Abstract
Models of addiction are based on neurobiological, behavioral, and pharmacological studies in animals, but translational support from human studies is limited. Studies are lacking in examining acute responses to alcohol in drinkers with alcohol use disorder (AUD), particularly in terms of relevant intoxicating doses and measurement of stimulating and rewarding effects throughout the breath alcohol concentration (BrAC) time curve. Participants were N = 60 AUD drinkers enrolled in the Chicago Social Drinking Project and examined in three random-order and blinded sessions for subjective and physiological responses to a beverage containing 0.0 g/kg, 0.8 g/kg, and 1.2 g/kg alcohol. BrAC in the alcohol sessions at 60 min was 0.09 g/dL and 0.13 g/dL, respectively. Both doses of alcohol produced significant biphasic effects on subjective measures of stimulation, euphoria, reward (liking and wanting), sedation, and neuroendocrine and cardiovascular factors. Increased pleasurable effects of alcohol were pronounced during the rising limb-to-peak BrAC and sedating effects emerged during the declining limb. Alcohol dose-dependently increased feel drug ratings and rewarding effects at peak BrAC or early declining limb, and physiological responses at the rising limb. Thus, rather than the notion of an overall tolerance, results show an alcohol response phenotype characterized by sensitivity to alcohol’s stimulating, rewarding and physiological effects. The results of this study may aid in the conceptualization of alcohol addiction as a disorder characterized by the persistence of enhanced hedonic alcohol responses rather than chronic tolerance and reward deficiency.
Similar content being viewed by others
Introduction
Alcohol consumption in humans is influenced by an individual’s sensitivity to the drug’s pharmacological properties. For decades, laboratory studies using controlled acute alcohol challenges have been instrumental in characterizing the subjective and behavioral responses associated with risky drinking and predictive of future alcohol use disorder (AUD) development. Among young adults, heavy drinkers are more sensitive than light drinkers to the euphoric, hedonic, and motivational properties of alcohol and less sensitive to its sedative effects [1,2,3]. Further, this subjective response phenotype is predictive of hazardous drinking and AUD symptomatology 6 and 10 years later [3, 4]. Prominent etiological models of addiction theorize that with chronic alcohol consumption, subjective and behavioral responses undergo critical adaptations. These proposed neuroadaptations include a transition from initially heightened alcohol reward to a reward deficit state [5, 6], the development of an overall tolerance to alcohol’s effects [7, 8], or an emergence of a sensitized motivational salience state [9]. While such models are based on neurobiological, behavioral, and pharmacological studies in animals, translational support from human studies is limited.
One reason for paucity of studies testing these theories is that individuals with AUD have largely been excluded from alcohol challenge research. Early studies in alcohol-dependent drinkers in the 1960s/1970s tested small samples of white males who were entering treatment, hospitalized, or imprisoned and lacked standardized or validated measures of alcohol subjective response [10,11,12]. Thus, evaluations were limited to observational reports and clinical interviews, noting that people with alcohol dependence demonstrated “a tendency to become more talkative and boisterous” at high blood alcohol ranges (100–200 mg/100cc [10];) with increased anxiety and depression [11, 13] and elevated cortisol [14]. Given arising ethical concerns, this line of research was ostensibly arrested for the next several decades. Eventual guidance from the National Advisory Council’s recommendations on alcohol administration in humans [15, 16] allowed a second wave of research examining oral or intravenous alcohol responses in AUD drinkers. However, studies are few and findings are mixed: relative to non-dependent drinkers, alcohol-dependent drinkers showed a blunted sedation response to alcohol [17] and both enhanced [18] or equivocal [17, 19] alcohol-induced stimulation. Importantly, there have been no dose-response studies of alcohol’s subjective and physiological effects in AUD drinkers with administration of doses reflective of their drinking patterns, i.e., ≥0.8 g/kg, or with appropriate placebo controls, baseline assessment, and reliable and valid measures of biphasic alcohol responses.
To our knowledge, the Chicago Social Drinking Project (CSDP) is the only placebo-controlled study examining responses to an intoxicating dose of alcohol (0.8 g/kg) in AUD drinkers. Young adults who developed AUD over the course of a decade of testing exhibited initially heightened euphoric, hedonic, and motivational responses to alcohol [3] compared with those who did not develop AUD. These heightened acute pleasurable responses to alcohol were maintained or potentiated during re-examination testing 10 years later [3]. However, the sample was modest as only a subset (21%; n = 39) met criteria for AUD after a decade and acute responses to only one dose of alcohol (vs. placebo) were examined.
Thus, the present study was a dose-response evaluation of alcohol’s subjective and physiological effects in the third cohort of CSDP comprised of young adult excessive drinkers with AUD. To compare results to prior studies, we examined responses to a 0.8 g/kg high dose of alcohol as well as to a 1.2 g/kg very high dose that more closely approximates excessive drinking levels characteristic of AUD. This dose was recently shown to be feasible and safe in this sample [20]. Primary outcomes were validated subjective measures sensitive to alcohol’s effects, including stimulation, sedation, liking and wanting examined across the breath alcohol concentration (BrAC) curve. Secondary outcomes were complementary subjective measures as well as physiological measures shown to be sensitive to alcohol intoxication in generally healthy (non-addicted) drinkers, including cortisol, mean arterial blood pressure, and heart rate (HR) [21,22,23,24]. Based on prior work, we hypothesized dose-dependent increases in stimulation, reward (liking and wanting), and sedation with the very high dose producing the greatest effects. For the secondary physiological responses, we expected that the very high alcohol dose would acutely increase cortisol levels, blood pressure, and HR relative to the high alcohol and placebo doses.
Materials and methods
Design
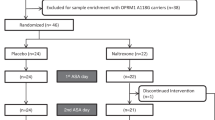
The study used a within-subject oral administration laboratory paradigm to evaluate subjective and physiological responses to two alcohol doses and placebo in young adult drinkers with AUD. Participants were part of the third cohort enrolled in the CSDP between June 2016 and March 2019. They attended three individual experimental sessions during which they received a beverage containing a placebo, high alcohol (0.8 g/kg) dose, and a very high alcohol (1.2 g/kg) dose that was 50% higher than the usual fixed high dose employed in prior studies in heavy and AUD drinkers [1, 2, 25,26,27] (for details, see [20]). The high dose and placebo sessions were in random order and double-blinded, while the very high dose session was randomized as the first or last session and single-blinded as it required a longer duration (7–8 h vs. 5 h for the other sessions) to account for an extended declining limb. During each session, participants completed measures before and at various time points after consuming their blinded beverage. All sessions were conducted at the Clinical Addictions Research Laboratory at the University of Chicago and were separated by at least 48 h. The study was fully approved by the University of Chicago Institutional Review Board (IRB) and all participants provided written and oral informed consent at screening.
Eligibility and screening
Recruitment occurred via online advertisements and word-of-mouth referrals. Inclusion criteria were: age 21–35 years, weight 110–220 pounds, good general health, and not pregnant or lactating; no current major psychiatric concerns; able to refrain from smoking for 8 h, not currently seeking treatment for alcohol-related problems, and meeting 2 or more AUD symptoms in the past year; and engaging in at least 11 heavy drinking episodes [defined as ≥5 drinks for men (≥4 for women) according to NIAAA and SAMHSA guidelines] per month and consuming ≥28 (≥21 for women) alcoholic drinks per week. The latter drinking quantity and frequency cutoffs were employed in order to assure heavier drinking patterns than in prior CSDP cohorts of heavy social drinkers [1].
Candidates meeting basic study inclusion criteria as determined via an initial telephone screen were scheduled for a 2 h in-person screening to verify eligibility. Surveys and interviews ascertained information on demographics, physical health, recent substance use, past month cigarette and alcohol use [via Timeline Follow Back [28]], and AUD symptoms and mental health [via Structured Clinical Interview for DSM-IV, non-patient version [29]]. Additional surveys included the Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA-Ar; [30]), Alcohol Use Disorder Identification Test (AUDIT; [31]), Drinker Inventory of Consequences Revised (Dr-InC 2 R); [32]), Beck Depression Inventory [33], Spielberger Trait Anxiety Inventory [34], and a two-generational biological family history (FH) tree for AUD. Family history (FH) was defined as positive by at least one biological first-degree relative or two or more second-degree relatives with AUD, and negative if no AUD for two generations, or undetermined. Details regarding screening and variable coding are published elsewhere [20] and included in Supplemental Materials.
The majority of candidates attending the in-person screening visit (66/81; 82%) were deemed eligible; those ineligible did not meet alcohol drinking criteria (n = 8), had a benzodiazepine or opioid use disorder (n = 2), a major psychiatric disorder (n = 4), or were not in the age range (n = 1). Of the 66 eligible candidates, five did not attend the first study session and one attended only one session. Thus, the final sample size was N = 60 participants.
Laboratory sessions
The methodology was standard across all laboratory challenge sessions, with the exception of the dose administered and the session length (see “Very High Alcohol Dose Session”). Study sessions began between 10:00 am and 3:00 pm. Participants were instructed to abstain from alcohol and medications for at least 24 h prior to the experimental session, as well as caffeine, cigarettes, and food for 3 h prior. Upon arrival to the session, the participant completed a breathalyzer test (Alco-Sensor III, Intoximeter, St. Louis, MO) to verify compliance with recent alcohol abstinence and the CIWA-Ar to assess alcohol withdrawal symptoms. Participants also provided a urine sample for drug toxicology and pregnancy testing (in women). After these arrival measures, the participant consumed a snack at 20% daily kilocalorie needs per body weight (55% carbohydrates, 10% protein, and 35% fat) [35] to reduce the possibility of alcohol-induced nausea. This was followed by the participant completing baseline subjective, objective, and performance measures (the latter measures are outside the scope of the present manuscript and will be presented in a separate paper).
Beverage administration
All study sessions followed identical beverage administration procedures. To reduce alcohol expectancy effects, the study employed the alternative substance paradigm [36] whereby participants were told that the beverage might contain a stimulant, a sedative, alcohol, or a placebo, or two substances in combination. At experimental time 0 (~45 min after arrival), the participant began the 15 min beverage consumption period. The participant received beverages in lidded, clear plastic cups in three equal portions and consumed each portion over three consecutive 5 min intervals with the research assistant present to engage in light conversation and ensure that the beverages were consumed. Beverages consisted of 190-proof ethanol (1% volume for placebo as a taste mask, 16% volume for alcohol beverages) mixed with water, a flavored drink mix, and a sucralose-based sugar substitute. Doses for women were adjusted to 85% of those for men to account for sex differences in total body water [37].
The participant completed post-beverage subjective and objective measures at 30-, 60-, 120-, and 180 min following beverage consumption. Between time points, the participant could watch movies or read magazines from a standardized list provided by the study. As a validation check, at the 60 min timepoint, participants indicated what they believed to be the active contents of the beverage they consumed: 48% correctly identified placebo, and 24% and 25% identified alcohol as the only active ingredient for the high and very high dose, respectively. Before discharging the participant, the experimenter confirmed BrAC level was ≤0.04 g/dL and completed a behavioral checklist for overt signs of intoxication on alertness, orientation, coordination, and gait. A ride-share service transported the participant home after each session. At the end of the final session, the participant was debriefed, given instructions for follow-up and compensated for their time.
Very high alcohol dose session
The very high dose session procedures (for details see [20]) were identical to the placebo and high dose conditions with the exception of logistical adjustments. This session was blinded only for the participant as it required a longer duration of 7–8 h (vs. 4–5 h for the other two sessions) to allow for the BrAC to descend to ≤0.04 g/dL before the participant was released.
Measures
Subjective measures
The primary subjective dependent measures were stimulation and sedation subscale scores from the 14-item Biphasic Alcohol Effects Scale (BAES) [38, 39] and two items from the Drug Effects Questionnaire (DEQ; visual analogue scale of 0–100; [40]) including hedonic reward (“do you LIKE the effect you are feeling now?”) and motivational salience (“would you like MORE of what you consumed, right now?”). Additional subjective measures were included to complement the primary outcomes and these included general drug effects (DEQ item: “do you FEEL any drug effects?), and subscales from the Addiction Research Center Inventory (ARCI; [41]), including the A (amphetamine scale, stimulation), PCAG (pentobarbital-chlorpromazine-alcohol group, sedative effects), and MBG (morphine-benzedrine group, euphoric effects). All surveys instructed participants to respond based on their current mood state and the instructions did not reveal the beverage content [39]. See Table S2 for correlations between the various subjective measure subscales.
Objective measures
At each post-drinking time point, breathalyzer tests (AlcoSensor IV; Intoximeter, St. Louis, Missouri) were taken but programed to read 0.000 mg/dL with actual BrAC values downloaded later to reduce experimenter bias. At both baseline and post-drinking time points, automated vital signs readings (mean arterial pressure (MAP) and HR) were obtained (CARESCAPE V100 Dinamap, GE Healthcare, Chicago, IL). Saliva samples were collected by a cotton salivette (Salimetrics LLC, Carlsbad, CA) and then stored in a –80 °C freezer. Samples were assayed for cortisol using a high-sensitivity enzyme immunoassay that was standardized and validated at The University of Chicago Clinical Research Center Core Laboratory with a 2% intra-assay coefficient of variation. For additional details regarding objective measures, see Supplemental Materials.
Statistical analyses
Examination of the subjective response data for normality revealed one participant who was an outlier (>3 SD from the mean) and thus was excluded from these analyses. For objective measures, 1.6% of cortisol values were identified as outliers (>3 SD from the mean), and these data were normalized to 3 SD of the mean. Sample contamination or missing data resulted in two more participants being removed from cortisol analysis. Generalized Estimating Equations (GEE) [42] models were conducted for the primary dependent variables, i.e., subjective stimulation, sedation, like and want more, and included dose (placebo, high dose alcohol, and very high dose alcohol),time (treated as a continuous variable), and their interaction. We used the Bonferroni method to correct Type-I error for the primary outcomes. Similar GEE analyses were used for the secondary outcomes. Significant main effects and interactions from GEE were further examined with post-estimation comparisons. All analyses included the covariates of session order, sex, FH, cigarette smoking, and cannabis use, and outcomes are summarized in Table 2. An additional analysis examining sex as a between-subjects factor revealed few significant differences with the exception of men having higher early declining limb alcohol wanting to the very high dose (sex × dose × time, p = 0.047).
Results
The sample averaged 26.8 ± 0.5 (SEM) years of age (range 21–35 years) with 14.2 ± 0.2 years of education. They were nearly equally divided on sex (55% male), racially and ethnically diverse (55% White, 27% Black, 18% Other; 27% Hispanic) (see Table 1 for demographic and drinking characteristics), with a majority (70%) having positive FH for AUD. Participants consumed alcohol on 21.8 of the 28 days (78%) prior to enrollment with a mean of 41.9 ± 2.6 alcoholic drinks consumed per week. Nearly two-thirds of days (63%) in the past month were heavy drinking days (≥5 drinks for men, ≥4 for women), with 28% of days meeting or exceeding the threshold for high intensity drinking (≥10 drinks for men, ≥8 drinks for women) [43]). All participants met past year criteria for AUD; 60% had severe AUD (6 + symptoms), 20% had moderate AUD (4–5 symptoms), and 20% had mild AUD (2–3 symptoms).
Participants endorsed alcohol-related problems and consequences consistent with harmful alcohol use [31, 44], with a mean AUDIT score of 19.9 (±0.9) and a DrInC-2R score of 33.6 (±2.9). Notably, participants were not experiencing alcohol withdrawal at either screening or session baseline assessments: 84% of the sample had CIWA = 0 at session arrival (across the three sessions), 11% had CIWA = 1, and 5% had CIWA between 2 and 8. The mean reported time since last alcoholic drink prior to sessions was 2.5 ± 1.7 SD days. Mean hepatic enzyme levels were within the normal range at study enrollment (see Table 1). As none met criteria for comorbid major psychiatric disorders, Beck Depression Inventory and Spielberger Trait Anxiety Inventory scores were in the non-clinical range. Regular cigarette smoking (≥1 pack/week) and weekly cannabis use was reported by 42 and 47% of the sample, respectively.
As expected, BrAC levels increased rapidly for both alcohol doses, with the very high dose producing a mean peak BrAC (at 60 min after the onset of drinking) that was 50% greater than the high dose, i.e., 0.130 g/dl ± 0.003 vs. 0.089 ± 0.002, respectively (Fig. 1A). Feel drug ratings were higher during the BrAC rising limb than the declining limb for both alcohol doses (Fig. 1B). There was a dose-response pattern observed throughout testing, i.e., the very high dose producing the greatest feel drug ratings and both alcohol doses eliciting higher ratings than placebo (dose × time, Χ2 = 21.73, p < 0.001).
Means ± SEM for breath alcohol concentrations (BrAC) (A) and feel drug ratings (B) across doses. The shaded bar indicates the alcohol drinking interval from time 0–15 min. Post-estimation results for dose × time interaction: on figure, significant differences between high dose vs. very high dose indicated by *p < 0.05 and **p < 0.01. In addition, for BrAC: 30 min < 60 min > 120 min > 180 min for the high and very high dose, ps < 0.001. For feel drug: 30 min = 60 min > 120 min > 180 min for high and very high doses, ps < 0.05.
For the primary outcomes, the high and very high alcohol doses (vs. placebo) significantly increased ratings on all subjective measures, with some notable differences between the doses (see Table 2 for GEE results). Both alcohol doses (vs. placebo) produced the prototypical biphasic effects on stimulation, with increases observed during the rising limb-to-peak BrAC interval, i.e., 30–60 min (dose × time, p < 0.001; Fig. 2A). Stimulation then declined with decreasing BrAC and by 120 min, there were no differences across doses.
Means ± SEM for subjective alcohol responses, including stimulation (A) from the Biphasic Alcohol Effects Scale; alcohol liking (B) and wanting (C) from the Drug Effects Questionnaire; and sedation (D) from the Biphasic Alcohol Effects Scale. The shaded bar indicates the alcohol drinking interval from time 0 to 15 min. Post-estimation results for dose x time interaction: on figure, significant differences between high dose vs. very high dose indicated by *p < 0.05 and **p < 0.01. In addition, for stimulation and liking: 30 min = 60 min > 120 min = 180 min for the high and very high doses, ps ≤ 0.001. For wanting: 30 min = 60 min > 120 min = 180 min for the high dose and 60 min > 180 min for the very high dose, ps ≤ 0.013.For sedation: 30 min = 60 min = 120 min = 180 min for the high dose and 30 min = 60 min < 120 min for the very high dose, ps ≤ 0.018.
Relative to placebo, the high and very high alcohol doses also increased liking (dose × time, p < 0.001) and wanting (dose × time, p = 0.009). Both doses increased liking at 30 min, and the very high dose continued to increase liking at 60 and 120 min (Fig. 2B). By 180 min, there were no longer dose-dependent differences in alcohol-induced liking. For motivational salience, both doses increased wanting through all time points (vs. placebo), with the very high dose potentiating wanting during the early declining limb at 120 min (Fig. 2C). Sedation ratings were higher at baseline for the very high dose session (p = 0.026) and these high levels were carried forward throughout the session with alcohol-induced increases noted during the latter time intervals (dose × time, p = 0.039; Fig. 2D).
Secondary subjective outcomes supported these findings: relative to placebo, both alcohol doses increased ARCI MBG (euphoria) and A (stimulation) scale ratings during the early post-consumption period at 30 min and the PCAG (sedation) scale at 120 and 180 min, with a more sustained PCAG increase for the very high dose at the end of the session [dose × time, ps < 0.001; Fig. 3A–C]. For physiological responses, cortisol was higher at baseline for the very high dose session (vs. high dose, p < 0.001; vs. placebo, p > 0.05) and remained higher at 30 and 60 min vs. the high dose and placebo, but not during the latter time points (dose × time, p < 0.001; Fig. 3D). The very high dose sharply increased MAP at 30 min, but this effect was transient as by 120 and 180 min, MAP was lower for both alcohol doses than placebo (dose × time, p < 0.001; Fig. 3E). For HR, results showed characteristic alcohol-induced increases for both doses throughout the BrAC relative to placebo (dose × time, p < 0.001; Fig. 3F). The physiological responses at each dose did not show significant patterns of association to the subjective measures after adjusting for multiple comparisons.
Means ± SEM for secondary subjective alcohol responses, including ARCI A (amphetamine scale; range 0–11) (A), MBG (morphine-benzedrine group; euphoric effects, range 0–16) (B), and PCAG (pentobarbital-chlorpromazine-alcohol group; sedative effects, range 0–15) (C). The shaded bar indicates the alcohol drinking interval from time 0 to 15 min. Post-estimation results for dose × time interaction: on figure, significant differences between high dose vs. very high dose indicated by *p < 0.05 and **p < 0.01. In addition, for ARCI A and MBG: 30 min = 60 min > 120 min = 180 min for the high and very high doses, ps ≤ 0.005. For PCAG: 30 min = 60 min < 120 min and 30 min < 180 min for the high dose and 30 min = 60 min < 120 min = 180 min for the very high dose ps ≤ 0.001. Means ± SEM for salivary cortisol (D), mean arterial pressure (E), and heart rate (F) responses. The shaded bar indicates the alcohol drinking interval from time 0 to 15 min. Post-estimation results for dose × time interaction: on figure, significant differences between high dose vs. very high dose indicated by *p < 0.05 and **p < 0.01. In addition, for cortisol: 30 min > 120 min = 180 min for the high dose and 30 min > 60 min > 120 min = 180 min for the very high dose, ps ≤ 0.037. For mean arterial pressure, 30 min > 60 min = 120 min = 180 min for the high dose 30 min > 60 min > 120 min = 180 min for the very high dose, ps ≤ 0.006. For heart rate: 30 min = 60 min =120 min = 180 min for the high and very high doses.
Discussion
These data represent the first placebo-controlled, dose-response examination with oral alcohol challenge in AUD drinkers. Both the 0.8 g/kg and 1.2 g/kg doses of alcohol produced significant biphasic effects on subjective measures of stimulation, euphoria, reward (liking and wanting), sedation, and neuroendocrine and cardiovascular factors. Increased pleasurable effects of alcohol at both doses were pronounced during rising limb-to-peak BrAC and increased sedative effects emerged during the declining limb—such biphasic alcohol effects have been shown in prior studies in heavy social drinkers [1,2,3]. Further, the very high alcohol dose potentiated high dose subjective ratings of feel drug, liking and wanting at peak and early declining BrAC (Fig. 1B, Fig. 2B, C) and cortisol and MAP during the rising BrAC limb (Fig. 3D–F). Thus, results show an alcohol response phenotype in young adult AUD drinkers characterized by sensitivity to alcohol’s stimulating, rewarding and physiological effects. There may also be implications for intervention, at least in young adult AUD drinkers, to consider targeting the persistence of hedonic alcohol responses rather than reward deficiency processes and a general tolerance to all of alcohol’s effects.
The study addressed critical issues to advance our understanding of alcohol reward and tolerance in AUD in two notable ways. First, results demonstrate that alcohol reward sensitivity is maintained in young adults with AUD. This finding is consistent with our recent alcohol challenge re-examination study in heavy drinkers progressing to AUD over a decade [3]. Considering this body of work within the theoretical framework of the allostasis model of addiction [45], the observation of pronounced euphoric and hedonic effects of alcohol supports a possible persistence of the purported binge-intoxication stage that occurs prior to, or in tandem with the subsequent negative affect/withdrawal stage of addiction. Of note, participants in the present study were not experiencing significant negative affect or withdrawal symptoms prior to their alcohol challenge, so determination of the negatively reinforcing effects of alcohol was not possible. Nevertheless, the study findings have potential ramifications for the relatively understudied nature of alcohol positive reinforcement in persons with AUD as most neurobiological research on mechanisms of rewarding alcohol effects have been conducted in healthy social drinkers [46,47,48]. Even at a dose (i.e., very high dose producing peak BrAC at 0.13 g/dl, 50% higher than the legal limit for impaired driving) that may be aversive for lighter social drinkers, AUD drinkers continue to experience pleasurable effects of alcohol, which may contribute to the excessive drinking that is characteristic of human AUD at a stage without significant negative affect or withdrawal symptoms.
Second, the results have important implications for understanding chronic functional tolerance, which is well-documented for alcohol’s depressant effects (i.e., motor impairment, hypothermia, and sedation [46,47,48,49]), yet not in terms of alcohol’s stimulating and rewarding effects. The present study sample averaged 42 alcoholic drinks weekly with frequent heavy and high intensity drinking episodes exceeding the high dose (0.8 g/kg) threshold. Therefore, we predicted, consistent with the DSM-5 criterion of tolerance, the high dose would produce subthreshold desirable subjective responses relative to the very high (1.2 g/kg) dose. However, the high dose elicited significant “feel drug” effects as well as increases in stimulation, euphoria, liking, and wanting in this sample of AUD drinkers, most of whom at the severe range of the disorder. Further, the positive subjective response increases to the high dose were of a much higher magnitude than previously shown in light social drinkers but comparable to that observed in heavy social drinkers who eventually develop AUD [1, 3, 50], with lower alcohol sedation than shown previously in either light or heavy social drinkers [1]. The observed sensitivity to alcohol’s positive effects in AUD drinkers suggests further research and consideration of clarifying the DSM-5 criterion to query separately for tolerance to alcohol’s sedating and impairing effects of alcohol apart from its the desirable and pleasant effects [7, 8].
In addition to clinically evaluating theories of allostasis and tolerance, on a broader level, the present study provides a comprehensive investigation of the biobehavioral response to alcohol in AUD drinkers, examining both subjective and physiological indicators of AUD pathology. The sample exhibited sensitivity to alcohol-induced autonomic arousal, with both doses producing similar HR increases throughout the BrAC and dose-dependent increases during the rising limb for cortisol and MAP. While prior work has identified potential physiological correlates of subjective alcohol responses in non-AUD social drinkers [21, 51,52,53,54], the biomarkers assessed in this sample of AUD drinkers did not show consistent associations with subjective responses. Numerous factors, including sample characteristics, route of alcohol administration, and choice of subjective measures may explain the differential findings. Alternatively, as fundamentally different systems underlie the processing of physiological and subjective alcohol responses, there may not be a direct relationship between these responses. Nevertheless, this remains a rich area for future research in establishing objective markers of excessive alcohol use.
The study results should be considered in light of the study strengths and limitations. Strengths include use of a placebo control and the alternative substance paradigm to minimize expectancy effects, comparison of two intoxicating doses of alcohol to examine tolerance and threshold effects, and use of reliable and valid measures of alcohol effects throughout the BrAC curve. In terms of limitations, the sample consisted of young adults to allow for comparisons across previous studies of young adult social drinkers [1, 3, 27, 55] and non-treatment seeking AUD drinkers [17, 18]. Despite the sample’s mean age of 27 years, most had severe (60%) or moderate (20%) AUD, however it remains an empirical question whether the observed positive alcohol responses would remain stable, increase, or decrease in chronic AUD drinkers through middle-age and older lifespan years, and whether drinking to alleviate withdrawal or negative affect (“dark side of addiction”) would eventually emerge. Additionally, subjective results by self-report may be confounded by response bias, demand characteristics, and variable item comprehension, but these were mitigated by providing clear instructions and not revealing the beverage contents. Last, as with any oral challenge study, there is a trade-off between internal and external validity as alcohol pharmacokinetics are more variable with oral than intravenous dosing [56]. However, controlling for BrAC at each dose did not change the significant findings and oral consumption aligns with the route of administration and experiences of drinkers [57].
In sum, the present study used a well-validated, placebo-controlled dose-ranging laboratory alcohol challenge paradigm to characterize acute subjective and physiological alcohol responses in young adult AUD drinkers from the third cohort of CSDP. At both high and very high alcohol doses, AUD drinkers were sensitive to alcohol stimulation, euphoria, liking and wanting, cardiovascular and acute neuroendocrine effects. These data, coupled with our recent 10-year re-examination study, demonstrate a persistence of alcohol’s pleasurable effects in those with AUD [3], providing direct evidence of acute reward sensitivity to two intoxicating alcohol doses in AUD drinkers in their young to early middle-adult years. The purported overall alcohol tolerance in AUD drinkers, at least among persons in their third decade of life, was not evident across study measures. This study overcomes a long history of insufficient acute alcohol testing in persons meeting criteria for AUD that has hampered scientific data critical to testing and modifying existing theories or proposing new theories of alcohol response phenotypes, as they relate to the development and maintenance of addiction. These data are also relevant to improving the efficacy of pharmacological and behavioral intervention strategies, as development of more agents that block or reduce alcohol’s rewarding effects [58] may be important therapeutic targets for drinkers with AUD.
References
King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389.
King AC, Hasin D, O’Connor SJ, McNamara PJ, Cao D. A prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol Psychiatry. 2016;79:489–98.
King A, Vena A, Hasin DS, DeWit H, O’Connor SJ, Cao D. Subjective responses to alcohol in the development and maintenance of alcohol use disorder. Am J Psychiatry. 2021;178:560–71.
King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry. 2014;75:798–806.
Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science (80-). 1997;278:52–8.
Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry. 2013;4:1–18.
Tabakoff B, Cornell N, Hoffman PL. Alcohol tolerance. Ann Emerg Med. 1986;15:1005–12.
Kalant H. Current state of knowledge about the mechanisms of alcohol tolerance. Addict Biol. 1996;1:133–41.
Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91.
Mendelson JH, Mello NK. Experimental Analysis of Drinking Behavior of Chronic Alcoholics. Ann N. Y Acad Sci. 1966;133:828–45.
Nathan PE, O’Brien JS. An experimental analysis of the behavior of alcoholics and nonalcoholics during prolonged experimental drinking: a necessary precursor of behavior therapy? Behav Ther. 1971;2:455–76.
Goodwin DW, Hill SY, Powell B, Viamontes J. Effect of alcohol on short term memory in alcoholics. Br J Psychiatry. 1973;122:93–4.
Mendelson JH, La Dou J, Solomon P. Experimentally induced chronic intoxication and withdrawal in alcoholics: part 3.Psychiatric findings. J Stud Alcohol Drugs. 1964;25:40–52.
Mendelson JH, Stein S. Serum Cortisol Levels in Alcoholic and Nonalcoholic Subjects During Experimentally Induced Ethanol Intoxication. Psychosom Med. 1966;28:616–26.
National Advisory Council on Alcohol Abuse and Alcoholism. Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation. 1989.
National Advisory Council on Alcohol Abuse and Alcoholism. Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation. 2005.
Bujarski S, Ray LA. Subjective response to alcohol and associated craving in heavy drinkers vs. alcohol dependents: an examination of Koob’s allostatic model in humans. Drug Alcohol Depend. 2014;140:161–7.
Thomas SE, Drobes DJ, Voronin K, Anton RF. Following alcohol consumption, nontreatment-seeking alcoholics report greater stimulation but similar sedation compared with social drinkers. J Stud Alcohol. 2004;65:330–5.
Bujarski S, Hutchison KE, Prause N, Ray LA. Functional significance of subjective response to alcohol across levels of alcohol exposure. Addict Biol. 2017;22:235–45.
Vena A, Howe M, Fridberg D, Cao D, King AC. The feasibility, tolerability, and safety of administering a very high alcohol dose to drinkers with alcohol use disorder. Alcohol Clin Exp Res. 2020;44:2588–97.
Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacol (Berl). 2001;157:20–30.
Ireland MA, Vandongen R, Davidson L, Beilin LJ, Rouse IL. Acute effects of moderate alcohol consumption on blood pressure and plasma catecholamines. Clin Sci (Lond). 1984;66:643–8.
Holdstock L, De Wit H. Individual differences in responses to ethanol and d-amphetamine:a within-subject study. Alcohol Clin Exp Res. 2001;25:540–8.
Schuckit MA, Gold E, Risch C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Arch Gen Psychiatry. 1987;44:942–5.
Kahler CW, Metrik J, Spillane NS, Day A, Leventhal AM, McKee SA, et al. Acute effects of low and high dose alcohol on smoking lapse behavior in a laboratory analogue task. Psychopharmacol (Berl). 2014;231:4649–57.
King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–35.
Wardell JD, Ramchandani VA, Hendershot CS. Drinking motives predict subjective effects of alcohol and alcohol wanting and liking during laboratory alcohol administration: a mediated pathway analysis. Alcohol Clin Exp Res. 2016;40:2190–8.
Sobell LC, Sobell MB. Measuring Alcohol Consumption. Timeline Follow-Back. In: Litten RZ, Allen JP, editors. Meas. Alcohol Consum., Totowa, NJ: Humana Press; 1992. p. 41–72.
First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis 1 Disorders, Research Version, Patient Edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 1995.
Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84:1353–7.
Babor TF, De La Fuente JR, Saunders JB, Grant M. Substance Abuse Disorders Identification Test: Guidelines for use in Primary Health Care. World Health Organization. Geneva, Switzerland; 2001.
Miller WR, Tonigan JS, Longabaugh R. The Drinker Inventory of Consequences (DrInC): An instrument for assessing adverse consequences of alcohol abuse. Project MATCH Monograph Series, Vol. 4. DHHS Publication No. 95-3911. Rockville MD: NIAAA, 1995.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An Inventory for Measuring Depression. Arch Gen Psychiatry. 1961;4:561.
Spielberger CD, Gorsuch R, Lushene RE, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA:Consulting Psychologists Press, Inc.; 1983.
Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39:5–41.
Conrad M, McNamara P, King A. Alternative substance paradigm: Effectiveness of beverage blinding and effects on acute alcohol responses. Exp Clin Psychopharmacol. 2012;20:382–9.
Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33:27–39.
Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–6.
Rueger SY, McNamara PJ, King AC. Expanding the utility of the biphasic alcohol effects scale (BAES) and initial psychometric support for the brief-BAES (B-BAES). Alcohol Clin Exp Res. 2009;33:916–24.
Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacol (Berl). 2013;227:177–92.
Haertzen CA, Hickey JE. Addiction Research Center Inventory (ARCI): Measurement of Euphoria and Other Drug Effects. In: Bozarth MA. (editors) Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer, New York, NY. https://doi.org/10.1007/978-1-4612-4812-5_24 (1987).
Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22.
Patrick ME. A call for research on high-intensity alcohol use. Alcohol Clin Exp Res. 2016;40:256–9.
Ray LA, Bujarski S, Yardley MM, Roche DJO, Hartwell EE. Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Abus. 2017;43:703–10.
Koob GF, Powell P, White A. Addiction as a coping response: hyperkatifeia, deaths of despair, and COVID-19. Am J Psychiatry. 2020;177:1031–7.
Pohorecky LA, Brick J, Carpenter JA. Assessment of the development of tolerance to ethanol using multiple measures. Alcohol Clin Exp Res. 1986;10:616–22.
Lê AD, Kiianmaa K. Characteristics of ethanol tolerance in alcohol drinking (AA) and alcohol avoiding (ANA) rats. Psychopharmacol (Berl). 1988;94:479–83.
Miller MA, Hays LR, Fillmore MT. Lack of tolerance to the disinhibiting effects of alcohol in heavy drinkers. Psychopharmacol (Berl). 2012;224:511–8.
Schuckit MA. Reactions to alcohol in sons of alcoholics and controls. Alcohol Clin Exp Res. 1988;12:465–70.
Vena AA, King AC. Testing the allostasis theory in light, heavy, and AUD drinkers. Res Soc Alcohol. Minneapolis, MN; 2019.
Brunelle C, Barrett SP, Pihl RO. Relationship between the cardiac response to acute intoxication and alcohol-induced subjective effects throughout the blood alcohol concentration curve. Hum Psychopharmacol. 2007;22:437–43.
Vatsalya V, Momenan R, Hommer DW, Ramchandani VA. Cardiac reactivity during the ascending phase of acute intravenous alcohol exposure and association with subjective perceptions of intoxication in social drinkers. Alcohol Clin Exp Res. 2014;38:1247–54.
Vatsalya V, Stangl BL, Ramchandani VA. Assessment of skin blood flow following acute intravenous alcohol, and association with subjective perceptions, in social drinkers. Alcohol Clin Exp Res. 2019;43:405–10.
Brkic S, Söderpalm B, Gordh AS. High cortisol responders to stress show increased sedation to alcohol compared to low cortisol responders: An alcohol dose–response study. Pharm Biochem Behav. 2016;143:65–72.
Weafer J, Ross TJ, O’Connor S, Stein EA, de Wit H, Childs E. Striatal activity correlates with stimulant-like effects of alcohol in healthy volunteers. Neuropsychopharmacology. 2018;43:2532–8.
Cyders MA, Plawecki MH, Corbin W, King A, McCarthy DM, Ramchandani VA, et al. To infuse or ingest in human laboratory alcohol research. Alcohol Clin Exp Res. 2020;44:764–76.
Fridberg DJ, Cao D, King AC. Alcohol subjective responses in heavy drinkers: Measuring acute effects in the natural environment versus the controlled laboratory setting. Alcohol Clin Exp Res. 2021;45:1287–97.
Ray LA, Green RJ, Roche DJO, Magill M, Bujarski S. Naltrexone effects on subjective responses to alcohol in the human laboratory: a systematic review and meta-analysis. Addict Biol. 2019;24:1138–52.
Acknowledgements
The authors would like to extended appreciation to Patrick McNamara for project coordination, Nandini Kaluvakolanu for running the salivary cortisol assays, Jon Grant, M.D. for medical supervision and oversight, and Patrick Smith, Eric Giger, and James Faria for their role in data collection and database management. This research was supported by grant R01-AA013746 (AK) from the National Institute on Alcohol Abuse and Alcoholism and T32-DA043469 (AV) from the National Institute on Drug Abuse.
Author information
Authors and Affiliations
Contributions
AK conceived of the study design, supervised collection, management, analysis, and interpretation of the data, and led the drafting and final approval of the paper. AV substantially contributed to data interpretation and paper writing. MH and AF drafted sections of the paper and contributed to presentation of the results. DC provided statistical support. Authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
King, A.C., Vena, A., Howe, M.M. et al. Haven’t lost the positive feeling: a dose-response, oral alcohol challenge study in drinkers with alcohol use disorder. Neuropsychopharmacol. 47, 1892–1900 (2022). https://doi.org/10.1038/s41386-022-01340-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01340-2