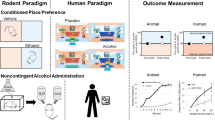
Abstract
Alcohol use disorder (AUD) is a significant public health concern, contributing to a myriad of social, psychological, and physiological issues. Despite substantial efforts within the alcohol research field, promising preclinical findings have failed to translate to clinical use, highlighting the necessity to develop safe and effective pharmacological probes with the ability to be used in preclinical and clinical research. Yohimbine, an α2 adrenergic receptor antagonist, is a well-validated pharmacological tool that has been widely employed in alcohol studies to evaluate noradrenergic activation. This scoping systematic review examines published literature in rodent and human studies involving the use of yohimbine relevant to alcohol research. We conducted a systematic literature review of MEDLINE, Embase, Web of Science Core Collection, CINAHL, PsycInfo, and Cochrane Central Register of Controlled Trials to identify: (1) Experimental Characteristics and Methodology, (2) Sex Differences, (3) Neurochemical Systems and Brain Regions, and (4) Discussion of Applications for Medication Development. Sixty-seven (62 preclinical and 5 clinical) studies were identified meeting the stated criteria, comprising extensive evidence supporting the use of yohimbine as a safe, titratable pharmacological agent for translational alcohol research. Support for the use of yohimbine as a fully translational tool, however, is hindered by limited available findings from human laboratory studies, as well as a dearth of studies examining sex differences in yohimbine’s mechanistic actions. Additional consideration should be given to further translational modeling, ideally allowing for parallel preclinical and clinical assessment of yohimbine, methodological assessment of neurochemical systems and brain regions.
Similar content being viewed by others
Introduction
An estimated 35.9 million adults per year suffer from alcohol use disorder (AUD) in the United States [1]. Alcohol research has found that dysregulation of the stress system greatly contributes to the reinforcement of alcohol-related behaviors [2, 3]. Currently, there are no FDA-approved medications that target the stress system [4], however, pharmacotherapies targeting the noradrenergic system have been suggested as promising AUD therapeutic interventions [5,6,7].
Activation of the noradrenergic system has been implicated as a component of stress-induced anxiety [8] and alcohol-seeking behaviors [5, 9,10,11,12]. Yohimbine, an α2 adrenergic receptor antagonist, is commonly utilized in alcohol research to pharmacologically probe the noradrenergic system in preclinical [13,14,15,16] and in human laboratory studies [17, 18]. Importantly, the drug increases peripheral [17] and central [13] noradrenergic activity. As yohimbine can be safely administered in animals and humans, it has the potential to be a strong translational pharmacological probe in alcohol research. There are, however, several underdeveloped parameters that may account for reducing the translational efforts of the yohimbine for probing the noradrenergic systems across species. Two major limitations include: the lack of inclusion of sex as a biological variable (SABV) [19] and limited studies examining brain functioning after peripheral yohimbine administration [20, 21].
Although sex differences within alcohol-related behaviors [22, 23], AUD [24], and noradrenergic activation [25, 26] have been well-established, very few studies using yohimbine utilized females, or had the power to include SABV in the analysis. Preclinical and clinical studies suggest that sex differences are largely important in the regulation of alcohol-related behaviors, suggesting that SABV should be investigated in the noradrenergic regulation of AUD [23, 27, 28]. Similarly, although yohimbine has been utilized as a probe to evaluate the response in many brain regions associated with reinstatement of alcohol seeking and consumption in rodents, there are limited investigations that examined the central effect of yohimbine within a clinical context.
The goal of this systematic review is to evaluate published yohimbine literature in alcohol studies, with particular emphasis on findings that inform yohimbine’s peripheral administration on neural mechanistic actions, and assess the value of yohimbine as a translational pharmacological probe for AUD research. We included primary literature of AUD preclinical models and randomized controlled trials (RCTs) involving yohimbine as a pharmacological probe for noradrenergic activation, and provided a detailed overview of: (1) Experimental Characteristics and Methodology, (2) Sex Differences, (3) Neurochemical Systems and Brain Regions, and (4) Discussion of Applications for Medication Development.
While yohimbine has been extensively utilized within animal and human alcohol research for decades, to our knowledge, a systematic review comprehensively assessing the experimental characteristics and yohimbine-related findings to improve translational efforts has not been conducted. We hypothesize that yohimbine is a strong, safe, and titratable translational pharmacological probe, that despite extensive characterization in preclinical models, has not been thoroughly examined as a translational probe for alcohol human laboratory studies. The results from this study will inform future research on the yohimbine’s cross species properties, to improve its use as a translational pharmacological tool to employ in alcohol research with the purpose of facilitating the development of novel pharmacotherapies for AUD.
Methods
The systematic scoping review design was reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [29], and the plan was pre-registered with Open Science Frame (OSF, https://osf.io/6vjzd).
Search methods
A literature search was conducted by the Brown University librarians, who constructed comprehensive search strategies in MEDLINE, Embase, Web of Science Core Collection, CINAHL, PsycInfo, and Cochrane Central Register of Controlled Trials. The search strategies used a combination of controlled vocabulary terms and keywords to describe two parameters: “yohimbine” AND “alcohol OR ethanol”. Searches were conducted on February 8th, 2021 and updated on November 15th, 2021. All retrieved studies were exported to EndNote for Windows version X9.3.3 (Clarivate Analytics), and duplicates were removed [30]. The de-duplicated results were uploaded to Covidence for screening and were subsequently reviewed by two independent reviewers in accordance with the PRISMA [29]. Detailed descriptions of search methods are included within the Supplementary Materials.
Eligibility criteria and study selection
We included in vivo preclinical studies and RCTs that administered yohimbine to probe the noradrenergic system to study alcohol-related behaviors. Relevant studies were first screened by the title and abstract and we applied the following criteria: abstract written in English and study conducted in rodents or humans. If criteria determination could not be made based on the title and abstract, the full text of the study was reviewed. Studies were excluded for the following reasons: (1) not reported in English, (2) duplicate study results, (3) not an original study (i.e., review), (4) alcohol/ethanol not studied, (5) incorrect drug used (i.e., not yohimbine), (6) yohimbine not administered in a laboratory paradigm of alcohol research, and (7) not peer-reviewed manuscripts. Title and abstract screening, full text review, and data extraction were completed by two independent reviewers (DEC and TRV-K), with discrepancies resolved by two consensus reviewers for the rodent studies (RC, NC) and one consensus reviewer (CLH-K) for clinical studies. Full description of study inclusion/exclusion criteria are shown within the PRISMA flow diagram (Fig. 1).
A systematic literature search of MEDLINE, Embase, Web of Science Core Collection, CINAHL, PsycInfo, and Cochrane Central Register of Controlled Trials was conducted on February 8th, 2021, and updated on November 15th, 2021. A combination of controlled vocabulary terms and keywords were used to describe two parameters: “yohimbine” AND “alcohol OR ethanol”. This produced 66 studies, with one additional study meeting the criteria was identified during peer review (this study was not returned through the systematic literature search as it did not contain the search terms within the abstract/title [32]). Studies were excluded for the following reasons: (1) study not reported in English, (2) duplicate study results, (3) not an original study (i.e., review), (4) alcohol/ethanol not studied, (5) incorrect drug used (i.e., not yohimbine), (6) yohimbine not administered in a laboratory paradigm of alcohol research, and (7) not peer-reviewed manuscripts.
Study characteristics assessments and outcomes
For all studies, we reported: experimental paradigm, yohimbine dose and route of administration, central nervous system (CNS) response, neurochemical systems and brain regions probed, and sex differences. For rodent models, we also reported: rodent strain and genetic background, (Supplementary Table S1) and for human studies, participant description and AUD diagnosis (Supplementary Table S2).
Risk of bias assessments
Risk of bias assessments were conducted by two independent reviewers (DEC and TRV-K), with discrepancies resolved by consensus. Bias in rodent studies was assessed by examining allocation concealment and randomization, blinding, inclusion and exclusion criteria, sample size, ethical compliance, and statistical methods. Assessment findings were reported simply as “Yes” if the criteria were met and reported, or “No” (Table 1) [31]. RCTs were assessed for selection, performance, detection, attrition, reporting biases, and described as high-risk, low-risk, or unclear (Table 2) [29].
Results
Of the 1135 articles initially identified in the literature search, 67 studies satisfied the criteria for inclusion in this systematic review after full-text screening, including one additional preclinical study which was identified during peer review [32]. Sixty-two studies were conducted in rodents, and five studies were on humans (PRISMA diagram, Fig. 1). Excluded studies that were identified prior to the screening process are also reported in the (Supplementary Table S3).
Preclinical studies
Experimental characteristics and methodology
Of the 62 studies conducted in rodents, 53 included rats and nine studies included mice. Rodents of various genetic backgrounds were broadly categorized as alcohol-preferring (AP) or not. Of the studies utilizing rodents not genetically selected to prefer alcohol: eight used Sprague-Dawley [16, 33,34,35,36,37,38,39], 12 Wistar [14, 16, 40,41,42,43,44,45,46,47,48,49], and 12 Long-Evans [13, 50,51,52,53,54,55,56,57,58,59,60] rats; two used Swiss-Webster [61, 62], three Albino [63,64,65], three C57/BL6 [66,67,68] mice. Studies involving AP rodents: nine used Marchigian Sardinian Alcohol-Preferring (msP) rats [32, 69,70,71,72,73,74,75,76], five inbred alcohol-preferring (iP) rats [77,78,79,80,81], and one used rats descended from Finnish AA line [82]. Several studies utilized two rodent strains: two Wistar and Long-Evans rats [83, 84]; two studies had a non-preferring line as controls: one Wistar and alcohol-preferring (P) [85], and one Wistar and msP [86] rats; one study used two AP lines: one P and HAD-2 rats [15]; one used Long-Sleep and Short-Sleep mice [87]. Only one study did not specify the strain of the rats used [88] (Supplementary Table S1).
In most rodent studies (N = 59), yohimbine was administered through intraperitoneal (IP) injection, with dose from 0.001 mg/kg [88] to 10 mg/kg [64, 88] (Fig. 2). Other methods of yohimbine injection include subcutaneous (SC, 2 mg/kg) [82], intravenous (IV, 1 mg/kg) [39], and intracerebroventricular (ICV, 1 μg) [87] administration. Fifteen experiments investigated multiple doses of yohimbine [15, 33, 42, 44, 46, 59, 61,62,63, 65, 68, 84,85,86, 88] (Supplementary Table S2).
Yohimbine was primarily administered intraperitoneally (IP) in the 59 studies included in this figure. Three studies, not included in this figure administered yohimbine in the following dosages and methods: subcutaneous injection of 2 mg/kg [82], venous cannula injection of 1 mg/kg [39], and intracerebroventricular injection of 1 μg [87].
Yohimbine was primarily used to investigate the impact of noradrenergic activation on alcohol self-administration and reinstatement of alcohol seeking.
Sex differences
Of the 62 rodent studies, only seven included females [33, 35, 63, 68, 70, 76, 82], and only four examined sex differences [33, 35, 68, 70]. Alcohol self-administration was included in the studies, however, yohimbine administration was tested only in reinstatement of alcohol seeking.
In Sprague-Dawley rats, chronically exposed to corticosterone in adolescence, females were more sensitive to yohimbine-induced reinstatement than males [33]. Additionally, in a yohimbine administration paired to alcohol-related cues paradigm, females showed enhanced reinstatement of alcohol seeking compared to males [33]. However, chronic and acute hormone manipulation (female: estradiol and male: testosterone) did not affected the yohimbine + cue-induced reinstatement of alcohol seeking [35]. Recently, it was reported that in msP rats, females exhibited more pronounced yohimbine-induced reinstatement of alcohol seeking compared to males [70]. In C57BL/6 J mice, yohimbine-induced reinstatement of alcohol seeking was reduced by IP oxytocin, both in male and females [68].
Neurochemical systems and brain regions
Extensive rodent work utilized yohimbine to study neurochemical and receptor systems: corticotropin releasing factor (CRF), opioid, neurokinin1 (NK1), peroxisome proliferator-activated receptor γ (PPARγ), endocannabinoid, and orexin. Additionally, several studies included analysis of multiple brain regions evaluated by Fos expression profiles.
CRF system and hypothalamic–pituitary–adrenal (HPA) axis activation
A wealth of studies demonstrated that yohimbine-induced increase of alcohol drinking and seeking is associated with mechanisms involving the activation of the CRF system [14, 36, 45, 52, 58, 67, 78, 86, 89]. Administration of yohimbine significantly increases expression of CRF mRNA in the dorsal region of the bed nucleus of the stria terminalis (BNST) and the central nucleus of the amygdala (CeA) of Wistar rats [89], and the paraventricular nucleus (PVN) of C57BL/6 J mice [67]. Downstream of the HPA axis, administration of yohimbine increased plasma corticosterone levels in the alcohol-trained animals compared to controls [58]. Yohimbine administration was tested both in alcohol-self administration and reinstatement of alcohol seeking.
Self-administration
Studies in Long-Evans rats trained to self-administer alcohol, reported that viral-mediated downregulation of CRF binding protein (CRFBP) in the CeA reduced alcohol consumption compared to controls, however this manipulation was not sufficient to attenuate yohimbine-induced increases in alcohol self-administration [52]. Pretreatment with the CRF1 receptor antagonist, antalarmin, blocked yohimbine-induced increases in alcohol self-administration in both Wistar [14, 86] and msP rats [86].
Reinstatement
Yohimbine-induced reinstatement of alcohol seeking was attenuated by infusions of d-Phe CRF (non-specific CRF receptor antagonist) into the median raphe nucleus of Wistar rats [45]. In iP rats, yohimbine-induced reinstatement of alcohol seeking was reduced by infusions into the nucleus incertus (NI) of CP376395 (CRF1 receptor antagonist), but not by astressin-2B (CRF2 receptor antagonist) [78]. Glucocorticoids, that are downstream key regulators of the CRF system, were also investigated [13, 34] through yohimbine-induced reinstatement of alcohol seeking. In Long-Evans rats, systemic and intra-CeA, but not intra-basolateral amygdala (BLA), infusions of mifepristone (glucocorticoid receptor antagonist) suppressed yohimbine-induced reinstatement of alcohol seeking without changing plasma corticosterone level [13]. Administration of corticosterone in adolescent Sprague-Dawley rats was shown to enhance yohimbine stress-induced reinstatement in alcohol-reinforced rats, compared to controls [34]. Also, intra-BLA infusion of a G-protein coupled receptor kinase 2 (GRK2) inhibitor reduced yohimbine-induced alcohol seeking [34]. The only mouse study using yohimbine to probe the role of the CRF system in alcohol seeking indicated that overexpression of CRF1 receptor in calcium-calmodulin-dependent kinase II (αCaMKII) neurons in the CeA increased yohimbine-induced reinstatement of alcohol seeking [36].
Opioid, neurokinin, PPARγ, orexin, and endocannabinoid system
Within the investigation of the opioid, neurokinin, PPARγ, orexin, and endocannabinoid system, yohimbine administration was tested only in reinstatement of alcohol seeking.
Opioid
Naltrexone (non-selective opioid receptor antagonist) did not significantly impact yohimbine-induced reinstatement in msP rats [74]. However, studies conducted in Long-Evans rats indicated that pretreatment with nor-BNI (κ-opioid) [50], and SoRI-9409 (δ-opioid) [53] receptor antagonists, reduced yohimbine-induced reinstatement without increasing plasma corticosterone levels [53]. Yohimbine-induced reinstatement of alcohol seeking was significantly blocked by administration of MT-7716 (potent and selective NOP receptor agonist) in msP rats [32], and by administration of SR-8993 (NOP receptor agonist) in Wistar rats [40]. Intriguingly, it was later reported that LY2940094 and LY2817412 (potent and selective NOP antagonists), also attenuated yohimbine-induced reinstatement of alcohol seeking in msP rats [70, 76]. LY2817412 was efficacious when administered in the ventral tegmental area (VTA), and CeA, but not the accumbens (NAc), both in male and female msP rats [70].
Neurokinin
Consistent with literature linking the NK1 receptor system to the motivation for alcohol, it was found that administration of NK1 receptor antagonists L822429 reduced yohimbine-induced reinstatement of alcohol seeking in msP and Wistar rats [69], [47]. Similarly, in Long-Evans rats, L822429 attenuated yohimbine-induced reinstatement of alcohol seeking, but not alcohol intake [84]. Interestingly, in P rats (with an innate upregulation of the NK1 system), intra-CeA infusion of L822429 attenuated reinstatement of alcohol seeking [85]. Whereas, in Wistar rats viral-mediated overexpression of NK1 in the CeA enhanced the sensitivity to yohimbine-induced increases in alcohol drinking and seeking [85].
PPARγ
The PPARγ pathway was examined in msP rats in four studies utilizing yohimbine with the administration of pioglitazone (selective PPARγ agonist) [71, 72, 74], and the extracts from andrographis paniculata plant and andrographolide (its major active compound) [75]. Pioglitazone attenuated yohimbine-induced reinstatement of alcohol seeking, but not by alcohol cue [72, 74]. Pioglitazone’s actions were shown to be region specific in the CeA, VTA, RMTg, but not the NAc shell, injections of pioglitazone inhibited yohimbine-induced reinstatement [71]. Finally, extracts from andrographis paniculata plant and andrographolide also significantly reduced yohimbine-induced reinstatement [75].
Orexin
First, it was shown that in iP rats, orexin neurons in the NI were activated by yohimbine-induced reinstatement of alcohol seeking [77]. Then, NI bilateral microinjections of TCS-OX2-29 (orexin2 receptor antagonist) attenuated yohimbine-induced reinstatement of alcohol seeking, while intra-NI injection SB-334867 (orexin 1 receptor antagonist) showed no significant effect [77]. Systemic administration of SB-334867 in Long-Evans rats similarly attenuated yohimbine-induced reinstatement of alcohol seeking [54].
Endocannabinoid
CB1-receptor-mediated activity was investigated using a selective fatty acid amide hydrolase (FAAH) inhibitor (URB597), which had no effect on yohimbine-induced relapse in Wistar rats [49].
Brain regions
Several studies examined yohimbine-induced changes in Fos expression throughout the brain [16, 41, 67, 81, 89] in different rat strains and mostly evaluated in reinstatement of alcohol seeking induced by yohimbine. Only one study evaluated both yohimbine-induced alcohol self-administration and then reinstatement induced by yohimbine after extinction of the operant response. In Wistar rats, dopaminergic and noradrenergic neuron lesions of the dorsal or ventral NA bundles by neurotoxin 6-hydroxydopamine (6-OHDA) did not impact yohimbine-induced alcohol self-administration or reinstatement [42].
In iP rats, Fos immunohistochemistry analysis across 42 brain regions following yohimbine-induced reinstatement of alcohol seeking showed that the yohimbine robustly activated neurons in the prefrontal cortex (PFC), extended amygdala, hypothalamus, thalamus, and the hippocampus [81]. In another study, yohimbine-induced reinstatement of alcohol seeking increased Fos activation in the CeA, which was attenuated by intra‐CeA injections of a selective Relaxin Family Peptide Receptor 3 antagonist (R3(B1‐22)R) [79]. Yohimbine-induced reinstatement, also led to increased Fos expression in cocaine and amphetamine regulated transcript (CART) containing neurons in the capsular (CeC) and lateral (CeL) part of the CeA [80]. Yohimbine-induced reinstatement of alcohol seeking was attenuated by direct injection of the neutralizing CART antibody into the CeA [80].
In Wistar rats yohimbine-induced neuronal activation in the CeA and NAc, but not in the BLA [41]. In situ hybridization analysis showed that yohimbine significantly increased cFos expression in brain regions associated with alcohol and drug seeking, such as the anterior cingulate cortex (ACg), orbitofrontal cortex (OFC), NAc core, shell, dorsal and ventral BNST, PVN, BLA, and CeA [89]. Many of those brain regions overlapped in C57BL/6 J mice [67].
In Long-Evans rats, yohimbine blocked alcohol-induced cFos expression in the Edinger-Westphal nucleus in a dose-dependent manner [66]. Additional studies investigating the role of yohimbine in discrete brain regions included electrolytic and chemical lesioning. Lesions in the lateral habenula were found to block yohimbine-induced reinstatement [51]. Whereas lesions of the RMTg had no significant impact on yohimbine-induced reinstatement of alcohol seeking [56].
Finally, in Sprague-Dawley rats, yohimbine significantly increased Fos immunoreactive expression in the NAc [16], while injection with prazosin, an α1 antagonist [16], prior to yohimbine resulted in significantly fewer Fos-immunoreactive cells in the NAc.
Medication development
Several drugs targeting the noradrenergic system were investigated in conjunction with yohimbine to evaluate both in alcohol-self administration and reinstatement.
Self-administration
In Wistar rats, baclofen (γ-aminobutyric acid (GABA)-B agonist) blocked yohimbine-induced increases in alcohol self-administration [48], and WAY 100,635 (selective serotonin (5-HT) 1 A receptor antagonist) reduced yohimbine-induced self-administration [42]. Clonidine (non-selective α2 receptor agonist), however, was not sufficient to attenuate yohimbine-induced increases in alcohol self-administration [42].
Reinstatement: Prazosin (α1 receptor antagonist) blocked yohimbine-induced reinstatement of alcohol seeking, and guanfacine (α2 A receptor agonist) successfully attenuated yohimbine-induced reinstatement of alcohol seeking both in Wistar and Long-Evans rats [83]. The yohimbine-induced reinstatement of alcohol seeking was successfully reversed by administration of clonidine in: Wistar rats [42, 46], selectively bred Long-Sleep and Short-Sleep mice [87], alcohol-preferring Finnish AA rats [82], and Sprague-Dawley rats [38]. WAY 100,635 also attenuated yohimbine-induced reinstatement of alcohol seeking [42]. Systemic and intra-CeA infusion of mifepristone, suppressed yohimbine-induced reinstatement of alcohol seeking in Long-Evans [13]. Finally, the administration of AP-202 (selective nicotinic α4β2-receptor antagonist), was unable to reverse yohimbine-induced reinstatement of alcohol seeking in Sprague-Dawley rats [37].
Clinical studies
Experimental characteristics and methodology
Individuals with diagnosis of AUD were included in the RCTs analyzed, except in one study in which all participants were healthy individuals [17]. Three RCTs [90,91,92] were conducted in an inpatient setting with the same 22 patients, from the West Haven Veterans Administration Medical Center and one with 25 patients at the NIH Clinical Research Center [18]. Two RCTs [91, 92] included control subjects. Ethnic background (56% white) of the participants were only provided in one study [18]. The mean age ranged from 39 [90,91,92] to 44 [18] years. None of the RCTs administered yohimbine combined to a behavioral paradigm (e.g., alcohol self-administration or cue reactivity). Nor did any study use yohimbine for brain imaging or medication development. The route of administration of yohimbine was reported in all RCTs (IV, 0.4 mg/kg for 10 min) [17, 18, 90,91,92].
Sex differences
Two clinical studies included women; a study that evaluated the craving response (without cue reactivity) after yohimbine challenge (N = 24, 12.5% women) [18] and a study that evaluated the co-administration of alcohol and yohimbine on severity of acute intoxication (N = 12, 71% women) [17]. However, neither of these studies addressed sex differences. The remaining three RCTs analyzed were conducted with only men [90,91,92].
Neurochemical systems and brain regions
Within the RCTs examined, no study utilized imaging paradigms to investigate the brain activities following a pharmacological challenge with yohimbine. Similarly to the rodent studies, plasma cortisol levels (in four out of the five studies) were measured to determine the impact of yohimbine on the downstream of HPA axis [17, 18, 90, 91]. Administration of yohimbine robustly activated the HPA axis, with increase of cortisol level both in individuals with AUD [17, 18, 90, 91] and controls [17], as well as increased anxiety [17, 90, 91], and alcohol craving [18]. Only one study [18] demonstrated that yohimbine had an effect on alcohol craving on patients going through withdrawal.
Medication development
In a RCT conducted in treatment seeking individuals with AUD, administration of yohimbine was compared to the serotonergic compound meta-chlorophenylpiperazine (mCPP), and placebo (double blind, counterbalanced) to assess the effect of acamprosate administered for 14 days on craving. Both yohimbine and mCPP successfully induced craving compared to placebo, with stronger effect in patients with severe AUD [18]. The study did not observe the effect of acamprosate in reducing craving in this experimental condition.
Safety and tolerability
No serious adverse reactions to yohimbine administration were reported in any rodent or human studies. Three RCTs [17, 90, 91] examined the effect of yohimbine on hemodynamic parameters, with increase of systolic and diastolic blood pressure and heart rate [91]. Four of the five studies [17, 18, 90, 91] measured yohimbine’s effect on anxiety and stress related feelings, with yohimbine either increasing nervousness [91] and anxiety [17] or having no significant effect [18].
Risk of bias assessments
The rodent studies on average met six out of the 13 criteria to assess bias. Selection bias for all five human studies was determined to be low-risk, as all studies were randomized, and allocation was concealed (Table 2). Literature sources, process, and limitations are fully described in the Supplementary (Tables S2, S3, and S4).
Discussion
In the alcohol field, yohimbine as a pharmacological agent to investigate the noradrenergic system has been employed in numerous studies with greatly varied aims and methodology. To our knowledge, this is the first systematic review evaluating primary literature on yohimbine in alcohol research, in rodents and humans. Sixty-two studies in rodents and five RCTs have been evaluated. Summarized below are key findings, and assessment of limitations to inform future research using yohimbine and improve the robustness of translational efforts and alignment of findings.
Main findings
Yohimbine is a well validated preclinical pharmacological probe for AUD research
The evaluated Experimental Characteristics and Methodology Results of this review showed that extensive preclinical research supports yohimbine as an efficacious pharmacological probe for the activation of noradrenergic system to investigate alcohol-related behaviors. There are however, limited human laboratory studies that have implemented similar research endeavors.
First, recent advocacy for preclinical models that have a higher capability for clinical translation is reflected in the studies analyzed in this review, which included rodent models genetically predisposed to consume alcohol. It has been well established that risk for AUD in humans has significant genetic contributions, which supports the necessity of preclinical models in AUD research that more closely model genetic contributions to the disorder [93, 94]. Only two studies included in this review directly compared yohimbine’s actions in an experimental paradigm between AP (AUD model) and not genetically-selected rats (control) [85, 86], and one study compared two AP strains [15].
In the highly characterized msP rats line, researchers extended the evaluation of yohimbine effect to specifically test the two msP-derived lines carrying the wildtype (GG) and the point mutation (AA) in the CRFR1 causing the overexpression of this receptor [86]. Administration of multiple doses of yohimbine, elicited increased operant alcohol self-administration and reinstated alcohol seeking in both AA and GG msP rats and in Wistar controls. Interestingly, however, the highest dose tested (2.5 mg/kg) failed to reinstate alcohol seeking in AA msP rats [86]. Further, despite earlier studies indicating P rats express greater sensitivity to alcohol seeking and consumption, a study within the review, showed that yohimbine elicited reinstatement of alcohol seeking in both in P and HAD-2 rats, however the HAD-2 rodents consumed more alcohol [15]. Similarly, studies using yohimbine have been conducted in different mice strains. Yohimbine activated the HPA axis response in Long-Sleep mice, as indicated by increased plasma corticosterone levels, but not in Short-Sleep mice; however, yohimbine did not alter alcohol-induced adrenocortical responses in either line [15]. The only study that showed specific differences between AP and Wistar rats reported that after yohimbine administration, P rats showed increased CeA neuronal activation and higher sensitivity to yohimbine-induced reinstatement compared to Wistar controls [85]. Overall, findings from reviewed studies generally support that yohimbine exerts similar actions in AP and control rodents in inducing alcohol self-administration and reinstatement of alcohol seeking (although in rats with an overactive CRF system, yohimbine seems to work at lower doses) [86], with one study observing greater yohimbine sensitivity in P rats compared to Wistar [85].
Regarding the study population in human laboratories, two clinical studies included individuals with AUD and healthy controls [91, 92]. In both studies, both groups showed similar responses to yohimbine, as measured through hemodynamic parameters. However, yohimbine-induced changes in plasma cortisol, prolactin, and plasma 3-methoxy-4-hydroxyphenylglycol (MHPG) were greater in individuals with AUD compared to healthy controls. From translational perspective, the cortisol effect is in line with preclinical data that have shown that administration of yohimbine increased plasma corticosterone levels in the alcohol-trained animals compared to controls [58].
Unfortunately, alcohol behavioral paradigms paired with yohimbine administration have been extensively investigated only in preclinical research. To this end, there are two ongoing RCTs that are examining the effect of yohimbine paired with alcohol cue reactivity, in conjunction with pharmacological treatment of an α1 receptor blocker (doxazosin: NCT02243709) and a glucocorticoid receptor blocker (mifepristone: NCT04135846).
Administration of yohimbine in preclinical studies was primarily through IP injections, while all RCTs administered yohimbine through IV. Only rodent models allow for ICV administration of yohimbine, which is an administration method essential for evaluating the mechanistic effects of the drug within the CNS, and no rodent or human study administered yohimbine orally. In preclinical models, yohimbine was most often utilized at a dosage of 1.25 mg/kg, with dosages ranging from 0.0001–10 mg/kg. Several studies comparing multiple doses of yohimbine similarly found that the dose of 1.25 mg/kg was ideal to produce the desired yohimbine-induced reinstatement of alcohol seeking within AP and non-preferring rodent strains [15, 33, 42, 44, 59, 84, 85]. Conversely, only one dosage of yohimbine, 0.4 mg/kg, was investigated in the clinical studies, highlighting the need for investigation of various doses within RCTs. From a translational perspective, a study on oral yohimbine in non-human primates showed increase in motor activation and affective response, but not in a dose-response manner [95]. Yohimbine oral dose of 32.4 mg is currently under investigation in the two RCTs mentioned above (NCT02243709 and NCT04135846).
Sex differences exist in yohimbines effects on alcohol-related behaviors
Numerous preclinical and clinical studies have indicated that sex differences exist in AUD and alcohol-related behaviors [24, 28, 96, 97], particularly in stress-related alcohol consumption [23, 98]. The three studies within this review that examine sex differences support previous evidence indicating that differences exist in yohimbine effects on alcohol-related behaviors [33, 35, 68], with female rodents exhibiting greater baseline susceptibility compared to males, and increased sensitivity to yohimbine-induced reinstatement of alcohol seeking, as well as other drugs [99,100,101].
There is also a dearth of clinical research examining sex differences in the utilization of yohimbine within human AUD research. Although no analysis of sex differences was conducted in any studies that met the criteria for the systematic review, two studies [17, 18] included female participants. However, the sample size was not large enough to analyze SABV. Currently there are several active clinical trials utilizing yohimbine in healthy individuals (NCT04180969) and other substance use disorders (NCT04231708, NCT04051619, NCT04181515), which may be able to further elucidate the role that sex differences play in yohimbine’s mechanistic actions.
From translational perspective, studies examining cocaine use disorder, found that high levels of endogenous progesterone attenuate drug cues after yohimbine administration in women, compared to women with low progesterone levels [102, 103]. These findings taken together illustrate the need for future research to include SABV, specifically in the investigation of stress-induced alcohol-related behaviors, and further examine sex differences within preclinical and clinical AUD models to better inform personalized medicine approaches.
Neurochemical systems and brain regions
Numerous neurochemical and receptor systems have been examined within the context of stress and alcohol-related behaviors, with this relationship further explored in several notable reviews [104,105,106]. It has been shown that chronic alcohol treatment selectively increases α2 adrenergic receptor densities [107]. Rodent studies, however, have reported conflicting results on the receptor expression at peripheral and central level. For example, IP yohimbine administration in male Wistar-Kyoto rats increases α2 receptor density in the kidneys [108], but ICV injection of yohimbine in male Wistar rats decreases α2 receptor density in hippocampal neurons [109]. Future studies, however, could further elucidate this relationship by examining yohimbine-induced alterations in α2 receptor density within an alcohol research paradigm.
Additionally, the HPA axis response in animal models (corticosterone) has been closely aligned with the neuroendocrine response (cortisol) in individuals with AUD [110,111,112]. As shown in the included studies, yohimbine administration in both preclinical and clinical settings demonstrated increases in corticosterone and cortisol levels respectively. Interestingly, it has been suggested that yohimbine’s effects on alcohol-related behaviors are independent of yohimbine’s actions on the HPA axis [14], which was further supported through findings in another study, which proposed that the primary action of yohimbine in reinstatement studies is to increase salience of the selected cues [113]. Taken together, those neuroendocrine results, support the translational efforts of measuring glucocorticoids response both in preclinical and clinical studies, ideally using aligned paradigms. In fact, studies evaluating the relationship between the HPA axis’s effect on increasing salience of alcohol cues and noradrenergic activation by yohimbine may utilize these peripherally measured glucocorticoids to elucidate the CNS mechanistic actions, which are extremely difficult to study in human laboratory studies.
Medication development
The noradrenergic system, with projections through key limbic and forebrain areas involved in the arousal, reinforcement, and stress processes involved with the development and maintenance of AUD [114], has been proposed as a pharmacological target for the stress contributing to AUD [5, 115].
Under normal conditions, noradrenergic activation has been associated with the regulation of stress, anxiety, vigilance, and arousal through the release of norepinephrine from the locus coeruleus [5, 116]. However, in individuals with AUD, noradrenergic dysregulation resulting in elevated norepinephrine levels has been suggested as a contributor to alcohol-related behaviors, like alcohol craving and consumption [115, 117]. Further, preclinical [16, 83] and clinical [118,119,120,121] AUD studies utilizing α1 noradrenergic receptor antagonists have been effectively used to reduce alcohol consumption and seeking. Thus, targeting of the noradrenergic system with yohimbine to increase available norepinephrine provides researchers with a valuable pharmacological tool to study the role of the noradrenergic system within AUD. One RCT was the first to utilize yohimbine and compare to mCPP, and placebo to challenge the FDA-approved medication for AUD, acamprosate [18], highlighting the importance of understanding the craving component and noradrenergic activation in developing pharmacotherapies for AUD [122].
Rodent models have provided the building blocks for AUD medication development. Therefore, investigating interspecies differences between human and rodent α2 adrenoceptor genetic variation is critical to fully utilize yohimbine as translational tool. Studies evaluating the effects of mutations on ligand selectivity in mouse and human α2A receptors demonstrated that point mutation at the extracellular loop impacts the binding preferences of yohimbine for the human (K1 increased 6-fold) and mouse α2A receptors [123]. In terms of physiological response, however, human research showed that genetic variation of α2 receptor loci (α2A, α2B, and α2C), did not affect yohimbine-induced increase in blood pressure [124].
Limitations
The wide variety in preclinical rodent models used to study the effect of yohimbine on alcohol drinking and seeking limits the generalizations of the findings highlighted by the present analysis. It is important to acknowledge that yohimbine, as pharmacological stressor has also been utilized in preclinical [125] and clinical [126, 127] setting outside the AUD field with translational aspect that are not reported here (not AUD research).
Limited preclinical investigation of SABV and a complete lack of clinical studies examining sex differences represents a significant limitation of yohimbine-related AUD research. Additionally, generalization of the clinical findings to the overall population is hindered in that three of the five RCTs were conducted within the exact same participant group [90,91,92]. In the preclinical studies examined, very few studies looked at neural network analysis or imaging. This made it difficult to examine the actions of peripheral yohimbine administration in the brain.
Also, there are seminal studies on yohimbine that were not included in this systematic review because they did not meet the inclusion criteria (e.g., paradigm not tested for an alcohol outcome) that provide additional information for future research. For example, a study evaluating the dose-response of yohimbine’s effects on reinstatement of food/drug seeking and operant self-administration, demonstrated that yohimbine’s effect may be independent of the food/drug reward during training sessions, and therefore may not be stress-associated pathways [113].
Translational summary
Despite the reported safety and tolerability data and the common exploration of alcohol research domains (consumption, craving, and neural response), none of the reviewed studies included a translational paradigm across preclinical and clinical setting. Despite the several alignments between rodent and human studies (e.g., corticosterone/cortisol; AUD models and AUD diagnosis), the lack of integration between preclinical and clinical paradigms provided fragmented and often incomplete results. Experimental characteristics and methods with parallel examination of yohimbine in AUD preclinical models and in alcohol human laboratory studies would reduce the knowledge gap when evaluating sex differences, action on neural response, and of applications for medication development.
Future directions
Yohimbine as a pharmacological probe within translational alcohol research would greatly benefit from the development of future studies, within integrative parallel paradigms of AUD. Such studies would serve to reduce the translational gap, allowing for more rapid identification of promising clinical targets for AUD and the development of pharmacotherapies through clinical trials. Further, although development of genetically-selected rodent models of AUD has allowed for preclinical models that more closely represent the genetic variances contributing to AUD within humans, further studies directly comparing the rodent strains used within AUD research is necessary to gain a more in depth understanding of the role genetic alterations play in AUD.
Additionally, this area of research would benefit from further study investigating the interaction between specific neurotransmitter systems and yohimbine, to further parse the role of noradrenergic activation in alcohol seeking behaviors. This topic has been highlighted in a recent review, which provides a thorough overview of the neurobiology of alcohol seeking behavior, providing essential insight on neurobiological mechanisms mediating alcohol seeking, and the role of respective neurotransmitter systems [104].
Conclusion
In summary, this systematic scoping review broadly examines the alcohol research literature utilizing yohimbine. Findings support the use of yohimbine as a safe, titrable pharmacological probe for AUD. Further, considerations must be made to reduce the translational gap between preclinical and clinical studies. To that end, utilization of males and females, genetic rodent models of AUD vs non-selected control lines, clinical AUD trials involving a range of yohimbine dosages, and studies conducting preclinical and clinical research in parallel would serve to greatly advance the utility and understanding of yohimbine as a pharmacological probe.
References
Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry. 2015;72:757–66. https://doi.org/10.1001/jamapsychiatry.2015.0584.
Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–59. https://doi.org/10.1176/appi.ajp.2007.05030503.
Sinha R. How does stress lead to risk of alcohol relapse? Alcohol Res. 2012;34:432–40.
Spierling SR, Zorrilla EP. Don’t stress about CRF: assessing the translational failures of CRF(1)antagonists. Psychopharmacol (Berl). 2017;234:1467–81. https://doi.org/10.1007/s00213-017-4556-2.
Haass-Koffler CL, Swift RM, Leggio L. Noradrenergic targets for the treatment of alcohol use disorder. Psychopharmacol (Berl). 2018;235:1625–34. https://doi.org/10.1007/s00213-018-4843-6.
Skelly MJ, Chappell AE, Carter E, Weiner JL. Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: Possible role of disrupted noradrenergic signaling. Neuropharmacology. 2015;97:149–59. https://doi.org/10.1016/j.neuropharm.2015.05.025.
Kenna GA, Haass-Koffler CL, Zywiak WH, Edwards SM, Brickley MB, Swift RM, et al. Role of the α1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addict Biol. 2016;21:904–14. https://doi.org/10.1111/adb.12275.
McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87:605–20. https://doi.org/10.1016/j.neuron.2015.07.002.
Szabadi E. Functional neuroanatomy of the central noradrenergic system. J Psychopharmacol. 2013;27:659–93. https://doi.org/10.1177/0269881113490326.
Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996;23:28–38.
Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996;23:39–51.
Haass-Koffler CL, Bartlett SE. Stress and addiction: contribution of the corticotropin releasing factor (CRF) system in neuroplasticity. Front Mol Neurosci. 2012;5:91 https://doi.org/10.3389/fnmol.2012.00091.
Simms JA, Haass-Koffler CL, Bito-Onon J, Li R, Bartlett SE. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology. 2012;37:906–18. https://doi.org/10.1038/npp.2011.268.
Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, et al. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacol (Berl). 2007;195:345–55. https://doi.org/10.1007/s00213-007-0905-x.
Bertholomey ML, Verplaetse TL, Czachowski CL. Alterations in ethanol seeking and self-administration following yohimbine in selectively bred alcohol-preferring (P) and high alcohol drinking (HAD-2) rats. Behav Brain Res. 2013;238:252–8. https://doi.org/10.1016/j.bbr.2012.10.030.
Funk D, Coen K, Tamadon S, Li Z, Loughlin A, Lê AD. Effects of prazosin and doxazosin on yohimbine-induced reinstatement of alcohol seeking in rats. Psychopharmacol (Berl). 2016;233:2197–207. https://doi.org/10.1007/s00213-016-4273-2.
McDougle CJ, Krystal JH, Price LH, Heninger GR, Charney DS. Noradrenergic response to acute ethanol administration in healthy subjects: comparison with intravenous yohimbine. Psychopharmacol (Berl). 1995;118:127–35. https://doi.org/10.1007/bf02245830.
Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, et al. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. 2011;36:1178–86. https://doi.org/10.1038/npp.2010.253.
Guizzetti M, Davies DL, Egli M, Finn DA, Molina P, Regunathan S, et al. Sex and the lab: an alcohol-focused commentary on the NIH initiative to balance sex in cell and animal studies. Alcohol Clin Exp Res. 2016;40:1182–91. https://doi.org/10.1111/acer.13072.
Moningka H, Lichenstein S, Worhunsky PD, DeVito EE, Scheinost D, Yip SW. Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research. Neuropsychopharmacology. 2019;44:259–73. https://doi.org/10.1038/s41386-018-0232-4.
Verplaetse TL, Cosgrove KP, Tanabe J, McKee SA. Sex/gender differences in brain function and structure in alcohol use: A narrative review of neuroimaging findings over the last 10 years. J Neurosci Res. 2021;99:309–23. https://doi.org/10.1002/jnr.24625.
Kezer CA, Simonetto DA, Shah VH. Sex differences in alcohol consumption and alcohol-associated liver disease. Mayo Clin Proc. 2021;96:1006–16. https://doi.org/10.1016/j.mayocp.2020.08.020.
Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, et al. Sex differences in stress-related alcohol use. Neurobiol Stress. 2019;10:100149 https://doi.org/10.1016/j.ynstr.2019.100149.
Agabio R, Pisanu C, Gessa GL, Franconi F. Sex differences in alcohol use disorder. Curr Med Chem. 2017;24:2661–70. https://doi.org/10.2174/0929867323666161202092908.
Mulvey B, Bhatti DL, Gyawali S, Lake AM, Kriaucionis S, Ford CP, et al. Molecular and functional sex differences of noradrenergic neurons in the mouse locus coeruleus. Cell Rep. 2018;23:2225–35. https://doi.org/10.1016/j.celrep.2018.04.054.
Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32:709–23. https://doi.org/10.1007/s10571-012-9824-4.
Flores-Bonilla A, Richardson HN. Sex differences in the neurobiology of alcohol use disorder. Alcohol Res. 2020;40:04. https://doi.org/10.35946/arcr.v40.2.04.
Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001;158:188–97. https://doi.org/10.1176/appi.ajp.158.2.188.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700 https://doi.org/10.1136/bmj.b2700.
Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104:240–3. https://doi.org/10.3163/1536-5050.104.3.014.
Krauth D, Woodruff TJ, Bero L. Instruments for assessing risk of bias and other methodological criteria of published animal studies: a systematic review. Environ Health Perspect. 2013;121:985–92. https://doi.org/10.1289/ehp.1206389.
Ciccocioppo R, Stopponi S, Economidou D, Kuriyama M, Kinoshita H, Heilig M, et al. Chronic treatment with novel brain-penetrating selective NOP receptor agonist MT-7716 reduces alcohol drinking and seeking in the rat. Neuropsychopharmacology. 2014;39:2601–10. https://doi.org/10.1038/npp.2014.113.
Bertholomey ML, Nagarajan V, Torregrossa MM. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacol (Berl). 2016;233:2277–87. https://doi.org/10.1007/s00213-016-4278-x.
Bertholomey ML, Stone K, Lam TT, Bang S, Wu W, Nairn AC, et al. Phosphoproteomic analysis of the amygdala response to adolescent glucocorticoid exposure reveals G-Protein coupled receptor kinase 2 as a target for reducing motivation for alcohol. Proteomes. 2018;6. https://doi.org/10.3390/proteomes6040041.
Bertholomey ML, Torregrossa MM. Gonadal hormones affect alcohol drinking, but not cue+yohimbine-induced alcohol seeking, in male and female rats. Physiol Behav. 2019;203:70–80. https://doi.org/10.1016/j.physbeh.2017.10.025.
Broccoli L, Uhrig S, von Jonquieres G, Schönig K, Bartsch D, Justice NJ, et al. Targeted overexpression of CRH receptor subtype 1 in central amygdala neurons: effect on alcohol-seeking behavior. Psychopharmacol (Berl). 2018;235:1821–33. https://doi.org/10.1007/s00213-018-4908-6.
Cippitelli A, Brunori G, Schoch J, Armishaw CJ, Wu J, Zaveri NT, et al. Differential regulation of alcohol taking and seeking by antagonism at α4β2 and α3β4 nAChRs. Psychopharmacol (Berl). 2018;235:1745–57. https://doi.org/10.1007/s00213-018-4883-y.
Mao L, Abdel-Rahman AA. Synergistic behavioral interaction between ethanol and clonidine in rats: role of alpha-2 adrenoceptors. J Pharm Exp Ther. 1996;279:443–9.
Russ RD, Abdel-Rahman AA, Wooles WR. Ethanol exhibits alpha receptor blocking-like properties in anesthetized rats. Proc Soc Exp Biol Med. 1989;190:1–6. https://doi.org/10.3181/00379727-190-42821.
Aziz AM, Brothers S, Sartor G, Holm L, Heilig M, Wahlestedt C, et al. The nociceptin/orphanin FQ receptor agonist SR-8993 as a candidate therapeutic for alcohol use disorders: validation in rat models. Psychopharmacol (Berl). 2016;233:3553–63. https://doi.org/10.1007/s00213-016-4385-8.
Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, et al. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacol (Berl). 2010;208:417–26. https://doi.org/10.1007/s00213-009-1741-y.
Dzung Lê A, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacol (Berl) 2009;204:477–88. https://doi.org/10.1007/s00213-009-1481-z.
Gass JT, Olive MF. Reinstatement of ethanol-seeking behavior following intravenous self-administration in Wistar rats. Alcohol Clin Exp Res. 2007;31:1441–5. https://doi.org/10.1111/j.1530-0277.2007.00480.x.
Lê AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacol (Berl). 2005;179:366–73. https://doi.org/10.1007/s00213-004-2036-y.
Le AD, Funk D, Coen K, Li Z, Shaham Y. Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement of alcohol seeking in rats. Addict Biol. 2013;18:448–51. https://doi.org/10.1111/j.1369-1600.2011.00374.x.
Parale MP, Kulkarni SK. Studies with alpha 2-adrenoceptor agonists and alcohol abstinence syndrome in rats. Psychopharmacol (Berl). 1986;88:237–9. https://doi.org/10.1007/bf00652247.
Sequeira MK, Nelson BS, Fulenwider HD, King CE, Nennig SE, Bohannon JB, et al. The neurokinin-1 receptor mediates escalated alcohol intake induced by multiple drinking models. Neuropharmacology 2018;137:194–201. https://doi.org/10.1016/j.neuropharm.2018.05.005.
Williams KL, Nickel MM, Bielak JT. Baclofen blocks yohimbine-induced increases in ethanol-reinforced responding in rats. Pharm Biochem Behav. 2016;144:20–5. https://doi.org/10.1016/j.pbb.2016.02.010.
Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, et al. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacol (Berl). 2008;198:449–60. https://doi.org/10.1007/s00213-008-1104-0.
Funk D, Coen K, Lê AD. The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. Brain Behav. 2014;4:356–67. https://doi.org/10.1002/brb3.222.
Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA. Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS One. 2014;9:e92701 https://doi.org/10.1371/journal.pone.0092701.
Haass-Koffler CL, Henry AT, Melkus G, Simms JA, Naemmuddin M, Nielsen CK, et al. Defining the role of corticotropin releasing factor binding protein in alcohol consumption. Transl Psychiatry 2016;6:e953 https://doi.org/10.1038/tp.2016.208.
Nielsen CK, Simms JA, Bito-Onon JJ, Li R, Ananthan S, Bartlett SE. The delta opioid receptor antagonist, SoRI-9409, decreases yohimbine stress-induced reinstatement of ethanol-seeking. Addict Biol. 2012;17:224–34. https://doi.org/10.1111/j.1369-1600.2010.00295.x.
Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacol (Berl). 2008;199:109–17. https://doi.org/10.1007/s00213-008-1136-5.
Richards JK, Simms JA, Bartlett SE. Conditioned cues and yohimbine induce reinstatement of beer and near-beer seeking in Long-Evans rats. Addict Biol. 2009;14:144–51. https://doi.org/10.1111/j.1369-1600.2008.00139.x.
Sheth C, Furlong TM, Keefe KA, Taha SA. Lesion of the rostromedial tegmental nucleus increases voluntary ethanol consumption and accelerates extinction of ethanol-induced conditioned taste aversion. Psychopharmacol (Berl). 2016;233:3737–49. https://doi.org/10.1007/s00213-016-4406-7.
Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35:1453–63. https://doi.org/10.1038/npp.2010.15.
Simms JA, Richards JK, Mill D, Kanholm I, Holgate JY, Bartlett SE. Induction of multiple reinstatements of ethanol- and sucrose-seeking behavior in Long-Evans rats by the α-2 adrenoreceptor antagonist yohimbine. Psychopharmacol (Berl). 2011;218:101–10. https://doi.org/10.1007/s00213-011-2451-9.
Tabbara RI, Rahbarnia A, Lê AD, Fletcher PJ. The pharmacological stressor yohimbine, but not U50,488, increases responding for conditioned reinforcers paired with ethanol or sucrose. Psychopharmacol (Berl). 2020;237:3689–702. https://doi.org/10.1007/s00213-020-05647-0.
Williams KL, Harding KM. Repeated alcohol extinction sessions in conjunction with MK-801, but not yohimbine or propranolol, reduces subsequent alcohol cue-induced responding in rats. Pharm Biochem Behav. 2014;116:16–24. https://doi.org/10.1016/j.pbb.2013.11.020.
Koechling UM, Smith BR, Amit Z. Differential effects of catecholamine antagonists on ethanol-induced excitation in mice. Psychopharmacol (Berl). 1990;102:234–8. https://doi.org/10.1007/bf02245927.
Koechling UM, Amit Z. Effects of CA antagonists on ethanol-induced excitation in habituated and nonhabituated mice: interaction with stress factors? Pharm Biochem Behav. 1993;44:791–6. https://doi.org/10.1016/0091-3057(93)90007-g.
Liljequist S, Berggren U, Engel J. The effect of catecholamine receptor antagonists on ethanol-induced locomotor stimulation. J Neural Transm. 1981;50:57–67. https://doi.org/10.1007/bf01254914.
Carlsson ML, Engberg G. Ethanol behaves as an NMDA antagonist with respect to locomotor stimulation in monoamine-depleted mice. J Neural Transm Gen Sect. 1992;87:155–60. https://doi.org/10.1007/bf01245017.
Edwards F, Schabinsky VV, Jackson DM, Starmer GA, Jenkins O. Involvement of catecholamines in acute tolerance to ethanol in mice. Psychopharmacol (Berl). 1983;79:246–50. https://doi.org/10.1007/bf00427821.
Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharm Exp Ther. 2002;302:516–24. https://doi.org/10.1124/jpet.102.036046.
Funk D, Li Z, Coen K, Lê AD. Effects of pharmacological stressors on c-fos and CRF mRNA in mouse brain: relationship to alcohol seeking. Neurosci Lett 2008;444:254–8. https://doi.org/10.1016/j.neulet.2008.08.043.
King CE, Becker HC. Oxytocin attenuates stress-induced reinstatement of alcohol seeking behavior in male and female mice. Psychopharmacol (Berl). 2019;236:2613–22. https://doi.org/10.1007/s00213-019-05233-z.
Ayanwuyi LO, Stopponi S, Ubaldi M, Cippitelli A, Nasuti C, Damadzic R, et al. Neurokinin 1 receptor blockade in the medial amygdala attenuates alcohol drinking in rats with innate anxiety but not in Wistar rats. Br J Pharmacol. 2015;172:5136–46. https://doi.org/10.1111/bph.13280.
Borruto AM, Fotio Y, Stopponi S, Petrella M, De Carlo S, Domi A, et al. NOP receptor antagonism attenuates reinstatement of alcohol-seeking through modulation of the mesolimbic circuitry in male and female alcohol-preferring rats. Neuropsychopharmacology. 2021;46:2121–31. https://doi.org/10.1038/s41386-021-01096-1.
Fotio Y, Borruto AM, Benvenuti F, Demopulos G, Gaitanaris G, Roberto M, et al. Activation of peroxisome proliferator-activated receptor γ reduces alcohol drinking and seeking by modulating multiple mesocorticolimbic regions in rats. Neuropsychopharmacology. 2021;46:360–7. https://doi.org/10.1038/s41386-020-0754-4.
Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, et al. Activation of nuclear PPARγ receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry. 2011;69:642–9. https://doi.org/10.1016/j.biopsych.2010.12.010.
Stopponi S, Somaini L, Cippitelli A, de Guglielmo G, Kallupi M, Cannella N, et al. Pregabalin reduces alcohol drinking and relapse to alcohol seeking in the rat. Psychopharmacol (Berl). 2012;220:87–96. https://doi.org/10.1007/s00213-011-2457-3.
Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M, et al. Activation of PPARγ by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcohol Clin Exp Res. 2013;37:1351–60. https://doi.org/10.1111/acer.12091.
Stopponi S, Fotio Y, Cifani C, Li H, Haass-Koffler CL, Cannella N, et al. Andrographis paniculata and Its Main Bioactive Ingredient Andrographolide Decrease Alcohol Drinking and Seeking in Rats Through Activation of Nuclear PPARγ Pathway. Alcohol Alcohol. 2021;56:240–9. https://doi.org/10.1093/alcalc/agaa136.
Rorick-Kehn LM, Ciccocioppo R, Wong CJ, Witkin JM, Martinez-Grau MA, Stopponi S, et al. A novel, orally bioavailable nociceptin receptor antagonist, LY2940094, reduces ethanol self-administration and ethanol seeking in animal models. Alcohol Clin Exp Res. 2016;40:945–54. https://doi.org/10.1111/acer.13052.
Kastman HE, Blasiak A, Walker L, Siwiec M, Krstew EV, Gundlach AL, et al. Nucleus incertus Orexin2 receptors mediate alcohol seeking in rats. Neuropharmacology. 2016;110:82–91. https://doi.org/10.1016/j.neuropharm.2016.07.006.
Walker LC, Kastman HE, Koeleman JA, Smith CM, Perry CJ, Krstew EV, et al. Nucleus incertus corticotrophin-releasing factor 1 receptor signalling regulates alcohol seeking in rats. Addict Biol. 2017;22:1641–54. https://doi.org/10.1111/adb.12426.
Walker LC, Kastman HE, Krstew EV, Gundlach AL, Lawrence AJ. Central amygdala relaxin-3/relaxin family peptide receptor 3 signalling modulates alcohol seeking in rats. Br J Pharmacol. 2017;174:3359–69. https://doi.org/10.1111/bph.13955.
Walker LC, Hand LJ, Letherby B, Huckstep KL, Campbell EJ, Lawrence AJ. Cocaine and amphetamine regulated transcript (CART) signalling in the central nucleus of the amygdala modulates stress-induced alcohol seeking. Neuropsychopharmacology. 2021;46:325–33. https://doi.org/10.1038/s41386-020-00807-4.
Walker LC, Kastman HE, Lawrence AJ. Pattern of neural activation following yohimbine-induced reinstatement of alcohol seeking in rats. Eur J Neurosci 2020;51:706–20. https://doi.org/10.1111/ejn.14431.
Opitz K. The effect of clonidine and related substances on voluntary ethanol consumption in rats. Drug Alcohol Depend. 1990;25:43–8. https://doi.org/10.1016/0376-8716(90)90139-6.
Lê AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, et al. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacol (Berl). 2011;218:89–99. https://doi.org/10.1007/s00213-011-2178-7.
Schank JR, King CE, Sun H, Cheng K, Rice KC, Heilig M, et al. The role of the neurokinin-1 receptor in stress-induced reinstatement of alcohol and cocaine seeking. Neuropsychopharmacology. 2014;39:1093–101. https://doi.org/10.1038/npp.2013.309.
Nelson BS, Fulenwider HD, Nennig SE, Smith BM, Sequeira MK, Chimberoff SH, et al. Escalated alcohol self-administration and sensitivity to yohimbine-induced reinstatement in alcohol preferring rats: potential role of neurokinin-1 receptors in the amygdala. Neuroscience. 2019;413:77–85. https://doi.org/10.1016/j.neuroscience.2019.06.023.
Ayanwuyi LO, Carvajal F, Lerma-Cabrera JM, Domi E, Björk K, Ubaldi M, et al. Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcohol drinking and seeking behaviors of marchigian sardinian alcohol-preferring rats. Front Psychiatry. 2013;4:23 https://doi.org/10.3389/fpsyt.2013.00023.
Zgombick JM, Erwin VG. Central mechanisms of ethanol-induced adrenocortical response in selectively bred lines of mice. Neuroendocrinology. 1987;46:324–32. https://doi.org/10.1159/000124840.
Reid LD, Delconte JD, Amendola CA, Nichols ML, Krupsky GW, Dharia NS, et al. alpha(2)-Adrenoceptor antagonists and propensity to take alcoholic beverages. Behav Pharmacol. 1994;5:485–93. https://doi.org/10.1097/00008877-199408000-00009.
Funk D, Li Z, Lê AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–43. https://doi.org/10.1016/j.neuroscience.2005.10.062.
Krystal JH, Webb E, Cooney N, Kranzler HR, Charney DS. Specificity of ethanollike effects elicited by serotonergic and noradrenergic mechanisms. Arch Gen Psychiatry. 1994;51:898–911. https://doi.org/10.1001/archpsyc.1994.03950110058008.
Krystal JH, Webb E, Cooney NL, Kranzler HR, Southwick SW, Heninger GR, et al. Serotonergic and noradrenergic dysregulation in alcoholism: m-chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry. 1996;153:83–92. https://doi.org/10.1176/ajp.153.1.83.
Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CA 3rd, et al. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorophenylpiperazine (mCPP). Psychopharmacol (Berl). 1997;131:207–15. https://doi.org/10.1007/s002130050285.
Edenberg HJ, Foroud T. Genetics and alcoholism. Nat Rev Gastroenterol Hepatol. 2013;10:487–94. https://doi.org/10.1038/nrgastro.2013.86.
Crabbe JC. Rodent models of genetic contributions to motivation to abuse alcohol. Nebr Symp Motiv. 2014;61:5–29. https://doi.org/10.1007/978-1-4939-0653-6_2.
Rosenblum LA, Coplan JD, Friedman S, Bassoff T. Dose-response effects of oral yohimbine in unrestrained primates. Biol Psychiatry. 1991;29:647–57. https://doi.org/10.1016/0006-3223(91)90134-8.
Moore CF, Lynch WJ. Alcohol preferring (P) rats as a model for examining sex differences in alcohol use disorder and its treatment. Pharm Biochem Behav. 2015;132:1–9. https://doi.org/10.1016/j.pbb.2015.02.014.
Barker JM, Taylor JR. Sex differences in incentive motivation and the relationship to the development and maintenance of alcohol use disorders. Physiol Behav. 2019;203:91–9. https://doi.org/10.1016/j.physbeh.2017.09.027.
Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp 2011;32:1998–2013. https://doi.org/10.1002/hbm.21165.
Li S, Zou S, Coen K, Funk D, Shram MJ, Lê AD. Sex differences in yohimbine-induced increases in the reinforcing efficacy of nicotine in adolescent rats. Addict Biol. 2014;19:156–64. https://doi.org/10.1111/j.1369-1600.2012.00473.x.
Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacol (Berl). 2011;216:53–62. https://doi.org/10.1007/s00213-011-2187-6.
Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend 2010;107:264–7. https://doi.org/10.1016/j.drugalcdep.2009.11.002.
Moran-Santa Maria MM, Sherman BJ, Brady KT, Baker NL, Hyer JM, Ferland C, et al. Impact of endogenous progesterone on reactivity to yohimbine and cocaine cues in cocaine-dependent women. Pharm Biochem Behav. 2018;165:63–9. https://doi.org/10.1016/j.pbb.2017.11.001.
Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergquist KT. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol. 2007;15:445–52. https://doi.org/10.1037/1064-1297.15.5.445.
Domi E, Domi A, Adermark L, Heilig M, Augier E. Neurobiology of alcohol seeking behavior. J Neurochem 2021;157:1585–614. https://doi.org/10.1111/jnc.15343.
Keyes KM, Hatzenbuehler ML, Grant BF, Hasin DS. Stress and alcohol: epidemiologic evidence. Alcohol Res 2012;34:391–400.
Powers RJ, Kutash IL. Stress and alcohol. Int J Addict. 1985;20:461–82. https://doi.org/10.3109/10826088509044926.
Getachew B, Hauser SR, Csoka AB, Taylor RE, Tizabi Y. Role of cortical alpha-2 adrenoceptors in alcohol withdrawal-induced depression and tricyclic antidepressants. Drug Alcohol Depend. 2017;175:133–9. https://doi.org/10.1016/j.drugalcdep.2017.03.004.
Sáiz J, Pazos A, Del Olmo E, Sáiz V, Sánchez A. Yohimbine-induced alterations in alpha(2)-adrenoceptors in kidney regions of the spontaneously hypertensive rats: an autoradiographic analysis. Pharm Rep. 2008;60:391–8.
Jahanshahi M, Nikmahzar E, Elyasi L, Babakordi F, Hooshmand E. α(2)-Adrenoceptor-ir neurons’ density changes after single dose of clonidine and yohimbine administration in the hippocampus of male rat. Int J Neurosci. 2018;128:404–11. https://doi.org/10.1080/00207454.2017.1389926.
Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–95. https://doi.org/10.31887/DCNS.2006.8.4/ssmith.
Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34:468–83.
Dai X, Thavundayil J, Santella S, Gianoulakis C. Response of the HPA-axis to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Psychoneuroendocrinology. 2007;32:293–305. https://doi.org/10.1016/j.psyneuen.2007.01.004.
Chen YW, Fiscella KA, Bacharach SZ, Tanda G, Shaham Y, Calu DJ. Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history. Addict Biol. 2015;20:690–700. https://doi.org/10.1111/adb.12164.
Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. https://doi.org/10.1016/j.neuron.2008.06.012.
Vazey EM, den Hartog CR, Moorman DE. Central noradrenergic interactions with alcohol and regulation of alcohol-related behaviors. Handb Exp Pharm. 2018;248:239–60. https://doi.org/10.1007/164_2018_108.
Atzori M, Cuevas-Olguin R, Esquivel-Rendon E, Garcia-Oscos F, Salgado-Delgado RC, Saderi N, et al. Locus ceruleus norepinephrine release: a central regulator of CNS spatio-temporal activation? Front Synaptic Neurosci. 2016;8:25 https://doi.org/10.3389/fnsyn.2016.00025.
Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–7. https://doi.org/10.1016/j.alcohol.2007.12.002.
Haass-Koffler CL, Goodyear K, Zywiak WH, Magill M, Eltinge SE, Wallace PM, et al. Higher pretreatment blood pressure is associated with greater alcohol drinking reduction in alcohol-dependent individuals treated with doxazosin. Drug Alcohol Depend. 2017;177:23–8. https://doi.org/10.1016/j.drugalcdep.2017.03.016.
Wilcox CE, Tonigan JS, Bogenschutz MP, Clifford J, Bigelow R, Simpson T. A randomized, placebo-controlled, clinical trial of prazosin for the treatment of alcohol use disorder. J Addict Med. 2018;12:339–45. https://doi.org/10.1097/adm.0000000000000413.
Simpson TL, Saxon AJ, Stappenbeck C, Malte CA, Lyons R, Tell D, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175:1216–24. https://doi.org/10.1176/appi.ajp.2018.17080913.
Milivojevic V, Angarita GA, Hermes G, Sinha R, Fox HC. Effects of prazosin on provoked alcohol craving and autonomic and neuroendocrine response to stress in alcohol use disorder. Alcohol Clin Exp Res. 2020;44:1488–96. https://doi.org/10.1111/acer.14378.
Haass-Koffler CL, Leggio L, Kenna GA. Pharmacological approaches to reducing craving in patients with alcohol use disorders. CNS Drugs. 2014;28:343–60. https://doi.org/10.1007/s40263-014-0149-3.
Laurila JM, Xhaard H, Ruuskanen JO, Rantanen MJ, Karlsson HK, Johnson MS, et al. The second extracellular loop of alpha2A-adrenoceptors contributes to the binding of yohimbine analogues. Br J Pharmacol. 2007;151:1293–304. https://doi.org/10.1038/sj.bjp.0707330.
Etzel JP, Rana BK, Wen G, Parmer RJ, Schork NJ, O’Connor DT, et al. Genetic variation at the human alpha2B-adrenergic receptor locus: role in blood pressure variation and yohimbine response. Hypertension. 2005;45:1207–13. https://doi.org/10.1161/01.HYP.0000166721.42734.49.
Gozzi A, Lepore S, Vicentini E, Merlo-Pich E, Bifone A. Differential effect of orexin-1 and CRF-1 antagonism on stress circuits: a fMRI study in the rat with the pharmacological stressor Yohimbine. Neuropsychopharmacology. 2013;38:2120–30. https://doi.org/10.1038/npp.2013.109.
Rosen MI, Kosten TR, Kreek MJ. The effects of naltrexone maintenance on the response to yohimbine in healthy volunteers. Biol Psychiatry. 1999;45:1636–45. https://doi.org/10.1016/s0006-3223(98)00259-5.
Greenwald MK, Lundahl LH, Steinmiller CL. Yohimbine increases opioid-seeking behavior in heroin-dependent, buprenorphine-maintained individuals. Psychopharmacol (Berl). 2013;225:811–24. https://doi.org/10.1007/s00213-012-2868-9.
Acknowledgements
The authors also would like to thank the Brown University librarians, Laura Pavlech and Kelsey Sawyer, for constructing the systematic database searches. They would also like to thank Paul S. Soliman for his contributions during manuscript revision.
Funding
DEC is supported by the National Institute on Alcohol Abuse and Alcoholism (R01 AA027760 and Research Supplements to Promote Diversity in Health-Related Research). CLH-K is supported by the National Institute on Alcohol Abuse and Alcoholism (K01 AA023867; R01 AA026589; R01 AA027760; R21 AA027614) and by the National Institute of General Medical Sciences (NIGMS), Center of Biomedical Research Excellence (COBRE, P20 GM130414). RC is supported by the PRIN 2017SXEXT5, and NC is supported by the Hetzler Foundation (Grant 202213).
Author information
Authors and Affiliations
Contributions
DEC and TRV-K served as two independent reviewers, RC and NC served as consensus reviewers for the animal studies, CLH-K as consensus reviewer for the human studies. DEC and CLH-K wrote the manuscript; CLH-K supervised the project; TRV-K, NC and RC contributed to the writing of the manuscript. All authors approved the final version of the manuscript and agree to be accountable for the content of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Curley, D.E., Vasaturo-Kolodner, T.R., Cannella, N. et al. Yohimbine as a pharmacological probe for alcohol research: a systematic review of rodent and human studies. Neuropsychopharmacol. 47, 2111–2122 (2022). https://doi.org/10.1038/s41386-022-01363-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01363-9