Abstract
Strategy switching is a form of cognitive flexibility that requires inhibiting a previously successful strategy and switching to a new strategy of a different categorical modality. It is dependent on dopamine (DA) receptor activation and release in ventral striatum and prefrontal cortex, two primary targets of ventral tegmental area (VTA) DA projections. Although the circuitry that underlies strategy switching early in learning has been studied, few studies have examined it after extended discrimination training. This may be important as DA activity and release patterns change across learning, with several studies demonstrating a critical role for substantia nigra pars compacta (SNc) DA activity and release once behaviors are well-learned. We have demonstrated that medial septum (MS) activation simultaneously increased VTA and decreased SNc DA population activity, as well as improved reversal learning via these actions on DA activity. We hypothesized that MS activation would improve strategy switching both early in learning and after extended training through its ability to increase VTA DA population activity and decrease SNc DA population activity, respectively. We chemogenetically activated the MS of male and female rats and measured their performance on an operant-based strategy switching task following 1, 10, or 15 days of discrimination training. Contrary to our hypothesis, MS activation did not affect strategy switching after 1 day of discrimination training. MS activation improved strategy switching after 10 days of training, but only in females. MS activation improved strategy switching in both sexes after 15 days of training. Infusion of bicuculline into the ventral subiculum (vSub) inhibited the MS-mediated decrease in SNc DA population activity and attenuated the improvement in strategy switching. Intra-vSub infusion of scopolamine inhibited the MS-mediated increase in VTA DA population activity but did not affect the improvement in strategy switching. Intra-vSub infusion of both bicuculline and scopolamine inhibited the MS-mediated effects on DA population activity in both the SNc and VTA and completely prevented the improvement in strategy switching. These data indicate that MS activation improves strategy switching once the original strategy has been sufficiently well-learned, and that this may occur via the MS’s regulation of DA neuron responsivity.
Similar content being viewed by others
Introduction
Cognitive flexibility is the ability to adjust behavior in response to changing environmental contingencies [1]. Deficits in cognitive flexibility represent core, predictive features in disorders such as addiction [2,3,4], depression [5], and schizophrenia [6]. Despite being pervasive across many psychiatric disorders [6,7,8,9,10,11,12,13], cognitive flexibility deficits are not effectively treated. Thus, treatment development for these deficits is a top priority, but first requires a more thorough understanding of the circuitry that underlies cognitive flexibility. Strategy switching is one commonly studied form of cognitive flexibility that requires inhibiting a previously successful discrimination strategy and then switching to a new strategy that is of a different categorical modality [14,15,16,17,18]. Some examples of this are measuring the ability to switch from a visual to an egocentric discrimination strategy [14] or switch from a tactile to an olfactory discrimination strategy [19]. The ability to perform a strategy switch after learning an initial discrimination strategy for 1–2 days requires dopamine (DA) receptor activation and release in prefrontal cortex (PFC) [16] and ventral striatum (VS) [15, 18], two primary targets of ventral tegmental area (VTA) DA projections [20, 21]. Few studies have examined how the circuitry underlying strategy switching changes after extended training, i.e. once the initial strategy has become well-learned, despite knowing that DA activity and release patterns change across learning. For example, Collins et al. found that DA release in VS predicted learning and performance speed during early learning (1–5 days), but not after extended training (6–7 days) [22]. Furthermore, initiation of an action sequence becomes dependent on the dorsolateral striatum (DLS) once it is well-learned [23]. In fact, the magnitude of phasic DA release from substantia nigra pars compacta (SNc) to the DLS predicts the initiation of a well-learned action [24] and increases as repetitions increase [25]. This suggests that the DA-related circuitry required to switch strategies after a behavior becomes well-learned may include a mechanism for affecting DA activity in SNc and/or DLS. Examining this question may be particularly important for the study of cognitive flexibility deficits in addiction, as this is a disorder characterized by both DA dysregulation and symptoms derived from excessive habit formation and a loss of flexible control over behavior [26,27,28,29].
The medial septum (MS) is a sub region of the basal forebrain that has been linked to the regulation of theta rhythmicity in the hippocampus [30, 31], as well as navigation [32,33,34,35] and learning [32,33,34,35]. Two of our previous studies led us to hypothesize that the MS might also play a role in strategy switching. First, MS activation improved spatial reversal learning, another form of cognitive flexibility, via a D1 receptor-mediated mechanism [36]. Second, MS activation simultaneously increased DA population activity in VTA and decreased it in SNc via the same specific pathway that precipitated the improvement in reversal learning [36,37,38]. DA population activity refers to the number of spontaneously active vs. inactive DA neurons, and changes in DA population activity are akin to state changes that can occur over minutes or hours. A change in the number of spontaneously active DA neurons means that the magnitude of phasic DA release will also change [39]. This is because DA release is driven by DA burst activity, which occurs over milliseconds, and DA neurons cannot burst without being spontaneously active [39]. Thus, by regulating DA population activity, MS activation could lead to increased DA release in VTA-projecting regions required for strategy switching, such as the VS [15, 18] and PFC [16], and decreased DA release in SNc-projecting regions required for maintaining well-learned action sequences, such as the DLS [23,24,25]. In this way, the MS’s regulation of DA population activity might create a state in which a strategy switch would happen more easily.
We hypothesized that MS activation would improve strategy switching both early in learning and after extended training through its actions on VTA and SNc DA population activity, respectively. We tested this hypothesis by chemogenetically activating the MS and measuring male and female rats’ ability to perform a strategy switch after 1, 10, or 15 discrimination learning days. Next, we replicated [37] a pharmacological manipulation in order to correlate the MS’s regulation of VTA and SNc DA population activity with its ability to improve strategy switching.
Materials and methods
Animals
Experiments were performed using adult female and male Sprague-Dawley rats (250–475 g, Envigo, Frederick, MD). Rats were housed in same-sex pairs with ad libitum access to food and water in a temperature- and humidity-controlled room until used. Experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh according to National Institute of Health Guide for the Care and Use of Laboratory Animals.
Viral construct and survival surgeries
We used a virus with the Gq-coupled designer receptor exclusively activated by a designer drug (DREADD) attached to the human synapsin promoter and the m-Cherry reporter (DR, AAV2–hSyn–hM3Dq–mCherry; Addgene, Watertown, MA), as well as an empty vector control virus (Con, AAV2-hSyn-EGFP, Figs. 1 and S.1). Prior to any behavioral training, rats were secured to a stereotaxic frame under anesthesia (isoflurane in oxygen, 5% induction and 2% maintenance) and a burr hole was drilled in the skull. A 5.0 µl Hamilton (Reno, NV) syringe with a 30-gauge needle was lowered at a 5° angle to avoid the sinus and terminated at the more posterior portion of the MS (AP: +0.6 mm, ML: ±0.55 mm, DV: −6.1 mm from dura). The syringe was connected to a Micro4 microsyringe pump controller (World Precision Instruments, Sarasota, FL). The viral infusion occurred over 9 min (0.1 µl of air + 0.8 µl virus, 0.1 µl/min) with an additional 9 min to allow for adequate viral diffusion. The 0.1 µl of air at the tip of the syringe was used to prevent the virus from leaking into the tissue while the syringe was slowly lowered into the MS.
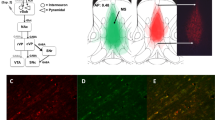
A Male and female rats were infused with an hM3Dq-containing (DR, AAV2–hSyn–hM3Dq–mCherry) or an empty vector control (Con, AAV2-hSyn EGFP) virus into the medial septum (MS, AP: +0.6, ML: ±0.55, DV: −6.1). Both viruses were well-expressed and contained within the MS. Coronal section drawings and associated micrographs illustrate the extent of the fluorescence signal within the MS (×2), and the enlarged micrographs shows expression in neurons within the MS (×10). B Experimental timeline is as follows: 6 weeks following the initial viral infusion surgery, all rats were food restricted and trained to nose-poke in response to a visual cue (white square) that appeared in one of two adjacent windows on a touch screen secured to an operant chamber. This “pre-training phase” took ~2 weeks to complete (completion measured by >75% response vs. omission). Rats began the discrimination training day(s) on the day following completion of pre-training. During discrimination training, rats learned a complex discrimination where they could use either an egocentric strategy (nose poke the left or right screen side, regardless of image) or a visual strategy (nose poke the target image, regardless of screen side) to earn a grain pellet reward. The 1-day training group received a systemic injection of clozapine-N-oxide (CNO; 3 mg/kg) or vehicle (Veh, saline) 30 min prior to the discrimination day. *This session continued until the rat reached a performance criterion of ten consecutive correct trials. The strategy learned during the discrimination day was counterbalanced across rats and there were no differences in learning rates between the two strategy types. The extended training groups performed either 10 or 15 consecutive discrimination training days where CNO or Veh injections were not given. These training days continued for 100 trials per day, regardless of performance. Contrary to the 1-day group, initial experiments revealed that rats reached a higher degree of performance, at a quicker rate, when learning an egocentric, compared to a visual, discrimination strategy. For example, rats learning the egocentric strategy responded correctly on more than 90% of the trials, on average, by discrimination training day 5, whereas rats learning the visual discrimination strategy never reached 90% correct, on average, across any of the 10 or 15 days of discrimination training. Thus, rats in the extended training groups were trained on only the egocentric discrimination strategy. All groups performed a strategy switch test the day after their discrimination day(s) ended (days 2, 11, or 16). The strategy switching test began with 20 “reminder” trials that rewarded the same strategy that had been learned during the discrimination day(s). Beginning with trial 21, the strategy that was rewarded switched to the other strategy. The test day continued until the rat reached the performance criterion of ten consecutive correct trials, until a 400-trial max threshold was reached, or 1 hour elapsed. Rats that did not reach criterion in 400 trials or 1 hour continued on subsequent days until criterion was reached. All subsequent test sessions did not include the initial 20 “reminder trials”, but rats did receive an injection of CNO or Veh prior to each additional test day.
Operant-based strategy switching
See Fig. 1B and Supplementary Methods for details. Briefly, rats learned a complex discrimination where they could use either an egocentric discrimination strategy (nose poke the left or right screen side, regardless of image) or a visual strategy (nose poke the target image, regardless of screen side) to earn a grain pellet reward. Following 1, 10, or 15 days of discrimination training on the same rewarded strategy, rats performed a strategy switch. This test began with 20 “reminder” trials that rewarded the strategy that had been learned during the discrimination day(s). On trial 21, the rewarded strategy switched to the opposite strategy. The test day continued until the rat reached the performance criterion of ten consecutive correct trials, until a 400-trial max threshold was reached, or 1 hour elapsed. The 1-hour time cap was used to prevent effects from diminishing DREADD efficacy. Rats that did not reach criterion in 400 trials or 1 hour continued on subsequent days until criterion was reached. Most rats reached criterion in 1 or 2 days; however, 3 females in the 10-day group took 3 days, 7 males and 8 females in the 15-day group took 3 days, 2 females in the 15-day group took 4 days, and 1 male and 1 female in the 15-day group took 5 days to reach criterion. All subsequent test days did not include the initial 20 “reminder trials”, but rats did receive an injection of CNO or Veh prior to each additional test day. In the final experiment, a different group of male and female rats underwent a second surgery to bilaterally implant cannulae into their ventral subiculum (vSub; AP: −6.0 mm, ML: ±4.5 mm, DL: −8.5 mm from skull). Rats recovered for 1 week following cannulation surgery and then began the 15-day discrimination training and strategy switch procedure as above.
Electrophysiological recordings
Following the conclusion of the strategy switching test, DA neuron activity in the VTA and SNc was assessed using the 9-electrode track protocol described previously [40, 41] (See Fig. S.4 and the supplementary methods for further detail). Briefly, DA neurons were identified and recorded for 1–3 min each using a variety of established criteria, including: low-pitched amplified tone with slow (2–10 Hz) irregular or bursting firing pattern, long duration (2–4 ms) biphasic action potential with initial segment-somatodendritic positive phase break, and a temporary inhibition of firing during tail or foot pinch [42,43,44]. The total number of spontaneously active DA neurons within each animal was counted and then normalized by dividing by the total number of tracks that were examined (active DA neurons per electrode recording track or DA neurons/ track).
Histology
Rats were euthanized, perfused transcardially with 4% paraformaldahyde, and then decapitated. Brains slices were harvested and mounted for immunofluorescent confirmation of Con and DR virus placement and spread. A mouse mCherry primary (1:8000, Abcam-ab125096) and goat anti-mouse secondary stain (alexa 488, 1:500; Abcam-ab150113) were used for confirmation of the hM3Dq receptor. A stain enhancer for the EGFP reporter molecule was not required.
Statistical analyses
In order to properly control for Clozapine-N-oxide (CNO, Tocris) and designer receptor effects, each experiment employed a 4-group design-DREADD virus with CNO injection (DR/CNO), empty vector control virus with CNO (Con/CNO), DREADD virus with vehicle injection (DR/Veh), and control virus with vehicle injection (Con/Veh). For the final intra-vSub infusion experiment, all four groups were comprised of rats that received intra-vSub infusions of saline, scopolamine, bicuculline, or both. No intra-vSub infusion impacted behavior or DA population activity in the control group rats (Con/CNO, DR/Veh, Con/Veh), as we previously demonstrated [37]. Therefore, these rats were combined within their respective group as shown in the figures (e.g., Con/Veh/Veh, Con/Veh/Scop, Con/veh/Bicuc, Con/Veh/both = Con/Veh*). All data are reported as mean ± SEM and were analyzed with analysis of variance. Post hoc analyses to determine individual group differences were performed using the Tukey’s, Sidak’s, or Fisher’s LSD test (indicated in figure legends). Experiments were performed with male and female rats, as indicated in the figures. A 95% confidence interval was performed for the 1, 10, and 15-day experiments to determine whether males and females were part of the same or separate intervals. For instances where they were part of the same intervals, the sexes are shown both separately and combined, and means and statistics are reported with sexes combined. For instances where they were part of separate intervals, males and females are only shown separated, and means and statistics are from the sexes separated.
Results
DREADD expression
Viral vectors (DR, AAV2–hSyn–hM3Dq–mCherry or Con, AAV2-hSyn-EFP) were highly expressed by cell bodies in the MS, and expression at the infusion site was well contained within the MS (Figs. 1A and S.1). Viral transfection also encompassed the vast majority of the anterior-posterior range of the MS, as shown previously with this same viral infusion protocol [36]. Rats in which viral expression was lateral or ventral to the MS were excluded from the below analyses.
MS activation has no effect on strategy switching after 1 day of discrimination training
To determine the effect of chemogenetic activation of the MS on discrimination learning and strategy switching, a DR or Con virus was infused into the MS of male and female rats (N = 15–18 rats/group). Rats performed each day (Fig. 1B) 30 min after a systemic injection of CNO (3 mg/kg) or Veh (saline). Chemogenetic activation of the MS did not affect discrimination learning in female rats (Fig. 2A, Mean ± SEM; Trials: Con/Veh: 121.5 ± 32.4, Con/CNO: 118.2 ± 17.1, DR/Veh: 62.8 ± 6.9, DR/CNO: 95.8 ± 26.4; p = 0.28; Errors: Con/Veh: 26.4 ± 7.2, Con/CNO: 29.2 ± 4.3, DR/Veh: 17.5 ± 2.1, DR/CNO: 22.5 ± 8.8; p = 0.59). MS activation also did not affect trial number in males (Con/Veh: 71.8 ± 14.0, Con/CNO: 83.7 ± 25.3, DR/Veh: 124.8 ± 24.1, DR/CNO: 61.4 ± 17.4; p = 0.14), but did produce a minor, but significant reduction in errors (Con/Veh: 19.6 ± 4.6, Con/CNO: 23.6 ± 7.6, DR/Veh: 38.18 ± 9.2, DR/CNO: 12.3 ± 4.2; p = 0.047). Prior to the strategy switch, all rats got the majority of the 20 “reminder” trials correct at the beginning of the session (trials correct out of 20-Con/Veh: 14.9 ± 0.7, Con/CNO: 15.1 ± 0.5, DR/Veh: 14.2 ± 0.7, DR/CNO: 14.9 ± 0.4; p = 0.40, not shown), suggesting good acquisition of the initial strategy. Chemogenetic activation of the MS had no effect on strategy switching in either sex (Fig. 2B; see legend for 95% CI). This was the case for both trials to criterion (sexes combined, Con/Veh: 108.2 ± 13.6, Con/CNO: 127.2 ± 25.0, DR/Veh: 136.4 ± 28.5, DR/CNO: 107.2 ± 14.7; p = 0.47) and errors (sexes combined, Con/Veh: 35.0 ± 4.8, Con/CNO: 40.3 ± 9.3, DR/Veh: 36.2 ± 7.8, DR/CNO: 30.3 ± 4.7; p = 0.75).
All experiments in this figure contain both male (open square) and female rats (open circle; N = 15–18 rats/group). A Chemogenetic activation of the MS did not affect learning rates in female rats, but did produce a slight reduction in discrimination errors in male rats (F3,40 = 2.89, p = 0.047) compared to the DR/Veh group *(p = 0.030). B Chemogenetic activation of the MS had no effect after the strategy switch (after trial 21), both in terms of trials to reach criterion (left, ten consecutive correct; F3,59 = 0.47, p = 0.71) and number of errors committed (right, F3,58 = 0.40, p = 0.75). A 95% confidence interval for trials and errors in DR/CNO rats revealed that MS activation had a similar effect in males (trials: 73.2, 142.9; errors: 20.4, 43.9) and females (Trials: −20.9, 228.4; errors: −7.3, 54.3), thus sexes are shown both separately and combined.
MS activation improves strategy switching after 10 days of discrimination training in female rats
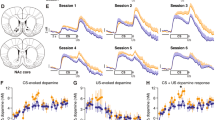
To compare the effect of MS activation on strategy switching after 1 discrimination day vs. extended training, a separate set of male and female rats were infused with the DR or Con virus in their MS. Rats were trained on the egocentric strategy for 100 trials per day for 10 consecutive days, but did not receive CNO or Veh injections during these training days. Analysis of the 10 discrimination days (Fig. 3A) revealed that female rats performed significantly better than males on day 3 (p = 0.003) and 4 (p = 0.013), suggesting that they learned the egocentric discrimination faster (day × sex interaction = p < 0.0001). Male rats’ learning did catch up, however, with their performance rates becoming indistinguishable from females from days 6–10 (Fig. 3A). On the 11th day, rats performed the strategy switch test 30 min after a systemic injection of CNO (3 mg/kg) or Veh (N = 10 rats per sex, per group). All rats performed similarly in the 20 “reminder” trials at the beginning of the session (Fig. S.2A). Data following the strategy switch on trial 21 showed that MS activation (DR/CNO group) significantly improved female, but not male, rats’ ability to perform the strategy switch, compared to the control groups (Fig. 3B). In DR/CNO females, both the number of trials to reach criterion (Con/Veh: 495.4 ± 57.4, Con/CNO: 532.6 ± 49.3, DR/Veh: 532.3 ± 57.2, DR/CNO: 248.3 ± 32.8; F3,36 = 7.46, p = 0.0005) and errors (Con/Veh: 178.2 ± 22.8, Con/CNO: 200.3 ± 20.9, DR/Veh: 172.3 ± 22.4, DR/CNO: 82.7 ± 11.6; F3,36 = 6.75, p = 0.001) were significantly reduced compared to all three control groups (all p’s < 0.05). Error type analysis (see supplemental methods for detail) revealed that female DR/CNO rats committed fewer perseverative (p = 0.002), but not never-reinforced (p = 0.13), errors (Fig. 3C). Female DR/CNO rats showed a significant increase in the time to make a correct (p = 0.0038), but not incorrect (p = 0.11), choice (Fig. S.2D). They also showed significantly lower premature screen nose-pokes during the inter-trial interval (Fig. S.2B, p = 0.041). These changes occurred without affecting the latency to collect the pellet reward (Fig. S.2C, p = 0.24). Although male DR/CNO rats did show a reduction in trials (Con/Veh: 538.1 ± 67.1, Con/CNO: 426.8 ± 57.9, DR/Veh: 422.0 ± 53.7, DR/CNO: 354.0 ± 25.1, F3,36 = 2.05, p = 0.12) and errors (Con/Veh: 221.6 ± 33.7, Con/CNO: 174.9 ± 26.0, DR/Veh: 175.7 ± 21.2, DR/CNO: 133.7 ± 9.7; F3,36 = 2.19, p = 0.11), neither effect reached statistical significance (Fig. 3B).
A 95% confidence interval for trials and errors in DR/CNO rats revealed that MS activation did not produce similar effects in males (trials: 297.1, 410.9; errors: 111.6, 155.8) and females (Trials: 174.2, 322.4; errors: 56.6, 108.8), thus sexes are shown separately (N = 10 rats per group, per sex). Male data points are represented by an open square and female data points with an open circle. A Female rats learned the egocentric discrimination faster, reaching asymptotic level of performance (defined as ≥92% correct) in fewer days than male rats (day × sex interaction: F9,666 = 5.62, p < 0.0001). *A post hoc Sidak’s test revealed this to be the case specifically at day 3 (p = 0.003) and 4 (p = 0.013). B MS activation (DR/CNO group) in females significantly reduced the number of trials to reach criterion (left, 10 consecutive correct, F3,36 = 7.46, p = 0.0005) and errors (right, F3,36 = 6.75, p = 0.001) compared to controls. ***A post hoc Tukey’s test revealed that the female DR/CNO rats were significantly reduced from all three of the control groups for both trials (vs. Con/Veh p = 0.007, vs. Con/CNO p = 0.002, vs. DR/Veh p = 0.002) and errors (vs. Con/Veh p = 0.009, vs. Con/CNO p = 0.001, vs. DR/Veh p = 0.016). Although male DR/CNO rats did show a minor reduction in trials and errors, neither effect reached statistical significance. C Analysis of error type revealed that female DR/CNO rats committed fewer perseverative (Con/Veh: 167.8 ± 22.4, Con/CNO: 186.7 ± 18.6, DR/Veh: 160.1 ± 22.1, DR/CNO: 74.1 ± 12.4; F3,35 = 6.16, p = 0.002), but not never-reinforced (Con/Veh: 10.4 ± 2.2, Con/CNO: 13.5 ± 3.4, DR/Veh: 12.1 ± 1.8, DR/CNO: 5.6 ± 1.7; F3,35 = 1.98, p = 0.13), errors. ***A post hoc Tukey’s test revealed that perseverative errors were reduced compared to all three control groups (vs. Con/Veh p = 0.011, vs. Con/CNO p = 0.002, vs. DR/Veh p = 0.021).
MS activation improves strategy switching in both sexes after 15 days of discrimination training
Because female rats learned the egocentric strategy faster in the prior experiment, they spent more days at a high, asymptotic level of performance. To test whether further discrimination training would reveal an effect in male rats, another set of male and female rats were infused with the DR or Con virus in their MS and trained for 15 consecutive 100-trial days. Different from the 10-day group, male and female rats learned the discrimination at a similar rate across the 15 days (Fig. 4A). On the 16th day, rats performed the strategy switch session as above. Also different from the 10-day discrimination training group, 95% confidence intervals revealed that MS activation produced a comparable degree of improvement in strategy switching in males (Trials: 329.3, 469.9; errors: 106.4, 170.9) and females (Trials: 183.1, 473.9; errors: 64.4, 165.3); thus, the combined means are reported (N = 12–14 rats/group, see legend for means separated by sex). MS activation significantly improved strategy switching performance by reducing both trials to reach criterion (Fig. 4B left, Con/Veh: 529.8 ± 61.2, Con/CNO: 616.7 ± 78.4, DR/Veh: 561.3 ± 66.5, DR/CNO: 376.9 ± 32.4; F3,47 = 3.03, p = 0.039) and errors (Fig. 4B right, Con/Veh: 207.0 ± 28.9, Con/CNO: 238.9 ± 28.2, DR/Veh: 226.6 ± 32.0, DR/CNO: 128.4 ± 11.5; F3,47 = 3.92, p = 0.014). Similar to the female DR/CNO rats in the 10-day experiment, DR/CNO rats of both sexes committed fewer perseverative (p = 0.010), but not never-reinforced (p = 0.93), errors (Fig. 4C). Choice latency was significantly increased in male, but not female, rats (Fig. S.3D). However, this was observed when the rats made both correct (p = 0.005) and incorrect (p = 0.007) choices, suggesting a general slowing of decision making, regardless of benefit. Reward collection latency was unaffected by MS activation in either sex (Fig. S.3C; p = 0.54). Both sexes of the DR/CNO group showed a significant reduction in premature screen nose-pokes during the inter-trial interval (p = 0.044; Fig. S.3B).
A 95% confidence interval revealed that MS activation produced a comparable degree of improvement in strategy switching in males (trials: 329.3, 469.9; errors: 106.4, 170.9) and females (Trials: 183.1, 473.9; errors: 64.4, 165.3); thus, the data are shown both separately and combined. Male data points are shown as open squares and female data points as open circles (N = 12–14 rats per group). A In contrast to the 10-day training rats, learning rates were not significantly different between sexes during the 15 days of discrimination training. B Chemogenetic activation of the MS reduced trials to criterion in males (left, N = 6–8 rats/group, Con/Veh: 618.8 ± 109.9, Con/CNO: 615.5 ± 100.1, DR/Veh: 618.0 ± 121.6, DR/CNO: 413.1 ± 35.5, F3,22 = 1.44, p = 0.26) and females (N = 6–7 rats/group, Con/Veh: 453.4 ± 56.4, Con/CNO: 617.8 ± 130.5, DR/Veh: 504.7 ± 58.4, DR/CNO: 328.5 ± 56.6, F3,21 = 2.16, p = 0.12), but neither reached statistical significance until combined (F3,47 = 3.03, p = 0.039). Chemogenetic activation of the MS reduced errors in males (right, Con/Veh: 266.8 ± 49.7, Con/CNO: 257.8 ± 40.5, DR/Veh: 257.7 ± 59.6, DR/CNO: 138.6 ± 13.6, F3,22 = 2.53, p = 0.083) and females (Con/Veh: 155.7 ± 19.6, Con/CNO: 220.0 ± 41.6, DR/Veh: 195.5 ± 23.7, DR/CNO: 114.8 ± 19.6, F3,21 = 2.80, p = 0.065), but both remained at #trend significance levels until combined (F3,47 = 3.92, p = 0.014). Once combined *a Tukey’s test revealed a significant reduction in trials compared to the Con/CNO group (p = 0.033), but not the other two (vs. Con/Veh p = 0.26 and vs. DR/Veh p = 0.14). **A Tukey’s test showed that DR/CNO rat errors were significantly reduced compared to the Con/CNO (p = 0.018) and DR/Veh (p = 0.043) groups, but not Con/Veh (p = 0.13). C DR/CNO rats of both sexes (combined) committed fewer perseverative (right, Con/Veh: 195.1.7 ± 27.8, Con/CNO: 226.0 ± 28.3, DR/Veh: 211.8 ± 29.5, DR/CNO: 115.1 ± 10.8, F3,47 = 4.26, p = 0.010), but not never-reinforced (left, Con/Veh: 11.9 ± 2.9, Con/CNO: 12.9 ± 3.3, DR/Veh: 14.8.5 ± 3.6, DR/CNO: 13.3 ± 2.5, F3,47 = 0.14, p = 0.93), errors. **A post hoc Tukey’s tests revealed a significant reduction in perseverative errors compared to the Con/CNO (p = 0.012) and DR/Veh (p = 0.036) groups, but only a trend compared to the Con/Veh group (p = 0.099).
The effects of MS activation on DA activity are blocked by pharmacological manipulation of the ventral subiculum
We hypothesized that MS activation improved strategy switching via its ability to regulate DA activity, similar to what we previously showed with reversal learning [36]. To first determine if MS activation affects DA population activity in female rats similar to what’s been shown previously in males [36,37,38], we recorded DA activity in females (see Fig. S.4) that had finished the strategy switching paradigm (N = 5–7 rats/group). Female control rats showed similar numbers of spontaneously active DA neurons in the SNc as previously reported [36, 37] in males (Fig. 5A left; DA neurons/track—Con/Veh: 1.8 ± 0.1, Con/CNO: 1.7 ± 0.1, DR/Veh: 1.7 ± 0.1). Chemogenetic activation of the MS significantly reduced the number of spontaneously active DA neurons in the SNc (DR/CNO: 0.9 ± 0.2; F3,20 = 13.5, p < 0.0001), and this, again, was similar to the reduction reported in males [36, 37]. This was also true when measuring in the VTA [36, 37, 45] for both female control rats (Fig. 5A right; Con/Veh: 0.8 ± 0.1, Con/CNO: 0.6 ± 0.2, DR/Veh: 1.0 ± 0.1) and after chemogenetic activation of the MS (DR/CNO: 1.6 ± 0.2; F3,21 = 8.05, p = 0.0009). MS activation did not significantly alter DA neuron firing rate or burst activity (not shown), as has been shown previously [36,37,38].
A Left, chemogenetic activation of the MS (DR/CNO group) significantly reduced DA neuron population activity in the SNc in female rats (open circles, N = 5–7 rats/group; F3,20 = 13.5, p < 0.0001). ***A Tukey’s test revealed that DA population activity was reduced compared to all three control groups (vs. Con/Veh p < 0.0001, vs. Con/CNO p = 0.0005, vs. DR/Veh p = 0.0002). Right, chemogenetic activation of the MS (DR/CNO group) significantly increased DA neuron population activity in the VTA in female rats (N = 5–7 rats/group; F3,21 = 8.05, p = 0.0009). **A Tukey’s test revealed that DA population activity was increased compared to Con/Veh (p = 0.0043) and Con/CNO (p = 0.001), but not DR/Veh (p = 0.12). B Left, intra-vSub infusions had no effect on baseline DA population activity when given to control rats and were combined (e.g., Con/Veh/Veh, Con/Veh/Scop, Con/veh/Bicuc, Con/Veh/both = Con/Veh*, etc.; N = 5–7 rats/group). The reduction in SNc DA population activity after MS activation was prevented by intra-vSub infusion of bicuculline (Bicuc) and both bicuculline and scopolamine (Both), but not by scopolamine (Scop). ***This effect was significant (F6,33 = 22.6, p < 0.0001), with a Tukey’s test showing that the DR/CNO/Veh and DR/CNO/Scop groups were significantly decreased from all other groups (vs. Con/Veh* p’s < 0.0001, vs. Con/CNO* p’s < 0.0001, vs. DR/Veh* p’s < 0.0001, vs. DR/CNO/Bicuc p < 0.0001 and p = 0.0003, vs. DR/CNO/Both p’s < 0.0001). Right, the increase in VTA DA population activity after MS activation was prevented by intra-vSub infusion of scopolamine (Scop) and both bicuculline and scopolamine (Both), but not by bicuculline (Bicuc). ***This effect was significant (F6,32 = 14.5, p < 0.0001), with a Tukey’s test showing that the DR/CNO/Veh and DR/CNO/Bicuc groups were significantly elevated from all other groups (vs. Con/Veh* p = 0.003 and 0.001, vs. Con/CNO* p = 0.023 and 0.009, vs. DR/Veh* p = 0.0003 and 0.0001, vs. DR/CNO/Scop p’s < 0.0001, vs. DR/CNO/Both p = 0.0003 and <0.0001, respectively). C Left, The reduction in trials to reach criterion (10 consecutive correct) after MS activation was inhibited by intra-vSub infusion of bicuculline (Bicuc), prevented by infusion of both bicuculline and scopolamine (Both), but not affected by infusion of scopolamine (Scop, N = 10–18 rats/group; overall F6,92 = 2.63, p = 0.021). *A Fisher’s LSD test showed that the DR/CNO/Veh group was reduced compared to the Con/CNO* (p = 0.007), DR/Veh* (p = 0.012), DR/CNO/Bicuc (p = 0017), and DR/CNO/Both (p = 0.006) groups. #DR/CNO/Scop rats remained similarly reduced as the DR/CNO/Veh group, showing a trend toward a reduction compared to Con/CNO* (p = 0.062), DR/Veh* (p = 0.087), and DR/CNO/Bicuc (p = 0.0998) groups, and a reduction compared to the DR/CNO/Both group (p = 0.040). Right, the reduction in errors after MS activation was prevented by infusion of both bicuculline and scopolamine (Both; F6,93 = 3.07, p = 0.009). Infusion of bicuculline showed a small inhibition of the reduction in errors, but this did not reach significance (vs. DR/CNO/Veh p = 0.22). Infusion of scopolamine had no effect on the reduction in errors. A Fisher’s LSD test showed that the DR/CNO/Veh and DR/CNO/Scop groups were *significantly reduced compared to the Con/CNO* (p = 0.0006 and 0.0013, respectively) and DR/Veh* (p = 0.023 and 0.029, respectively) groups and #showed a trend toward a significant reduction compared to the Con/Veh* (p = 0.087 and 0.094, respectively) and DR/CNO/Both (p = 0.079 and 0.082, respectively) groups. D Right, the reduction in perseverative errors after MS activation was prevented by infusion of both bicuculline and scopolamine (Con/Veh*: 156.3 ± 17.1, Con/CNO*: 201.3 ± 20.9, DR/Veh*: 167.1 ± 17.6, DR/CNO/Veh: 104.0 ± 12.2, DR/CNO/Scop: 104.3 ± 19.2, DR/CNO/Bicuc: 134.2 ± 14.4, DR/CNO/both: 156.8 ± 33.1; F6,93 = 3.33, p = 0.005). Infusion of bicuculline or scopolamine alone did not affect the reduction in perseverative errors (vs. DR/CNO/Veh p = 0.28 and 0.99, respectively). *#A Fisher’s LSD test showed that the DR/CNO/Veh and DR/CNO/Scop groups were reduced compared to the Con/Veh* (p = 0.048 and 0.075, respectively), Con/CNO* (p = 0.0003 and 0.001, respectively), DR/Veh* (p = 0.019 and 0.034, respectively), and DR/CNO/Both (p = 0.085 and 0.11, respectively). Left, never-reinforced errors were not affected by any systemic or intra-vSub treatment (Con/Veh*: 9.5 ± 1.6, Con/CNO*: 14.1 ± 1.8, DR/Veh*: 12.6 ± 1.7, DR/CNO/Veh: 14.3 ± 3.2, DR/CNO/Scop: 10.0 ± 3.8, DR/CNO/Bicuc: 18.0 ± 3.7, DR/CNO/both: 18.3 ± 4.3, F6,93 = 1.51, p = 0.18).
We showed previously that the MS regulates DA population activity via a direct projection to the vSub [37], which then follows a known pathway [46,47,48,49] to the VTA and SNc [37]. Furthermore, we showed that infusion of scopolamine into the vSub selectively inhibited the increase in DA population activity in the VTA after pharmacological MS activation, but did not affect the decrease in SNc [37]. Infusion of bicuculline into the vSub selectively prevented the decrease in SNc, but did not affect the increase in VTA [37]. To confirm these findings chemogenetically, we used male and female rats that had been infused previously with the DR or Con virus in the MS and had completed behavioral experiments. Anesthetized rats were given a systemic injection of CNO or Veh and an intra-vSub infusion of vehicle (Veh; saline), scopolamine (Scop; 8 µg in 1 µl), bicuculline (Bicuc; 12.5 ng in 0.5 µl), or both bicuculline and scopolamine (Both) 30 and 10 min prior to recording, respectively (N = 5–7 rats/group). In a group containing male (open squares) and female (open circle) rats, DA population activity in the SNc of control rats was similar to what has been shown previously (Fig. 5B left; Con/Veh*: 1.8 ± 0.1, Con/CNO*: 1.8 ± 0.1, DR/Veh*: 1.7 ± 0.02). DA population activity was significantly decreased following chemogenetic activation of the MS (DR/CNO/Veh: 0.9 ± 0.1). Intra-vSub infusion of bicuculline or both bicuculline and scopolamine prevented the decrease in SNc DA population activity (DR/CNO/Bicuc: 1.6 ± 0.2, DR/CNO/both: 1.9 ± 0.03; F6,33 = 22.6, p < 0.0001), but scopolamine did not (DR/CNO/Scop: 0.9 ± 0.1). DA population activity in the VTA of control rats also was similar to what has been shown previously (Fig. 5B right; Con/Veh*: 1.0 ± 0.1, Con/CNO*: 1.1 ± 0.1, DR/Veh*: 0.9 ± 0.04). DA population activity was significantly elevated following chemogenetic activation of the MS (DR/CNO/Veh: 1.6 ± 0.1). Intra-vSub infusion of scopolamine or both drugs prevented the increase in VTA DA population activity (DR/CNO/Scop: 0.6 ± 0.1, DR/CNO/both: 0.8 ± 0.1; F6,32 = 14.5, p < 0.0001), but bicuculline did not (DR/CNO/Bicuc: 1.7 ± 0.2). No manipulation significantly affected DA neuron firing rate or burst activity (not shown), as has been shown previously [36,37,38].
MS activation-induced improvement in strategy switching is inhibited by intra-vSub infusion of bicuculline and prevented by infusion of both bicuculline and scopolamine
To determine whether the manipulation that selectively inhibited the MS’s regulation of DA population activity in the above experiment would also inhibit the MS-mediated improvement in strategy switching, a group containing male and female rats was infused with the DR or Con virus in their MS and bilaterally implanted with cannulae in vSub (N = 10–18 rats/group). Rats followed the 15-day discrimination training procedure outlined above (see Fig. S.5A for learning curves) because this led to similar effects in both sexes in the prior experiment. On the 16th day, rats were given a systemic injection of CNO or Veh, as well as bilateral vSub infusions of Veh, Scop, Bicuc, or Both, as above. All rats performed the majority of the 20 “reminder” trials correctly (see Fig. S.5B). MS activation (DR/CNO/Veh) again improved strategy switching, reducing both trials to reach criterion (Fig. 5C left; Con/Veh*: 444.4 ± 38.0, Con/CNO*: 579.2 ± 64.5, DR/Veh*: 564.9 ± 64.5, DR/CNO/Veh: 355.1 ± 37.7; DR/CNO/Veh vs. Con/Veh* p = 0.27, vs. Con/CNO* p = 0.007, vs. DR/Veh* p = 0.012) and errors (Fig. 5C right; Con/Veh*: 165.8.0 ± 17.6, Con/CNO*: 215.3 ± 20.9, DR/Veh*: 182.6 ± 18.4, DR/CNO/Veh: 118.3 ± 13.9; DR/CNO/Veh vs. Con/Veh* p = 0.087, vs. Con/CNO* p = 0.0006, vs. DR/Veh* p = 0.023). Intra-vSub infusion of scopolamine did not affect the improvement in strategy switching. Both trials-to-criterion (DR/CNO/Scop: 408.6 ± 53.7; vs. DR/CNO/Veh p = 0.57) and errors (DR/CNO/Scop: 114.3 ± 19.3; vs. DR/CNO/Veh p = 0.90) were similar to the DR/CNO/Veh group and remained reduced compared to the control groups (see legend). Intra-vSub infusion of bicuculline attenuated the improvement in strategy switching. DR/CNO/Bicuc rats required as many trials to reach criterion as the control rats (567.1 ± 52.9; vs. Con/Veh* p = 0.14, vs. Con/CNO* p = 0.89, vs. DR/Veh* p = 0.98), and significantly more than DR/CNO/Veh rats (p = 0.017). Interestingly, DR/CNO/Bicuc rats did not commit significantly more errors compared to DR/CNO/Veh rats (155.2 ± 16.1; vs. DR/CNO/Veh p = 0.22). Intra-vSub infusion of both bicuculline and scopolamine prevented the improvement in strategy switching mediated by MS activation. DR/CNO/Both rats had comparable trials-to-criterion as the control groups (619.9 ± 122.0; vs. Con/Veh* p = 0.053, vs. Con/CNO* p = 0.65, vs. DR/Veh* p = 0.54), which were significantly increased compared to DR/CNO/Veh rats (p = 0.006). However, DR/CNO/Both rats also committed a similar number of errors compared to controls (174.9 ± 37.0; vs. Con/Veh* p = 0.76, vs. Con/CNO* p = 0.19, vs. DR/Veh* p = 0.80), which trended toward a significant increase compared to both the DR/CNO/Veh (p = 0.079) and DR/CNO/Scop (p = 0.082) groups. MS activation (DR/CNO/Veh) improved performance by reducing perseverative (vs. Con/Veh* p = 0.048, vs. Con/CNO* p = 0.0003, vs. DR/Veh* p = 0.019), but not never-reinforced (overall p = 0.18), errors (Fig. 5D). This effect was blocked only by infusion of both bicuculline and scopolamine (p = 0.085), which did not affect never-reinforced errors. Infusion of scopolamine, bicuculline, or both in the DR/CNO, but not control, rats led to a slight, but statistically significant, increase in choice and reward collection times compared to the other four groups (Fig. S.5D, p’s < 0.05).
Discussion
Chemogenetic activation of the MS had no effect on strategy switching after 1 day of discrimination training. It is possible that the lack of effect in the 1-day group is due to a floor effect. Rats in the 1-day group got 70–75% of their “reminder trials” correct, suggesting that they had acquired the initial strategy prior to the switch. We also have shown an ability to improve reversal learning when the overall trial and error numbers were even lower [36] than those in the 1-day group. However, further study will be needed to test this. MS activation improved strategy switching after 10 days of discrimination training, but only in female rats. Female rats performed the strategy switch faster (fewer trials) and with fewer mistakes (specifically perseverative) compared to controls. MS activation improved strategy switching in both sexes after 15 days of training, again primarily by reducing perseverative errors. Infusion of bicuculline into the vSub inhibited the MS-mediated decrease in SNc DA population activity and attenuated the improvement in strategy switching. Intra-vSub infusion of scopolamine inhibited the MS-mediated increase in VTA DA population activity but did not affect the improvement in strategy switching. Intra-vSub infusion of both bicuculline and scopolamine inhibited the MS-mediated effects on DA population activity in both the SNc and VTA and prevented the improvement in strategy switching. These data indicate that MS activation improves strategy switching, but only once the original strategy has been sufficiently well-learned. They also suggest that the mechanism by which this occurs may be via the MS’s regulation of DA neuron responsivity.
Are there sex differences in the MS’s regulation of strategy switching and does this affect our interpretation of the results?
Chemogenetic activation of the MS significantly improved strategy switching performance in female rats after 10 days of discrimination training, but did not significantly improve strategy switching in males. Interestingly, this effect was demonstrated in a cohort where female rats initially learned the discrimination faster, performing significantly better than males at days 3 and 4. Males and females performed similarly from day 6 through day 10. This led us to hypothesize that the level of overtraining could be critical for the MS’s ability to improve strategy switching, such that 10 days was sufficient in female rats but not males. In a separate rat cohort, we extended discrimination training to 15 days and found that this was sufficient training to reveal an MS-mediated improvement in strategy switching in both sexes. Interestingly, male and female rats performed similarly across all 15 days of discrimination training in this cohort, as well as the other experiment that used the 15-day training protocol, suggesting they learned at similar rates in these cohorts. So, are there sex differences in the MS’s regulation of strategy switching or is this a simple cohort difference? And, does this affect our interpretation of the results?
A well-performed study by Chen et al. demonstrated that female rats tended to use an egocentric strategy when learning to discriminate between two novel stimuli [50]. Male rats tended to erratically shift strategies based on feedback from the previous trial [50]. This led to female rats learning discriminations faster, as was also seen in our 10-day group. However, few studies have compared MS functionality in male and female rodents, so there is little support for the notion that the MS’s regulation of strategy switching would be different between sexes. Indeed, data from this and our prior publications [36, 37] indicate that MS activation had a comparable effect on DA population activity in male and female rats, even in terms of magnitude of the effect. While our current data demonstrating the necessity of the MS’s regulation of DA population activity for its effect on strategy switching is correlational, it remains that we cannot provide any mechanistic evidence to support distinct MS functionality by sex. Thus, while data from Chen et al. provides good evidence for differing learning rates between sexes, the difference in learning rates in the 10-day group, in this study, seems more likely to be a cohort difference than a true sex difference. Further study will be needed to evaluate this.
Our interpretation of our results is that the initial strategy needs to be sufficiently well-learned for MS activation to improve strategy switching. Whether the female rats learned faster in the 10-day group because of their sex-based inclination toward the egocentric strategy [50] or because of a simple cohort difference, our results show that their learning rate did affect the MS’s ability to improve strategy switching. Similarly, whether both sexes performed equivalently in the 15-day groups because males were given 5 extra days of training to “catch up” with the females or because they simply learned at the same rate as the females from the beginning, our results again show that level of overtraining affects the MS’s ability to improve strategy switching. It will be important in future studies to define what level of training is sufficient for a strategy to be well-learned, across individual rats, as well as whether a sex difference exists. At present, however, we believe our interpretation is valid regardless of whether there are sex differences in the MS regulation of strategy switching or not.
How could the MS’s ability to downregulate DA population activity in SNc lead to an improvement in strategy switching?
Our data demonstrated that intra-vSub infusion of bicuculline both selectively prevented the MS’s ability to downregulate DA population activity in SNc and significantly inhibited the MS-mediated improvement in strategy switching. This led us to speculate that the primary mechanism by which the MS improves strategy switching is via its ability to downregulate DA population activity in SNc. We propose that this could occur as follows: as rewarded behaviors are repeated over time and learning progresses, motor action plans become stereotyped [51,52,53]. DA release that is initially seen in VS and is predictive of learning rate [21, 22], begins to dissipate [25]. This coincides with the emergence of a DA signal in the SNc to DLS pathway [25, 54,55,56]. Increased DA release in DLS, which is associated with general motor invigoration early in learning [21, 57], now increases the likelihood of initiating the previously learned, stereotyped action sequence [23, 51]. Furthermore, inhibition of the DLS severely inhibits execution of a well-learned behavior [23], while activation of DA release in DLS impairs reversal learning [58]. These data suggest that the MS’s reduction of SNc DA population activity may be improving strategy switching by decreasing the likelihood of initiating the stereotyped action sequence associated with the previous, well-learned strategy. Although this notion is speculative, we believe that it is supported by some of our secondary findings. First, MS activation improved strategy switching by reducing perseverative errors, but had no effect on never-reinforced errors in any group. This indicates that MS activation was reducing errors that were committed specifically by following the previously rewarded strategy. Second, several groups demonstrated an increase in choice latency following MS activation, which was not accompanied by an increase in reward collection latency in most cases. This suggests an MS-mediated slowing of decision-making, specifically, rather than a general slowing of movement. Finally, MS activation led to a reduction in premature nose-pokes during the inter-trial interval. This suggests that the rats were waiting until the stimuli were present before making a choice. Together, we interpret these results to mean that MS activation may have led the rats to be more deliberate, accurate, and possibly goal-directed, primarily as a result of the MS inhibiting the SNc-mediated initiation of the previously learned, stereotyped strategy.
Are DA population activity changes in VTA irrelevant to the MS’s ability to affect strategy switching?
Although intra-vSub infusion of scopolamine did not affect the MS’s improvement in strategy switching, it is important to note that infusion of both drugs inhibited the improvement in strategy switching to a greater degree than bicuculline alone. In fact, the reduction in errors, and specifically perseverative errors, was not blocked except for after the infusion of both bicuculline and scopolamine. This suggests that scopolamine’s inhibition of the MS activation-induced increase in VTA DA population activity likely had some effect on the MS’s improvement in strategy switching, albeit a secondary effect. Thus, we posit that once a strategy has been sufficiently well-learned, altering DA activity in the SNc is the more impactful result of MS activation. However, a secondary result of decreasing the likelihood of initiating the well-learned strategy/action sequence may be allowing a return of behavior to a “goal-directed” state, as suggested above. Once back in the goal-directed state, the MS’s increase in VTA DA activity may still provide some enhancement in learning rate for the new strategy, similar to what others have shown [22].
What is the MS’s role in cognitive flexibility and how might this function be applicable in psychiatric disorders?
In a previous publication, we demonstrated that MS activation improved spatial reversal learning in a T-maze [36]. Interestingly, this improvement was found after only 1 day of training and occurred despite the rats needing only 20–25 trials, on average, to perform. This finding led us to hypothesize that MS activation would also improve strategy switching after 1 day of training, but we found that MS activation had no effect on strategy switching until rats had been trained for 10–15 days. It is true that reversal learning and strategy switching are slightly different cognitive operations, one requiring a simple reversal within the same categorical modality and the other a switch to a strategy of a different categorical modality, respectively. It is also true that reversal learning and strategy switching are regulated by different subregions within the PFC and VS [14, 15, 17, 18, 59,60,61]. However, DA release and receptor activation are required for both [16, 18, 61, 62], and the MS-mediated improvement in both seems to rely on the MS’s ability to affect DA population activity in VTA and SNc [36]. So, why does MS activation seem to affect reversal learning and strategy switching differently, and what does this say about the MS’s role in cognitive flexibility? Work by others has demonstrated that egocentric strategies used in T-maze running can actually become stereotyped and dependent on the DLS and SNc relatively quickly [23, 51]. The data from this study implicates the MS’s regulation of SNc DA activity for its ability to improve strategy switching after extended training. Thus, it is possible that the common thread between the MS’s ability to improve strategy switching and spatial reversal learning is its ability to regulate DA population activity in SNc. If this is the case, the difference between our prior and current results may be as simple as the amount of time it takes each respective behavior to become dependent on the SNc. Further study will be needed to confirm this, but the combination of our current and previous findings leads us to posit that the MS’s role in cognitive flexibility is to reinstate flexibility once behavior has become stereotyped or habitual; i.e., once it is dependent on the SNc.
Habit formation is an adaptive response that minimizes cognitive processing for behaviors that have been well-learned and for which a positive outcome can be acquired for a stereotyped sequence of actions. However, excessive habit formation is maladaptive, can lead to a loss of flexible control over thoughts and behaviors, and may contribute to pathology in many psychiatric disorders, such as addiction [4, 26,27,28,29], obsessive-compulsive disorder [4, 63,64,65], compulsive eating disorders [66], as well as others [51, 52]. DA release in DLS, a primary target of the SNc, has been heavily implicated in both habit formation [27, 51, 52] and many of the disorders mentioned above [51, 55, 65, 67, 68]. Thus, if the MS’s role is to reinstate flexibility once behavior has become stereotyped and/or dependent on the SNc, it is possible that activation of the MS could be a therapeutically relevant avenue in the treatment of some aspects of these disorders.
Conclusions
This study demonstrates that MS activation improves strategy switching, but only once the initial strategy is sufficiently well-learned. Interestingly, this effect is demonstrated as a slowing of decision-making, reducing perseverative errors, in order to adapt to a new strategy more quickly. We propose that this is primarily due to the MS’s reduction of DA activity in the SNc, a region that is responsible for initiating a well-learned action sequence [23, 51]. Together with our previous findings, we propose that the MS’s ability to downregulate SNc DA activity is a means to destabilize the initiation of a well-learned, stereotyped action sequence and thus increase flexible adaptation to a set of changing environmental contingencies. Continued examination of the MS’s ability to promote flexibility will be important, as this MS-mediated pathway may have treatment relevance for a number of psychiatric disorders. This will be the subject of our future studies.
References
Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–68. https://doi.org/10.1146/annurev-psych-113011-143750.
Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology. 2012;219:607–20. https://doi.org/10.1007/s00213-011-2579-7.
Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology. 2008;197:421–31. https://doi.org/10.1007/s00213-007-1051-1.
Figee M, Pattij T, Willuhn I, Luigjes J, van den Brink W, Goudriaan A, et al. Compulsivity in obsessive-compulsive disorder and addictions. Eur Neuropsychopharmacol. 2016;26:856–68. https://doi.org/10.1016/j.euroneuro.2015.12.003.
MacPherson HA, Kudinova AY, Schettini E, Jenkins GA, Gilbert AC, Thomas SA, et al. Relationship between cognitive flexibility and subsequent course of mood symptoms and suicidal ideation in young adults with childhood-onset bipolar disorder. Eur Child Adolesc Psychiatry. 2021. https://doi.org/10.1007/s00787-020-01688-0.
Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res. 1999;37:251–70. https://doi.org/10.1016/s0920-9964(98)00156-x.
Mallorquí-Bagué N, Tolosa-Sola I, Fernández-Aranda F, Granero R, Fagundo AB, Lozano-Madrid M, et al. Cognitive deficits in executive functions and decision-making impairments cluster gambling disorder sub-types. J Gambl Stud. 2018;34:209–23. https://doi.org/10.1007/s10899-017-9724-0.
Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav 2005;81:319–30. https://doi.org/10.1016/j.pbb.2005.02.017.
Liang CS, Ho PS, Yen CH, Chen CY, Kuo SC, Huang CC, et al. The relationship between the striatal dopamine transporter and novelty seeking and cognitive flexibility in opioid dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2017;74:36–42. https://doi.org/10.1016/j.pnpbp.2016.12.001.
Francazio SK, Flessner CA. Cognitive flexibility differentiates young adults exhibiting obsessive-compulsive behaviors from controls. Psychiatry Res. 2015;228:185–90. https://doi.org/10.1016/j.psychres.2015.04.038.
Lima IMM, Peckham AD, Johnson SL. Cognitive deficits in bipolar disorders: implications for emotion. Clin Psychol Rev. 2018;59:126–36. https://doi.org/10.1016/j.cpr.2017.11.006.
Park J, Moghaddam B. Impact of anxiety on prefrontal cortex encoding of cognitive flexibility. Neuroscience. 2017;345:193–202. https://doi.org/10.1016/j.neuroscience.2016.06.013.
Allain P, Etcharry-Bouyx F, Verny C. Executive functions in clinical and preclinical Alzheimer's disease. Rev Neurol. 2013;169:695–708. https://doi.org/10.1016/j.neurol.2013.07.020.
Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. https://doi.org/10.1016/j.bbr.2008.02.008.
Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci. 2006;26:2449–57. https://doi.org/10.1523/JNEUROSCI.4431-05.2006.
Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. https://doi.org/10.1038/sj.npp.1300825.
Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem. 2008;89:567–73. https://doi.org/10.1016/j.nlm.2007.10.007.
Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–52. https://doi.org/10.1038/npp.2009.21.
Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4.
Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. https://doi.org/10.1038/npp.2009.129.
Collins AL, Saunders BT. Heterogeneity in striatal dopamine circuits: form and function in dynamic reward seeking. J Neurosci Res. 2020;98:1046–69. https://doi.org/10.1002/jnr.24587.
Collins AL, Greenfield VY, Bye JK, Linker KE, Wang AS, Wassum KM. Dynamic mesolimbic dopamine signaling during action sequence learning and expectation violation. Sci Rep. 2016;6:20231. https://doi.org/10.1038/srep20231.
Crego A, Štoček F, Marchuk AG, Carmichael JE, van der Meer M, Smith KS. Complementary control over habits and behavioral vigor by phasic activity in the dorsolateral striatum. J Neurosci. 2020;40:2139–53. https://doi.org/10.1523/JNEUROSCI.1313-19.2019.
da Silva JA, Tecuapetla F, Paixao V, Costa RM. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature. 2018;554:244–8. https://doi.org/10.1038/nature25457.
Willuhn I, Burgeno LM, Everitt BJ, Phillips PE. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc Natl Acad Sci USA 2012;109:20703–8. https://doi.org/10.1073/pnas.1213460109.
Ostlund SB, Balleine BW. On habits and addiction: an associative analysis of compulsive drug seeking. Drug Disco Today Dis Models. 2008;5:235–45. https://doi.org/10.1016/j.ddmod.2009.07.004.
Lerner TN. Interfacing behavioral and neural circuit models for habit formation. J Neurosci Res. 2020;98:1031–45. https://doi.org/10.1002/jnr.24581.
Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. https://doi.org/10.1038/nn1579.
Pierce RC, Vanderschuren LJ. Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neurosci Biobehav Rev. 2010;35:212–9. https://doi.org/10.1016/j.neubiorev.2010.01.007.
Huh CY, Goutagny R, Williams S. Glutamatergic neurons of the mouse medial septum and diagonal band of Broca synaptically drive hippocampal pyramidal cells: relevance for hippocampal theta rhythm. J Neurosci. 2010;30:15951–61. https://doi.org/10.1523/JNEUROSCI.3663-10.2010.
Yoder RM, Pang KC. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus. 2005;15:381–92. https://doi.org/10.1002/hipo.20062.
Roland JJ, Stewart AL, Janke KL, Gielow MR, Kostek JA, Savage LM, et al. Medial septum-diagonal band of Broca (MSDB) GABAergic regulation of hippocampal acetylcholine efflux is dependent on cognitive demands. J Neurosci. 2014;34:506–14. https://doi.org/10.1523/JNEUROSCI.2352-13.2014.
Pang KC, Jiao X, Sinha S, Beck KD, Servatius RJ. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: effects on proactive interference. Hippocampus. 2011;21:835–46. https://doi.org/10.1002/hipo.20799.
Lecourtier L, de Vasconcelos AP, Leroux E, Cosquer B, Geiger K, Lithfous S, et al. Septohippocampal pathways contribute to system consolidation of a spatial memory: sequential implication of GABAergic and cholinergic neurons. Hippocampus. 2011;21:1277–89. https://doi.org/10.1002/hipo.20837.
Tsanov M. Speed and oscillations: medial septum integration of attention and navigation. Front Syst Neurosci. 2017;11:67. https://doi.org/10.3389/fnsys.2017.00067.
Bortz DM, Gazo KL, Grace AA. The medial septum enhances reversal learning via opposing actions on ventral tegmental area and substantia nigra dopamine neurons. Neuropsychopharmacology. 2019;44:2186–94. https://doi.org/10.1038/s41386-019-0453-1.
Bortz DM, Grace AA. Medial septum differentially regulates dopamine neuron activity in the rat ventral tegmental area and substantia nigra via distinct pathways. Neuropsychopharmacology. 2018;43:2093–100. https://doi.org/10.1038/s41386-018-0048-2.
Bortz DM, Grace AA. Medial septum activation produces opposite effects on dopamine neuron activity in the ventral tegmental area and substantia nigra in MAM vs. normal rats. NPJ Schizophr. 2018;4:17. https://doi.org/10.1038/s41537-018-0059-3.
Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–7. https://doi.org/10.1016/j.tins.2007.03.003.
Bunney BS, Grace AA. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci. 1978;23:1715–27.
Bortz DM, Grace AA. Medial septum differentially regulates dopamine neuron activity in the rat ventral tegmental area and substantia nigra via distinct pathways. Neuropsychopharmacology. 2018. https://doi.org/10.1038/s41386-018-0048-2.
Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons-1. Identif Charact Neurosci. 1983;10:301–15.
Ungless MA, Grace AA. Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35:422–30. https://doi.org/10.1016/j.tins.2012.02.003.
Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984;4:2866–76.
Perez SM, Chen L, Lodge DJ. Alterations in dopamine system function across the estrous cycle of the MAM rodent model of schizophrenia. Psychoneuroendocrinology. 2014;47:88–97. https://doi.org/10.1016/j.psyneuen.2014.05.005.
Grace AA. Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 2012;62:1342–8. https://doi.org/10.1016/j.neuropharm.2011.05.011.
Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–22.
Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–73. https://doi.org/10.1038/nn1103.
Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–61. https://doi.org/10.1038/sj.npp.1300963.
Chen CS, Ebitz RB, Bindas SR, Redish AD, Hayden BY, Grissom NM. Divergent strategies for learning in males and females. Curr Biol. 2020;31:39–50.e4. https://doi.org/10.1016/j.cub.2020.09.075.
Smith KS, Graybiel AM. Habit formation. Dialogues Clin Neurosci. 2016;18:33–43.
Malvaez M. Neural substrates of habit. J Neurosci Res. 2019. https://doi.org/10.1002/jnr.24552.
Lerner TN. The effortless custody of automatism. Science. 2018;362:169. https://doi.org/10.1126/science.aav1250.
Willuhn I, Burgeno LM, Groblewski PA, Phillips PE. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci. 2014;17:704–9. https://doi.org/10.1038/nn.3694.
Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–41. https://doi.org/10.1016/j.neuron.2007.12.019.
Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–82.
Saunders BT, Richard JM, Margolis EB, Janak PH. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat Neurosci. 2018;21:1072–83. https://doi.org/10.1038/s41593-018-0191-4.
van der Merwe R, Nadel J, Copes-Finke D, Pawelko S, Scott J, Fox M, et al. Characterization of striatal dopamine projections across striatal subregions in behavioral flexibility. bioRxiv. 2021. https://doi.org/10.1101/2021.09.18.460922.
Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2009;204:396–409. https://doi.org/10.1016/j.bbr.2008.12.001.
Izquierdo A. Functional heterogeneity within rat orbitofrontal cortex in reward learning and decision making. J Neurosci. 2017;37:10529–40. https://doi.org/10.1523/JNEUROSCI.1678-17.2017.
Radke AK, Kocharian A, Covey DP, Lovinger DM, Cheer JF, Mateo Y, et al. Contributions of nucleus accumbens dopamine to cognitive flexibility. Eur J Neurosci. 2019;50:2023–35. https://doi.org/10.1111/ejn.14152.
Klanker M, Fellinger L, Feenstra M, Willuhn I, Denys D. Regionally distinct phasic dopamine release patterns in the striatum during reversal learning. Neuroscience. 2017;345:110–23. https://doi.org/10.1016/j.neuroscience.2016.05.011.
Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW, et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry. 2011;168:718–26. https://doi.org/10.1176/appi.ajp.2011.10071062.
Gillan CM, Morein-Zamir S, Urcelay GP, Sule A, Voon V, Apergis-Schoute AM, et al. Enhanced avoidance habits in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:631–8. https://doi.org/10.1016/j.biopsych.2013.02.002.
Voon V, Derbyshire K, Rück C, Irvine MA, Worbe Y, Enander J, et al. Disorders of compulsivity: a common bias towards learning habits. Mol Psychiatry. 2015;20:345–52. https://doi.org/10.1038/mp.2014.44.
Hildebrandt BA, Ahmari SE. Breaking it down: investigation of binge eating components in animal models to enhance translation. Front Psychiatry. 2021;12:728535. https://doi.org/10.3389/fpsyt.2021.728535.
Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci. 2008;31:415–37. https://doi.org/10.1017/S0140525X0800472X.
Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. https://doi.org/10.1016/j.bbr.2008.09.027.
Acknowledgements
We would like to thank Dr. Stan Floresco for his help in creating the strategy switching task, as well as his continued guidance and support.
Funding
This work was funded by NIMH grants 1F32MH115550 (DMB) and MH057440-11 (AAG).
Author information
Authors and Affiliations
Contributions
DMB and AAG planned all experiments. DMB, CMF, and CCP performed all experiments. All authors participated in manuscript writing and editing.
Corresponding author
Ethics declarations
Competing interests
AAG received funds from the following organizations: Lundbeck, Pfizer, Otsuka, Asubio, Autofony, Janssen, Alkermes, SynAgile, and Newron. DMB, CMF, and CCP declare no competing financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bortz, D.M., Feistritzer, C.M., Power, C.C. et al. Medial septum activation improves strategy switching once strategies are well-learned via bidirectional regulation of dopamine neuron population activity. Neuropsychopharmacol. 47, 2090–2100 (2022). https://doi.org/10.1038/s41386-022-01387-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01387-1