Abstract
Identification of biomarkers for psychiatric disorders remains very challenging due to substantial symptom heterogeneity and diagnostic comorbidity, limiting the ability to map symptoms to underlying neurobiology. Dimensional symptom clusters, such as anhedonia, hyperarousal, etc., are complex and arise due to interactions of a multitude of complex biological relationships. The primary aim of the current investigation was to use multi-set canonical correlation analysis (mCCA) to derive biomarkers (biochemical, physiological) linked to dimensional symptoms across the anxiety and depressive spectrum. Active-duty service members (N = 2,592) completed standardized depression, anxiety and posttraumatic stress questionnaires and several psychophysiological and biochemical assays. Using this approach, we identified two phenotype associations between distinct physiological and biological phenotypes. One was characterized by symptoms of dysphoric arousal (anhedonia, anxiety, hypervigilance) which was associated with low blood pressure and startle reactivity. This finding is in line with previous studies suggesting blunted physiological reactivity is associated with subpopulations endorsing anxiety with comorbid depressive features. A second phenotype of anxious fatigue (high anxiety and reexperiencing/avoidance symptoms coupled with fatigue) was associated with elevated blood levels of norepinephrine and the inflammatory marker C-reactive protein in conjunction with high blood pressure. This second phenotype may describe populations in which inflammation and high sympathetic outflow might contribute to anxious fatigue. Overall, these findings support the growing consensus that distinct neuropsychiatric symptom patterns are associated with differential physiological and blood-based biological profiles and highlight the potential of mCCA to reveal important psychiatric symptom biomarkers from several psychophysiological and biochemical measures.
Similar content being viewed by others
Introduction
Stress-related neuropsychiatric disorders such as major depressive disorder (MDD), anxiety disorders, and post-traumatic stress disorder (PTSD) are common and associated with high levels of comorbidity and within-disorder heterogeneity [1,2,3]. High disorder comorbidity and symptom heterogeneity suggest that approaches focusing on DSM5 diagnostic categories or on a circumscribed biomarker set could limit identification of likely complex relationships between clinically heterogeneous neuropsychiatric symptoms and their underlying biological signatures [4]. This is consistent with the concept that neuropsychiatric disorders are not distinct disorders, but instead are comprised of sets of neurobiological mechanisms across several units of analysis [5,6,7]. Since it is unlikely that any single biological mechanism that operates in isolation can explain the full range of symptoms of a given disorder, there is a need for development of alternative analytic approaches that address the dimensional nature of psychiatric symptoms and the array of neurobiological mechanisms that are likely contributors [8]. Such approaches can help identify sources of heterogeneity within a disorder or reveal comprehensive phenotype profiles to explain transdiagnostic symptom patterns. Use of unbiased data-driven approaches have begun to yield biological signatures of discrete profiles of stress-related neuropsychiatric symptoms [9,10,11].
Leverage of multivariate analytic approaches is increasingly used to identify interrelationships among multiple units of analysis, including between psychiatric symptoms and biological markers that univariate approaches are unable to capture [12,13,14]. One analytic technique that has received renewed interest in addressing psychiatric and neurobiological heterogeneity in neuropsychiatric disorders is canonical correlation analysis (CCA) [15, 16]. CCA a type of multivariate analysis that seeks to extract multiple sets of latent features called canonical variates (CVs) that when correlated, represent the maximized linear relationship between feature sets of variables, and has recently been applied to neuroimaging measures to identify brain-based dimensions of neuropsychiatric symptoms [17,18,19,20]. It can be expanded to include multiple feature sets, called multi-set CCA (mCCA), to identify multivariate patterns between multiple neurobiological modalities and psychiatric disorders that a more traditional two-way CCA would miss [21,22,23]. mCCA is like other multivariate fusion and latent variable approaches such as joint-independent components analysis (jICA), in that they are both data-driven approaches that do not require specific hypotheses and they both seek to find latent patterns across two or more variable feature sets [24]. Whereas jICA assumes a linked-relationship between each variable modality, mCCA is more flexible by allowing both linked and distinct interrelationships between two or more variables (see Ref. [23] for a review). However, applying mCCA to investigate stress-related symptoms is still relatively rare, partly due to the need for extensive, deep phenotype data sets with adequate power.
The aim of our current investigation was to use mCCA to derive linked relationships between dimensional stress-related psychiatric symptoms, psychophysiological measures and a set of biochemical assays in a large cohort of active-duty participants from the Marine Resiliency Study (MRS; N = 2592) [25]. MRS is a large prospective, longitudinal study of active-duty service members aimed to identify predictors of risk and resilience to combat stress. Here we focused on the pre-deployment time point which had the largest sample size available with three different features of data including biochemical (e.g., blood, saliva, and urine bioassays), psychophysiological (e.g., acoustic startle reflex, fear potentiated startle, blood pressure, heart rate variability), and questionnaire or interview derived psychiatric symptom data (e.g., depression, anxiety, and trauma-related reexperiencing, avoidance, and hypervigilance symptoms). Using these dataset features, we performed a three-way mCCA to define dimensional transdiagnostic psychiatric symptom components (See Fig. 1) that associated with specific biological marker sets.
After preprocessing, a data-reduction step was conducted on the psychophysiological and psychiatric symptom measures via principle components analysis (PCA). Next, the three features sets were entered into the mCCA to derive five canonical variates (CVs) for each feature set and for each subject. The first CVs for each feature set are correlated to form a multi-set canonical correlation (mCC), which represents the maximized linear relationship between the three data feature sets and can be represented as a correlation table. The remaining mCCs are calculated using the residuals from the prior mCC. Portions of this figure was created using BioRender.com (San Francisco, CA).
Materials and methods
Participants
Participants were recruited from infantry battalions deploying to either Iraq (2008) or Afghanistan (2009–2010). All active-duty members of these operational units were eligible. There were no exclusion criteria. Women were not included because female Marines were not part of infantry battalions at the time of testing (see Table 1 for sample details). A total of 2592 active-duty Marines and accompanying Navy personnel were enrolled in the pre deployment assessment. Participants missing multiple data points were removed (n = 88), leaving a total of 2504 for the primary analyses before preprocessing. Study procedures were approved by the institutional review boards of the Veteran’s Administration San Diego Healthcare System; the University of California, San Diego; and the Naval Health Research Center. All participants provided voluntary written informed consent. Complete MRS methods and demographic information are described elsewhere [25]. The measures relevant to the present study are presented here.
Psychiatric measures
PTSD symptoms were assessed with the Clinician-Administered PTSD Scale for DSM-IV (CAPS-IV) [26] the gold-standard clinical interview assessing for diagnostic criteria and severity of PTSD. All interviews were conducted by study personnel trained, certified, and supervised by a licensed psychiatrist (D.G.B). 13.18% of the sample met criteria for PTSD using partial DSM-IV PTSD criteria: >0 Cluster B symptom, >1 Cluster C symptoms, and >1 Cluster D symptoms, with minimum frequency ratings of 1 and minimum intensity ratings of 2 [27]. Depression symptoms were measured with the Beck Depression Inventory version 2 (BDI-II) [28]: 7.91% of the sample met criteria for moderate to severe depression (BDI-II score > 19). The BDI-II measures the presence of depressive symptoms within the past 2 weeks. Anxiety symptoms were assessed with the Beck Anxiety Inventory (BAI) [29], a reliable measure of general anxiety symptoms present within the past week which discriminates between anxiety vs. depressive symptoms fairly well [30]: 13.74% of the sample endorsed moderate to severe levels of anxiety (BAI score >15).
Psychophysiological measures
Modulation of acoustic startle reactivity was measured with three separate tasks. Before testing, each participant is screened for hearing impairment and fitted with headphones while seated in a comfortable chair facing a computer monitor. After electrode placement and verification, the participant completed the following startle tasks: (1) assessment of startle threshold using acoustic tones, (2) test of modulation of acoustic startle response while viewing emotional images or anticipating image presentation, and (3) test for pre-pulse inhibition and startle habituation [31,32,33,34]. Details of each task and pre-processing are reported in the Supplementary Materials. Cardiovascular measures included systolic and diastolic blood pressure, heart-rate, and heart rate variability (HRV) [35, 36].
Biochemical measures
Peripheral blood, urine, and saliva samples were collected from all participants. Blood-based assays were C-reactive protein, and neuropeptide-Y. Saliva-based assays were cortisol, cotinine, and α-amylase. Spot-urine assays were epinephrine, and norepinephrine [25, 37,38,39]. See Supplementary Materials for data collection and processing details.
Analysis
Pre-Processing and data reduction
The pre-processing of the psychophysiological and biochemical markers was conducted using standard procedures (see Supplemental Materials). For the remaining participants (n = 2504), missing data was imputed using predicted mean matching multiple imputation by chained equations using the mice package in R [40]. Missing datapoints for each variable were imputed within its respective feature set (i.e., missing cortisol value was imputed using biochemical variables and not psychophysiology or psychiatric symptoms). Missing datapoints were infrequent: Biochemical variable range: 0–80 missing datapoints; Psychophysiology variable range: 0 – 30; psychiatric symptoms variable range: 6–12 (see Supplementary Table 1 for details). Next, psychophysiological non-responders (e.g. no startle response) were removed (n = 422). Multivariate outliers were additionally removed (n = 58), leaving a final sample n = 2024. Variables were normalized using a best-model approach via the bestNormalize package in R [41]. Variance associated with age, battalion cohort, time of day of data collection, and ethnicity was removed via multiple regression prior to computing the mCCA. All pre-processing and analyses were conducted in R.
Data-reduction
Data reduction was performed to reduce data dimensionality and orthogonalize variables sets with elevated collinearity prior to computing the mCCA [15, 18]. The psychophysiological variables (nvar = 20; variance inflation factor [VIF] range=1.06–10.62) and psychiatric symptoms (nvar = 57; VIF range: 1.07–2.69) were entered into separate principal component analyses (PCAs) to derive a reduced set of principle components (PCs) for each feature set. The number of PCs derived was based upon Horn’s technique [42] using the paran package in R [43]. This technique compares the PCA eigenvalues to eigen values produced on a random number of datasets to adjust the sample error-induced inflation. PCs with adjusted eigenvalues greater than one were kept. For the psychophysiological feature set, five PCs were derived explaining 72.4% of the variance. Supplemental Table 2 shows the PCA loadings and names given to each PC. For the psychiatric symptom feature set, 10 PCs were derived explaining 52.3% of the variance. Supplementary Table 3 shows the PCA loadings and names for each component. The five psychophysiological PCs and the 10 psychiatric symptom PCs, along with the seven biochemical variables were then submitted to the mCCA. The biochemical feature set consisted of only seven variables and had low VIFs (range: 1.00–1.51), therefore data-reduction was not necessary.
Multi-set CCA
To examine the inter-relationships between psychophysiology and biochemical feature sets, and psychiatric clinical symptoms, we computed a multi-set canonical correlation analysis (mCCA) using R:mcancor via the nscancor package [44]. mCCA is an extension of a two-way CCA to allow for multiple data-set domains [22, 45]. Like traditional CCA [46], mCCA identifies linked relationships of canonical variates (CVs), such that the correlations among the CVs for the multiple domains are linearly maximized, in this case three data domains. The next set of canonical variables is found by again maximizing their correlation from the residuals produced from the prior multi-set canonical correlation, under the additional constraint that they must be uncorrelated to all previous ones until reaching the final canonical set (mCC = 5). These constraints are specified through iterative regression functions for each domain set. To derive an overall metric for each of the five canonical correlation multisets, we computed the sum of the three pairwise squared canonical correlations (R2) for each mCC. This metric indicates the shared variance explained by each canonical triplet and has a similar interpretation as R2 in multiple regression [47].
Permutation and bootstrapping
To determine the significance of mCCA correlation, we performed restricted, or multi-level block, permutation using the “permute” package in R [48]. We randomly scrambled subjects’ psychophysiological and biochemical data columns to break the association between subjects’ psychophysiology/biochemical measures with their clinical psychiatric symptom measures. To reduce potential inflation of significance testing caused by dependence in datasets [49], we restricted the permutations to within battalion cohort due to the shared military experience within each battalion. We re-ran mCCA for 5000 permutations to create a null distribution of mCCA values. We compared the original mCCA values to these re-aligned distributions. Any significant mCCA values would have to be greater than the correlations in the permuted datasets. Permuted P values were computed by determining the number of permuted canonical correlation values (mCCperm) that were greater than or equal to the observed canonical correlation (mCCobs) divided by the number of permutations: Pperm = (# mCCperm ≥ mCCobs) / 5000. We conducted permutations for each mCC triplet and each pair-wise CC. To reduce Type-I error, the permuted P values were corrected using Benjamini-Hochberg False Discovery Rate (FDR < 0.05). We additionally computed a bootstrap resampling procedure. We performed 5,000 random resamples and estimated the means, standard errors, and 95% confidence intervals for each mCCA value (See Supplementary Table 4 for the bootstrapped mean mCCs and the standard errors).
Results
Multi-set canonical correlation analysis
A multi-set canonical correlation analysis (mCCA) of psychiatric symptoms and biological measures revealed four significant canonical correlations (Table 2). In the remaining sections, we focus only on the first two mCCs. The first two mCCAs have moderately robust pairwise CCs, whereas the remaining mCCs were considerably weaker and potentially not scientifically meaningful [15]. We present the results for the remaining mCCs in Supplementary Materials Results and Supplementary Figs. 1–3.
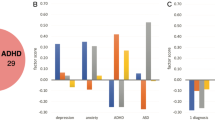
The first mCC revealed a significant multi-set relationship between psychiatric symptoms and physiological/biological measures mCC 1 R2 = 0.22, Ppermuted < 0.0002, FDRadjusted < 0.0005. Examination of the pairwise relationships between the canonical variates (CVs) indicated that mCCA 1 was driven predominately by the relationship between the psychiatric symptoms and psychophysiology, CC = 0.46, Ppermuted < 0.0002, FDRadjusted < 0.0002, CIboot = 0.33–0.46; Fig. 2A. The relationship between psychiatric symptoms and biochemical CVs was not significant, CC = 0.09, Ppermuted = 0.99, PFDR = 0.99, CIboot = 0.05–0.15; nor was the relationship between the psychophysiology and biochemical CVs significant, CC = 0.05, Ppermuted = 0.99, PFDR = 0.99, CIboot = 0.003–0.09. Examination of the canonical loadings for the first mCC indicate that the psychiatric symptom CV 1 (Fig. 2B) was characterized by a cluster of symptoms including dysphoric and anxious arousal as described by the 5-factor model of CAPS [50] which includes elevated dysphoric arousal, anxiety, and anhedonia (canonical loadings = 0.66, 0.41, and 0.39, respectively). The psychophysiology CV 1 (Fig. 3B) was associated with low blood pressure levels, general startle reactivity, and prepulse inhibition (canonical loadings = –0.52, –0.40, and –0.32, respectively). These findings suggest that individuals who reported higher levels of dysphoric and anxious arousal exhibited both lower blood pressure and startle reactivity.
A Scatterplot depicts the significant pairwise canonical correlation (CC) between the Psychophysiology canonical variate (CV) and the psychiatric symptom CV. Each dot represents an individual subject’s score as a function of the relationship between each CV pair. The inset reflects the permutation test (5000 permutations). The vertical dotted line indicates the exact pairwise canonical correlation value. P values were calculated using restricted permutation testing (Pperm). B For visualization purposes, univariate Pearson correlations of two examples of top-weighted psychophysiological and psychiatric symptom variables from mCC 1 are depicted. C Canonical loadings for the psychiatric symptom CV 1. The top three PCs are dysphoric arousal, anxiety, and anhedonia. C Canonical loadings for the psychophysiology CV 1. The top two PCs are general startle reactivity and blood pressure.
A Scatterplots depict the two significant pairwise canonical correlation (CC) between the biochemical canonical variate (CV) and the psychiatric symptom CV and the psychophysiological CV and the psychiatric CV. The insets reflect the permutation tests (5000 permutations). The vertical dotted lines indicate the exact pairwise canonical correlation value. P values were calculated using restricted permutation testing (Pperm). B For visualization purposes, residualized univariate Pearson correlations of the top-weighted biochemical, psychophysiological, and psychiatric symptom variables from mCC 2 are depicted. (C) Canonical loadings for the psychiatric symptom CV 2. The top three PCs are fatigue, anxiety, and reexperiencing/avoidance symptoms. (C) Canonical loadings for the for the biochemical CV 2. The top two PCs are norepinephrine and C-reactive protein (CRP). C Canonical loadings for the psychophysiology CV 2. The top two PCs are blood pressure and low startle threshold (Low thresh.).
The second mCC revealed a significant multi-set relationship between psychiatric symptoms and biological measures mCC 2 R2 = 0.05, Ppermuted < 0.0004 FDRadjusted < 0.0007; Table 2. This canonical finding was predominately driven by the relationship between the psychiatric symptoms and biochemical assays (CC = 0.19, Ppermuted = 0.0002, FDRadjusted = 0.0004, CIboot = 0.10–0.26; Fig. 3A) and between the psychiatric symptoms and the psychophysiology measures (CC = 0.11, Ppermuted = 0.004, FDRadjusted = 0.006, CIboot = 0.01–0.12; Fig. 3B). Examination of the canonical loadings indicate that the psychiatric symptom CV 2 (Fig. 3C left panel) was characterized by elevated symptom levels of fatigue, anxiety and by the symptoms of reexperiencing/avoidance (canonical loadings = 0.71, 0.30, and 0.29, respectively). The biochemical CV2 (Fig. 3C middle panel) was characterized by elevated norepinephrine and CRP (canonical loadings = 0.75 and 0.51). The psychophysiology CV 2 (Fig. 3C right panel) was associated with high blood pressure, and negatively associated with the low startle threshold PC (i.e. startle less likely when presented with weak startling stimuli, indicating high startle threshold) (canonical loadings = 0.87 and –0.48). These findings indicate that increases in self-reported fatigue, anxiety, and reexperiencing/avoidance symptoms of intrusive trauma imagery and avoidance were jointly associated with increases in norepinephrine, CRP levels, blood pressure, and high startle threshold. These findings remained robust even when not controlling for variation in age, race/ethnicity, time of day, and battalion cohort (See Supplementary Table S5).
Pearson correlations
Details for the correlational analysis between the PCs and the individual variables are reported in the Supplementary Materials.
PC correlations
The largest pairwise-correlation between psychophysiology PCs and psychiatric symptoms was between the blood pressure PC and the hypervigilance PC, R2 = –0.19, p = 7.6 × 10–18, PFDR = 2.0 × 10–16 (See Supplementary Fig. 3), an effect size lower than that observed in mCCA R1(CC = 0.46). The largest pairwise-correlation between biochemical and psychiatric systems was between norepinephrine and the fatigue PC, R2 = 0.03, r = 0.18, p = 2.02 × 10–15, which is on par with the biochemical and psychiatric symptom CC from mCCA R2 (CC = 0.19) (See Supplementary Fig. 4).
Item-level correlations
Like the correlations computed on the PCs, for mCC1, the individual psychiatric symptom items (22 items) were low to moderately correlated with psychophysiology (12 items), rs < 0.12 (range:–0.03–0.12), ps > 0.001 (uncorrected; See Supplementary Fig. S5). For mCC2, the individual psychiatric symptom items (15 items) were low to moderately correlated with the psychophysiology (6 items) and biochemical (2 items) measures that most defined the PCs underlying mCC2, rs < 0.14 (range: –0.06 to 0.14), ps > 0.001 (uncorrected; see Supplementary Fig. S6).
Discussion
The primary aim of the current investigation was to derive multivariate biomarkers linked to dimensional symptom measures of dysphoria, anxiety, fatigue, and trauma-related reexperiencing and avoidance. We identified linked patterns that characterized the relationship between these dimensional symptoms and biological signature sets. We identified a symptom cluster of dysphoric arousal and anhedonia that was associated with blunted startle reactivity and low blood pressure, and alternatively, a symptom cluster of fatigue and reexperiencing/avoidance symptoms associated with elevated norepinephrine and CRP, high blood pressure and high startle threshold. We also identified two other weaker but statistically significant relationships between psychiatric symptoms, psychophysiological, and biochemical measures (See Supplementary Materials). Using permutation testing and bootstrapping our results were shown to be reliable and reproducible. Overall, CCA also offers the potential to identify robust relationships with larger effect sizes and with stronger protection against Type 1 errors due to fewer multiple comparisons than when using traditional univariate analyses [16, 51]. As shown in the current investigation, we observed a robust relationship between psychiatric symptoms and psychophysiology CV (CC 1 = 0.46), whereas the highest univariate correlation was substantially weaker (r = –0.19). These results provide compelling support for the utility of the mCCA approach to identify novel and robust findings in complex multimodal datasets.
Using the mCCA approach, we derived psychiatric symptom phenotypes that were based on patterns between individual differences of psychophysiology and psychiatric symptoms. In other words, the psychiatric dimension of dysphoric arousal (characterized by anhedonia, dysphoric arousal, and anxiety) observed in mCC 1 is represented as a mixture of these symptoms and psychophysiology measures. The psychophysiology dimension was comprised of blunted measures of arousal, including low general startle reactivity and low blood pressure. The finding of a negative relationship between general startle response and low blood pressure with self-reported arousal may seem counterintuitive [52,53,54]. However, both startle hyporeactivity [55,56,57,58,59] and low blood pressure [60,61,62] are documented in individuals with a history of anxiety, dysphoria, and stress-related neuropsychiatric symptoms (see Lang et al., 2014 for a review [63]). Epidemiological studies support a link between low blood pressure and depression, particularly anhedonia symptoms [64]. In a large cohort (n = 60,799), individuals with comorbid anxiety and depression were more likely to have low blood pressure than individuals without these symptoms [65] and high baseline levels of anxiety and depression predicted low blood pressure 22 years later [62]. Furthermore, low blood pressure is associated with suicidal ideation [66] and is a risk-factor for late life depression [61]. The CCA also showed blunted startle associated with dysphoric arousal and anhedonia. Blunted startle responding can occur after chronic or long-standing stress [63, 67,68,69] and in adults endorsing early-life adversity [59, 70, 71]. In a recent review of psychophysiological phenotypes across anxiety and mood disorders, blunted heart rate and startle responses either at baseline or in response to threat is observed in populations with high chronic distress and depression symptoms [72]. Blunted psychophysiological reactivity may also reflect elevated dissociative symptoms in individuals experiencing chronic traumatic stress symptoms [73, 74]. Pre-deployment dissociative symptoms were not assessed in this investigation; therefore, it will be important for future studies to include a measure of dissociation to determine how it links to multimodal physiological markers. These previous findings have focused on traditional diagnostic categories (i.e., Major Depressive Disorder, PTSD), whereas our findings are focused on transdiagnostic phenotypes. Therefore, direct comparison of our findings to previous work needs to acknowledge this distinction. However, these prior findings are thus very much in line with the CCA identified in the current study that used an unbiased, data-driven approach across wide range of symptom measures and physiological and peripheral signaling markers. Taken together these findings suggest that there is a clear subpopulation of individuals with blunted physiological responses associated with combined dysphoric arousal and anhedonia, which may suggest specific mechanisms underlying this symptom pattern. The biological mechanism(s) for this association is unclear, however there is some data to suggest an inflexible corticolimbic-response including blunted amygdala reactivity or that poor amygdala synchrony to emotional stimuli contribute to the blunted physiological reactivity [75, 76] as well as dysregulation of the hypothalamic-pituitary-adrenal axis and renin-angiotensin systems that influence blood pressure [65].
We also identified a biomarker associated with anxious fatigue, high anxiety and reexperiencing/avoidance symptoms—a symptom profile that covaried with elevated norepinephrine and CRP, elevated blood pressure, and high startle threshold. Physiologically, autonomic imbalance, such as excess sympathetic drive, particularly under periods of stress, are known to contribute to increased blood pressure and inflammation [77,78,79,80,81,82]. Thus, our observed relationship between CRP, norepinephrine and blood pressure levels is not un-expected. The physiologic profile of high inflammation/sympathetic drive, associated with reexperiencing/avoidance symptoms and overall high anxiety/fear symptoms in humans is consistent with previous work, and may be exacerbated in those with more severe illness [79, 83,84,85]. These findings are also in line with descriptions of both high CRP and peripheral norepinephrine across anxiety and trauma disorders as well as during chronic stress (e.g. [86,87,88,89,90]) and fatigue [91]. A benefit of CCA and mCCA is that they can extract multiple components of latent variable patterns—relationships that exist in a high-dimensional dataset that would not otherwise be apparent if a non-latent variable approach was used [47]. The high sympathetic drive phenotype is a unique linear combination of psychiatric, biochemical, and psychophysiological variables that is orthogonal to the phenotype observed in mCC 1.
There are limitations to this study that should be considered. First, although the CCA approach may allow for detection of complex relationships, it requires a number of a-priori choices in model development and interpretation, including definition of the phenotype and delimitation of the variety and array of biological and behavioral markers to be included in the analytic model. This is a feature of most unbiased analytic approaches however, not just CCA [13]. Related, creation of robust data-driven and latent psychiatric phenotypes helps address clinical heterogeneity and aids in linking complex constructs across multiple domains. But such approaches also raise important questions about interpretability and applications in new datasets [92]. Enhancing the generalizability and clinical utility of latent variable psychiatric phenotypes will be a critical step for future investigations. Second, the sample consisted exclusively of active-duty male Marine/Navy servicemen and thus is not necessarily generalizable to civilians and females [93, 94]. Third, this population had good variance in symptoms and biomarker phenotypes, however it was predominantly a relatively healthy population, thus other or additional symptom-marker relationships may be detectable in a more clinically impaired sample. Fourth, the sample was predominately White and the results may not generalize to individuals from other racial groups. Future work will be needed to examine racial and ethnic differences in multimodal phenotypes since recent work has found racial differences in phenotypes associated with neuropsychiatric symptoms [95]. Fifth, another drawback of CCA is that it is based on the assumption that the relationships between the features are linear and therefore do not measure higher-order relationships [15]. Applying kernelized-CCA, other multivariate/machine learning approaches (e.g., independent components analysis [ICA], neural nets), or their combination (mCCA + ICA, deep CCA) may better identify non-linear relationships between variable sets than CCA alone [9, 21, 96, 97]. However, these limitations are balanced by notable strengths, including the large sample (N = 2024), the deeply phenotyped dataset, and the relative physical health of the population—reducing confounds of comorbid physical illnesses and other extraneous variables (note all peripheral biomarkers were controlled for the effects of age, time of assessment, cohort, and ethnicity).
Conclusion
High psychiatric disorder comorbidity and symptom heterogeneity suggests that the current diagnostic system is not capturing the range of patient’s symptom experience, which may hinder the identification of clinically useful biomarkers to guide treatment development [2, 98]. Work linking a single neurobiological measure to a single diagnostic disorder has had limited clinical utility [99], motivating the field to shift to a framework where the focus is on dimensional psychiatric symptoms, not diagnostic categories, and on multiple, not single, biological markers [5, 11, 100,101,102]. Clearly, new data-driven analytic strategies are needed to address the complex multivariate relationship between psychiatric symptoms and multiple biological markers [8, 103], with mCCA being well-suited to handle this challenge [47]. The current findings support the mission towards a dimensional model of neuropsychiatric symptoms grounded in neurobiology and highlight the potential of multivariate statistics to reveal important psychiatric symptom biomarkers derived from several psychophysiological and biochemical measures. Future work will be required to apply this approach with other high-dimensional datasets that are an inherent part of biological assays (e.g., neuroimaging, genome, epigenome), and to test how multimodal biomarkers relate to other measures (e.g., trauma history, psychosocial functioning), and to determine their predictive utility.
Data availability
Data will be available under institutional approved data use agreement.
Change history
30 September 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41386-022-01467-2
References
Allsopp K, Read J, Corcoran R, Kinderman P. Heterogeneity in psychiatric diagnostic classification. Psychiatry Res. 2019;279:15–22.
Feczko E, Miranda-Dominguez O, Marr M, Graham AM, Nigg JT, Fair DA. The Heterogeneity Problem: Approaches to Identify Psychiatric Subtypes. Trends Cogn Sci. 2019;23:584–601.
Galatzer-Levy IR, Bryant RA. 636,120 ways to have posttraumatic stress disorder. Perspect Psychol Sci 2013;8:651–62.
Kalia M, Costa e Silva J. Biomarkers of psychiatric diseases: current status and future prospects. Metabolism 2015;64:S11–S15.
Insel TR, Cuthbert BN. Brain disorders? Precisely: precision medicine comes to psychiatry. Science (80-). 2015;348:499–500.
Krueger RF, Kotov R, Watson D, Forbes MK, Eaton NR, Ruggero CJ, et al. Progress in achieving quantitative classification of psychopathology. World Psychiatry. 2018;17:282–93.
Liberzon I. Searching for intermediate phenotypes in posttraumatic stress disorder. Biol Psychiatry. 2018;83:797–9.
Mellem MS, Liu Y, Gonzalez H, Kollada M, Martin WJ, Ahammad P. Machine learning models identify multimodal measurements highly predictive of transdiagnostic symptom severity for mood, anhedonia, and anxiety. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:56–67.
Siegel CE, Laska EM, Lin Z, Xu M, Abu-Amara D, Jeffers MK, et al. Utilization of machine learning for identifying symptom severity military-related PTSD subtypes and their biological correlates. Transl Psychiatry. 2021;11:227.
Ben-Zion Z, Zeevi Y, Keynan NJ, Admon R, Kozlovski T, Sharon H, et al. Multi-domain potential biomarkers for post-traumatic stress disorder (PTSD) severity in recent trauma survivors. Transl Psychiatry. 2020;10:208.
Harnett NG, Stevens JS, Fani N, van Rooij SJH, Ely TD, Michopoulos V, et al. Acute Posttraumatic Symptoms Are Associated With Multimodal Neuroimaging Structural Covariance Patterns: A Possible Role for the Neural Substrates of Visual Processing in Posttraumatic Stress Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020. 2020. https://doi.org/10.1016/j.bpsc.2020.07.019.
Ferrante M, Redish AD, Oquendo MA, Averbeck BB, Kinnane ME, Gordon JA. Computational psychiatry: a report from the 2017 NIMH workshop on opportunities and challenges. Mol Psychiatry. 2019;24:479–83.
Bzdok D, Meyer-Lindenberg A. Machine learning for precision psychiatry: opportunities and challenges. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:223–30.
Janssen RJ, Mourão-Miranda J, Schnack HG. Making individual prognoses in psychiatry using neuroimaging and machine learning. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:798–808.
Wang H-T, Smallwood J, Mourao-Miranda J, Xia CH, Satterthwaite TD, Bassett DS, et al. Finding the needle in a high-dimensional haystack: canonical correlation analysis for neuroscientists. Neuroimage 2020;216:116745.
Zhuang X, Yang Z, Cordes D. A technical review of canonical correlation analysis for neuroscience applications. Hum Brain Mapp. 2020;41:3807–33.
Mihalik A, Ferreira FS, Moutoussis M, Ziegler G, Adams RA, Rosa MJ, et al. Multiple holdouts with stability: improving the generalizability of machine learning analyses of brain-behavior relationships. Biol Psychiatry. 2020;87:368–76.
Mihalik A, Ferreira FS, Rosa MJ, Moutoussis M, Ziegler G, Monteiro JM, et al. Brain-behaviour modes of covariation in healthy and clinically depressed young people. Sci Rep. 2019;9:11536.
Xia CH, Ma Z, Ciric R, Gu S, Betzel RF, Kaczkurkin AN, et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun. 2018;9:3003.
Stout DM, Buchsbaum MS, Spadoni AD, Risbrough VB, Strigo IA, Matthews SC, et al. Multimodal canonical correlation reveals converging neural circuitry across trauma-related disorders of affect and cognition. Neurobiol Stress. 2018;9:241–50.
Calhoun VD, Sui J. Multimodal fusion of brain imaging data: a key to finding the missing link(s) in complex mental illness. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:230–44.
Correa NM, Eichele T, Adali T, Li Y-O, Calhoun VD. Multi-set canonical correlation analysis for the fusion of concurrent single trial ERP and functional MRI. Neuroimage. 2010;50:1438–45.
Sui J, Adali T, Yu Q, Chen J, Calhoun VD. A review of multivariate methods for multimodal fusion of brain imaging data. J Neurosci Methods. 2012;204:68–81.
Sui J, Pearlson GD, Du Y, Yu Q, Jones TR, Chen J, et al. In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biol Psychiatry. 2015;78:794–804.
Baker DG, Nash WP, Litz BT, Geyer MA, Risbrough VB, Nievergelt CM. Predictors of risk and resiliencefor posttraumatic stress disorder among ground combat Marines:methods of the Marine Resiliency Study. Prev Chronic Dis. 2012;9:110134.
Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90.
Acheson DT, Geyer MA, Baker DG, Nievergelt CM, Yurgil K, Risbrough VB. Conditioned fear and extinction learning performance and its association with psychiatric symptoms in active duty Marines. Psychoneuroendocrinology 2015;51:495–505.
Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. J Pers Assess. 1996;67:588–97.
Beck AT, Steer RA Beck Anxiety Inventory Manual. San Antonio, TX: Harcourt Brace and Company; 1993.
Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol. 1994;103:103–16.
Acheson DT, Stein MB, Paulus MP, Ravindran L, Simmons AN, Lohr JB, et al. Effects of anxiolytic treatment on potentiated startle during aversive image anticipation. Hum Psychopharmacol Clin Exp. 2012;27:419–27.
Acheson DT, Stein MB, Paulus MP, Geyer MA, Risbrough VB. The effect of pregabalin on sensorimotor gating in ‘low’ gating humans and mice. Neuropharmacology 2012;63:480–5.
Strigo IA, Simmons AN, Matthews SC, Craig ADB. The relationship between amygdala activation and passive exposure time to an aversive cue during a continuous performance task. PLoS ONE. 2010;5:e15093–e15093.
Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60:402–9.
Minassian A, Geyer MA, Baker DG, Nievergelt CM, O’Connor DT, Risbrough VB. Heart rate variability characteristics in a large group of active-duty marines and relationship to posttraumatic stress. Psychosom Med. 2014;76:292–301.
Biswas N, Maihofer AX, Mir SA, Rao F, Zhang K, Khandrika S, et al. Polymorphisms at the F12 and KLKB1 loci have significant trait association with activation of the renin-angiotensin system. BMC Med Genet. 2016;17:21.
Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, et al. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71:423–31.
Miramontes-Gonzalez JP, Hightower CM, Zhang K, Kurosaki H, Schork AJ, Biswas N, et al. A new common functional coding variant at the DDC gene change renal enzyme activity and modify renal dopamine function. Sci Rep. 2019;9:5055.
Reijnen A, Geuze E, Eekhout I, Maihofer AX, Nievergelt CM, Baker DG, et al. Biological profiling of plasma neuropeptide Y in relation to posttraumatic stress symptoms in two combat cohorts. Biol Psychol. 2018;134:72–79.
van Buuren S, Groothuis-Oudshoorn CGM. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67.
Peterson RA, Cavanaugh JE. Ordered quantile normalization: a semiparametric transformation built for the cross-validation era. J Appl Stat. 2020;47:2312–27.
Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika 1965;30:179–85.
Dinno A. Exploring the Sensitivity of Horn’s Parallel Analysis to the Distributional Form of Random Data. Multivar Behav Res. 2009;44:362–88.
Sigg C, Fischer B, Ommer B, Roth V, Buhmann J Nonnegative CCA for Audiovisual Source Separation. 2007 IEEE Work. Mach. Learn. Signal Process. 2007, p. 253–8.
Kettenring JR. Canonical analysis of several sets of variables. Biometrika 1971;58:433–51.
Hotelling H. Canonical correlation analysis (CCA). Biometrika 1936;28:321–77.
Sherry A, Henson RK. Conducting and interpreting canonical correlation analysis in personality research: a user-friendly primer. J Pers Assess. 2005;84:37–48.
Simpson SL, Lyday RG, Hayasaka S, Marsh AP, Laurienti PJ. A permutation testing framework to compare groups of brain networks. Front Comput Neurosci. 2013;7:171.
Winkler AM, Webster MA, Vidaurre D, Nichols TE, Smith SM. Multi-level block permutation. Neuroimage 2015;123:253–68.
Pietrzak RH, Tsai J, Harpaz-Rotem I, Whealin JM, Southwick SM. Support for a novel five-factor model of posttraumatic stress symptoms in three independent samples of Iraq/Afghanistan veterans: a confirmatory factor analytic study. J Psychiatr Res. 2012;46:317–22.
Yu M, Cullen N, Linn KA, Oathes DJ, Seok D, Cook PA, et al. Structural brain measures linked to clinical phenotypes in major depression replicate across clinical centres. Mol Psychiatry. 2021. 2021. https://doi.org/10.1038/s41380-021-01039-8.
Kuhn M, Wendt J, Sjouwerman R, Büchel C, Hamm A, Lonsdorf TB. The neurofunctional basis of affective startle modulation in humans: evidence from combined facial electromyography and functional magnetic resonance imaging. Biol Psychiatry. 2020;87:548–58.
Grillon C, Davis M. Acoustic startle and anticipatory anxiety in humans: effects of monaural right and left ear stimulation. Psychophysiology. 1995;32:155–61.
Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501.
Taylor-Clift A, Morris BH, Rottenberg J, Kovacs M. Emotion-modulated startle in anxiety disorders is blunted by co-morbid depressive episodes. Psychol Med. 2011;41:129–39.
Taubitz LE, Robinson JS, Larson CL. Modulation of the startle reflex across time by unpleasant pictures distinguishes dysphoric from non-dysphoric women. Int J Psychophysiol. 2013;87:124–9.
Grillon C, Franco-Chaves JA, Mateus CF, Ionescu DF, Zarate CA. Major depression is not associated with blunting of aversive responses; evidence for enhanced anxious anticipation. PLoS ONE. 2013;8:e70969.
Glenn DE, Acheson DT, Geyer MA, Nievergelt CM, Baker DG, Risbrough VB, et al. High and low threshold for startle reactivity associated with PTSD symptoms but not PTSD risk: evidence from a prospective study of active duty Marines. Depress Anxiety. 2016;33:192–202.
Stout DM, Powell S, Kangavary A, Acheson DT, Nievergelt CM, Kash T, et al. Dissociable impact of childhood trauma and deployment trauma on affective modulation of startle. Neurobiol Stress. 2021;15:100362.
Licht CMM, de Geus EJC, Seldenrijk A, van Hout HPJ, Zitman FG, van Dyck R, et al. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension 2009;53:631–8.
Briggs R, Kenny RA, Kennelly SP. Does baseline hypotension predict incident depression in a cohort of community-dwelling older people? Data from The Irish Longitudinal Study on Ageing (TILDA). Age Ageing. 2017;46:648–53.
Hildrum B, Romild U, Holmen J. Anxiety and depression lowers blood pressure: 22-year follow-up of the population based HUNT study, Norway. BMC Public Health. 2011;11:601.
Lang PJ, McTeague LM, Bradley MM. Pathological anxiety and function/dysfunction in the brain’s fear/defense circuitry. Restor Neurol Neurosci. 2014;32:63–77.
Loas G. Anhedonia in Heart Disease. In: Ritsner MS, editor. Anhedonia A Compr. Handb. Vol. II, Dordrecht: Springer Netherlands; 2014. p. 291–299.
Hildrum B, Mykletun A, Stordal E, Bjelland I, Dahl AA, Holmen J. Association of low blood pressure with anxiety and depression: the Nord-Trøndelag Health Study. J Epidemiol Community Health. 2007;61:53–58.
Joung K, Cho S. Association of low blood pressure with suicidal ideation: a cross-sectional study of 10,708 adults with normal or low blood pressure in Korea. BMC Public Health. 2018;18:200.
Tang W, Jbabdi S, Zhu Z, Cottaar M, Grisot G, Lehman JF, et al. A connectional hub in the rostral anterior cingulate cortex links areas of emotion and cognitive control. Elife 2019;8:e43761.
Conti LH, Printz MP. Rat strain-dependent effects of repeated stress on the acoustic startle response. Behav Brain Res. 2003;144:11–18.
Schmeltzer SN, Vollmer LL, Rush JE, Weinert M, Dolgas CM, Sah R. History of chronic stress modifies acute stress-evoked fear memory and acoustic startle in male rats. Stress. 2015;18:244–53.
D’Andrea W, Pole N, DePierro J, Freed S, Wallace DB. Heterogeneity of defensive responses after exposure to trauma: Blunted autonomic reactivity in response to startling sounds. Int J Psychophysiol. 2013;90:80–89.
Zhu J, Lowen SB, Anderson CM, Ohashi K, Khan A, Teicher MH. Association of prepubertal and postpubertal exposure to childhood maltreatment with adult amygdala function. JAMA Psychiatry. 2019;76:843–53.
Hyde J, Ryan KM, Waters AM. Psychophysiological markers of fear and anxiety. Curr Psychiatry Rep. 2019;21:56.
Bremner JD. Acute and chronic responses to psychological trauma: where do we go from here? Am J Psychiatry. 1999;156:349–51.
Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, et al. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010;167:640–7.
Sambuco N, Costa VD, Lang PJ, Bradley MM. Assessing the role of the amygdala in fear of pain: neural activation under threat of shock. J Affect Disord. 2020;276:1142–8.
Tottenham N, Weissman MM, Wang Z, Warner V, Gameroff MJ, Semanek DP, et al. Depression risk is associated with weakened synchrony between the amygdala and experienced emotion. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:343–51.
Motta E Motta J, Souza LN, Vieira BB, Delle H, Consolim-Colombo FM, Egan BM, et al. Acute physical and mental stress resulted in an increase in fatty acids, norepinephrine, and hemodynamic changes in normal individuals: A possible pathophysiological mechanism for hypertension—Pilot study. J Clin Hypertens. 2021;23:888–94.
Geracioti TDJ, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–30.
Strawn JR, Ekhator NN, Horn PS, Baker DG, Geracioti TDJ. Blood pressure and cerebrospinal fluid norepinephrine in combat-related posttraumatic stress disorder. Psychosom Med. 2004;66:757–9.
Mishra I, Pullum KB, Thayer DC, Plummer ER, Conkright BW, Morris AJ, et al. Chemical sympathectomy reduces peripheral inflammatory responses to acute and chronic sleep fragmentation. Am J Physiol Regul Integr Comp Physiol. 2020;318:R781–R789.
Murray K, Godinez DR, Brust-Mascher I, Miller EN, Gareau MG, Reardon C. Neuroanatomy of the spleen: Mapping the relationship between sympathetic neurons and lymphocytes. PLoS ONE. 2017;12:e0182416.
Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve-an integrative interface between two supersystems: the brain and the immune system. Pharm Rev. 2000;52:595–638.
Baker DG, Nievergelt CM, O’Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology 2012;62:663–73.
Fonkoue IT, Marvar PJ, Norrholm S, Li Y, Kankam ML, Jones TN, et al. Symptom severity impacts sympathetic dysregulation and inflammation in post-traumatic stress disorder (PTSD). Brain Behav Immun. 2020;83:260–9.
Geracioti TDJ, Baker DG, Kasckow JW, Strawn JR, Jeffrey Mulchahey J, Dashevsky BA, et al. Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinology. 2008;33:416–24.
Friend SF, Nachnani R, Powell SB, Risbrough VB. C-Reactive Protein: Marker of risk for post-traumatic stress disorder and its potential for a mechanistic role in trauma response and recovery. Eur J Neurosci. 2020. https://doi.org/10.1111/ejn.15031. Epub ahead of print
Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J Psychosom Res. 2004;57:353–8.
Johnson TV, Abbasi A, Master VA. Systematic review of the evidence of a relationship between chronic psychosocial stress and C-reactive protein. Mol Diagn Ther. 2013;17:147–64.
Vogelzangs N, Beekman ATF, de Jonge P, Penninx BWJH. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249.
Wingenfeld K, Whooley MA, Neylan TC, Otte C, Cohen BE. Effect of current and lifetime posttraumatic stress disorder on 24-h urinary catecholamines and cortisol: results from the Mind Your Heart Study. Psychoneuroendocrinology, 2015;52:83–91.
Cho HJ, Kivimäki M, Bower JE, Irwin MR. Association of C-reactive protein and interleukin-6 with new-onset fatigue in the Whitehall II prospective cohort study. Psychol Med. 2013;43:1773–83.
Paulus MP, Thompson WK. The challenges and opportunities of small effects: the new normal in academic psychiatry. JAMA Psychiatry. 2019;76:353–4.
Husky MM, Mazure CM, Kovess-Masfety V. Gender differences in psychiatric and medical comorbidity with post-traumatic stress disorder. Compr Psychiatry. 2018;84:75–81.
Tannenbaum C, Boksa P. Sex: a key consideration in understanding the etiology of psychiatric disorders and improving treatment. J Psychiatry Neurosci. 2019;44:364–6.
Harnett NG, Wheelock MD, Wood KH, Goodman AM, Mrug S, Elliott MN, et al. Negative life experiences contribute to racial differences in the neural response to threat. Neuroimage. 2019;202:116086.
Sui J, He H, Pearlson GD, Adali T, Kiehl KA, Yu Q, et al. Three-way (N-way) fusion of brain imaging data based on mCCA+jICA and its application to discriminating schizophrenia. Neuroimage. 2013;66:119–32.
Lan Y-T, Liu W, Lu B-L Multimodal emotion recognition using deep generalized canonical correlation analysis with an attention mechanism. 2020 Int. Jt. Conf. Neural Networks, 2020, p. 1–6.
Huys QJM, Maia TV, Frank MJ. Computational psychiatry as a bridge from neuroscience to clinical applications. Nat Neurosci. 2016;19:404–13.
Woo C-W, Chang LJ, Lindquist MA, Wager TD. Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci. 2017;20:365–77.
Cuthbert BN. Research domain criteria: toward future psychiatric nosologies. Dialogues Clin Neurosci. 2015;17:89–97.
Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, et al. The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J Abnorm Psychol. 2017;126:454–77.
Dean KR, Hammamieh R, Mellon SH, Abu-Amara D, Flory JD, Guffanti G, et al. Multi-omic biomarker identification and validation for diagnosing warzone-related post-traumatic stress disorder. Mol Psychiatry. 2019. 2019. https://doi.org/10.1038/s41380-019-0496-z.
Zhou Z, Wu T-C, Wang B, Wang H, Tu XM, Feng C. Machine learning methods in psychiatry: a brief introduction. Gen Psychiatry. 2020;33:e100171–e100171.
Funding
This work was supported by the VA Center of Excellence for Stress and Mental Health (all authors). DGB, DMS, ANS and VBR are supported by a National Institute of Mental Health P50 Silvio Conte Center site award (P50 MH096889). The Marine Resilience Study was supported by Veterans Affairs Health Service Research and Development project SDR 09-0128, the Marine Corps, and the Navy Bureau of Medicine and Surgery (DGB, VBR). DMS is supported by a VA Career Development Award (IK2-CX001861). ANS is supported by a VA Merit Award (I01 CX001542). VBR is supported BX004312 (VA) W81XWH-17 (DOD) and AA026560 (NIH). CMN is supported by 2RO1MH106595 (NIH) and R01MH093500 (NIH). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Author information
Authors and Affiliations
Contributions
DGB & VBR conceptualized, initiated, and supervised completion of the MRS project. DGB, VBR, CMN, AXM, & NB completed variable preprocessing and data collation. DMS and ANS conceptualized analysis plan for this paper. DMS and ANS performed data analysis. DMS wrote the paper. DMS created the figures and tables. All authors contributed to data interpretation and manuscript revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to add author Arpi Minassian.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stout, D.M., Simmons, A.N., Nievergelt, C.M. et al. Deriving psychiatric symptom-based biomarkers from multivariate relationships between psychophysiological and biochemical measures. Neuropsychopharmacol. 47, 2252–2260 (2022). https://doi.org/10.1038/s41386-022-01303-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01303-7