Funding
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R01DA044756. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
All authors contributed to drafting the work or revising it critically for important intellectual content, gave final approval of the version to be published; and provided an agreement to be accountable for all aspects of the work. In addition: YZ provided contributions to the conception, the data acquisition, analysis and interpretation, and wrote the manuscript draft; AGM - the study concept and design, data acquisition, analysis and interpretation, and the project supervision; HB and JDM - data analysis and contributions to data acquisition and interpretation; AM provided contributions to the study concept and design; JER provided contributions to the study concept and data interpretation; KKSS provided contributions to the study conception, data acquisition and the project supervision.
Corresponding author
Ethics declarations
Competing interests
AGM discloses grants from the National Institute on Drug Abuse and consulting for Rose Research Center LLC on the project funded by Philip Morris International, outside the submitted work. JER discloses grants from the National Institute on Drug Abuse, research support from Foundation for a Smoke-Free World, Philip Morris International, Altria, JUUL Labs, consulting with Revive pharmaceuticals, and consulting and patent purchase agreement with Philip Morris International, related to smoking cessation and tobacco harm reduction. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zuo, Y., Mukhin, A.G., Berg, H. et al. Comparison of brain nicotine uptake from electronic cigarettes and combustible cigarettes. Neuropsychopharmacol. 47, 1939–1944 (2022). https://doi.org/10.1038/s41386-022-01410-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01410-5