Abstract
Cerebral beta amyloid (Aβ) deposition and late-life depression (LLD) are known to be associated with the trajectory of Alzheimer’s disease (AD). However, their neurobiological link is not clear. Previous studies showed aberrant functional connectivity (FC) changes in the default mode network (DMN) in early Aβ deposition and LLD, but its mediating role has not been elucidated. This study was performed to investigate the distinctive association pattern of DMN FC linking LLD and Aβ retention in cognitively normal older adults. A total of 235 cognitively normal older adults with (n = 118) and without depression (n = 117) underwent resting-state functional magnetic resonance imaging and 18F-flutemetamol positron emission tomography to investigate the associations between Aβ burden, depression, and DMN FC. Independent component analysis showed increased anterior DMN FC and decreased posterior DMN FC in the depression group compared with the no depression group. Global cerebral Aβ retention was positively correlated with anterior and negatively correlated with posterior DMN FC. Anterior DMN FC was positively correlated with severity of depression, whereas posterior DMN FC was negatively correlated with cognitive function. In addition, the effects of global cerebral Aβ retention on severity of depression were mediated by subgenual anterior cingulate FC. Our results of anterior and posterior DMN FC dissociation pattern may be pivotal in linking cerebral Aβ pathology and LLD in the course of AD progression. Further longitudinal studies are needed to confirm the causal relationships between cerebral Aβ retention and LLD.
Similar content being viewed by others
Introduction
Several studies have demonstrated the association between late-life depression (LLD) and Alzheimer’s disease (AD), but their temporal relationship is still controversial [1]. AD is known to increase risk of depression, but depression seems to increase risk of subsequent AD [2]. In terms of the latter relationship, a history of depression was reported to double the risk of dementia [3]. Contemporary disease models further suggest that depressive symptoms could be a causal factor accelerating AD progression or may even represent the earliest signs of AD [4].
Despite this important association between depression and AD, the neurobiological mechanisms underlying this link are poorly understood. Disturbance of the hypothalamic-pituitary-adrenal (HPA) axis by depression was proposed to be an important factor because it can accelerate hippocampal atrophy resulting in progressive cognitive decline [5]. Other studies demonstrated associations between LLD, ischemic white matter hyperintensity (WMH), and executive dysfunction suggesting ischemic damage to the brain as the core etiology [6, 7]. While these earlier studies focused on glucocorticoid-related hippocampal damage and the interaction between depression and vascular burden, more recent research suggested that cerebral beta-amyloid (Aβ) deposition may be one of the most important pathophysiology linking depression and AD [8].
Numerous studies including analyses of cerebrospinal fluid (CSF) and amyloid positron emission tomography (PET) have demonstrated associations between LLD and Aβ pathology. A study reported that CSF Aβ 1–42, which is known to inversely reflect cerebral Aβ deposition, is lowered in cognitively normal older adults with major depressive disorder compared with healthy controls [9]. A study using 11C‐labeled Pittsburgh Compound B ([11C]-PiB) demonstrated a positive correlation between depressive symptoms and cerebral Aβ deposition [10]. A 5-year longitudinal study showed that higher plasma Aβ 1–42 level at baseline predicted the development of LLD and conversion to probable or possible AD [11]. A more recent study examined relationships between cerebral Aβ burden and longitudinal measures of depressive symptoms [12]. This longitudinal study demonstrated that higher cerebral Aβ burden measured via PiB was associated with increased depressive symptoms over time in cognitively normal older adults. The study suggested that depressive symptoms may be an outcome of Alzheimer’s pathology at the preclinical stage.
Despite the above findings, a number of studies failed to identify neurobiological mechanisms linking LLD and Aβ pathology [13]. A study using 18F-flutemetamol (FMM) PET showed that there were no differences in cortical amyloid uptake or proportion of amyloid-positive subjects between healthy control and LLD groups [14]. This cross-sectional study showed that there were no associations between Aβ burden and occurrence of depression or hippocampal volume. Therefore, analysis of hippocampal volume decrement was unable to reveal the association between Aβ pathology and LLD. Another clinicopathological study showed that severity of depressive symptoms was not associated with Braak and Braak Neurofibrillary Stages [15].
A possible explanation for these contradictory results is that additional neurobiological mechanisms could mediate between cerebral Aβ pathology and LLD. The biomarker model of AD indicated that cerebral Aβ accumulation is necessary but not sufficient to produce the clinical symptoms in patients with AD trajectory [16]. Among several pathophysiological mechanisms mediating cerebral Aβ retention and clinical symptoms, functional network disruptions are known to reflect relatively earlier cognitive and behavioral symptoms from synaptic dysfunctions of the brain [17]. Accumulating evidences suggest that brain regions particularly vulnerable to early Aβ deposition overlap with a network of brain regions that together constitute the default mode network (DMN) [18]. Studies initially showed that high global Aβ deposition in the cerebral cortex was associated with general decrement of functional connectivity (FC) in the DMN [19, 20]. More recent studies have suggested that DMN responded differently to Aβ deposition, which showed decreased DMN FC in multiple posteromedial regions and compensatory or excitatory increases in DMN FC in numerous anteromedial regions [21]. It is now generally acknowledged that posterior DMN is mainly involved in episodic memory, whereas anterior DMN is involved in modulation of emotional behavior and self-referential processing [22]. In patients with depression, FC of anterior medial regions was reported to be increased in association with rumination, while FC of posterior medial regions was decreased in association with episodic memory dysfunction [23, 24]. Building on these previous studies, we postulated that aberrance of DMN may play an important role in the association between LLD and Aβ pathology.
The purpose of this study was to investigate associations between cerebral Aβ burden, depression, and aberrant FC of DMN in cognitively normal older adults. In addition, we also investigated the role of white matter damage in this complex relationship. We hypothesized that anterior-posterior dissociation pattern of DMN could be a common pathophysiological mechanism of cerebral Aβ deposition and LLD.
Methods
Subjects
A total of 235 older adults with normal cognition, consisting of 118 with depression (depression group) and 117 without depression (no depression group), were included in the study. Subjects were recruited from volunteers of the Catholic Aging Brain Imaging (CABI) database, which contains brain scans of patients who visited the outpatient clinic at Catholic Brain Health Center, Yeouido St. Mary’s Hospital, The Catholic University of Korea, between 2017 and 2019. The inclusion criteria for the depression group were as follows: (1) age ≥60 years; (2) diagnosis of major depressive disorder with 17-item Hamilton Depression Rating Scale (HAMD17) total score >10; (3); and normal cognitive function confirmed with the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD-K); (4) patients not taking any psychotropic medications (e.g., antidepressant, benzodiazepines and antipsychotics). Subjects in the no depression group were matched to the depression group with regard to age, handedness, and level of education. We excluded subjects with any current or past psychiatric diagnosis established by the Mini-International Neuropsychiatric Interview (MINI) [25]. The MINI was conducted separately by two psychiatric specialists to confirm inclusion and exclusion criteria. The details of inclusion and exclusion criteria are described in the supplementary material. The study was conducted in accordance with ethical and safety guidelines set forth by the Institutional Review Board of Yeouido St. Mary’s Hospital, The Catholic University of Korea (IRB number: SC18TESI0143), and all subjects provided written informed consent.
Positron emission tomography
Information regarding 18F-FMM production, data collection, and analytical results were described previously [26]. MRI in each individual was utilized for co-registration, defining regions of interest (ROIs), and for correction of partial volume effects associated with expansion of the cerebrospinal spaces due to cerebral atrophy. Analysis of 18F-FMM PET data was performed using the standardized uptake value ratio (SUVR) 90 min post-injection, with the pons as the reference. SUVRs of the six cortical ROIs (frontal, superior parietal, lateral temporal, striatum, anterior cingulate cortex, and posterior cingulate cortex/precuneus) were averaged to define global cerebral Aβ burden. The PET scan was conducted within 4 weeks of clinical screening and cognitive function test. Consistent with cutoff values used in previous 18F-FMM PET studies, we used neocortical SUVR of 0.62 as the cutoff between high and low [26]. However, amyloid positivity was confirmed by visual reading from two separate nuclear medicine radiologists.
Acquisition of MRI
MRI data were collected by the Department of Radiology, Yeouido St. Mary’s Hospital, The Catholic University of Korea, using a 3T Siemens MAGETOM Skyra machine and eight-channel Siemens head coils (Siemens Medical Solutions, Erlangen, Germany). The detailed parameters are described in the supplementary material.
Data analysis
fMRI data preprocessing
Resting-state fMRI data preprocessing was carried out using the functional connectivity toolbox v18 (CONN, https://www.nitrc.org/projects/conn/). The pre-processing pipeline included motion estimation and correction, structural segmentation and normalization, functional outlier detection and scrubbing, and functional spatial smoothing with a 6-mm Gaussian kernel. An anatomical component-based noise correction (aCompCor) procedure was used to remove possible confounders, including blood-oxygen-level-dependent (BOLD) signals from the white matter and CSF, realignment parameters (six motion parameters and six first-order temporal derivatives), scrubbing parameters (maximum inter-scan movement and identified invalid scans), and task-design effects. The waveform of each brain voxel was filtered using a bandpass filter (0.009 < f < 0.08 Hz) to reduce the effects of low-frequency drift.
Independent component analysis (ICA)
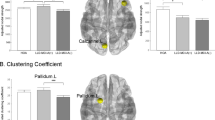
Group independent component analysis (ICA) enables voxel-wise testing of component images or fitting of a model to component time courses [27]. We selected anterior and posterior DMN as intrinsic-connectivity-networks (ICNs) of interest using spatial correlation against a set of previously defined maps [28]. Anterior DMN was identified as the spatial map comprising the anterior cingulate, middle temporal gyrus, temporal pole, and ventromedial frontal gyrus (Fig. 1A). Posterior DMN was identified as the spatial maps comprising the precuneus, and parahippocampal gyrus, hippocampus, inferior temporal gyrus, angular gyrus, and superior frontal gyrus (Fig. 1B). The detailed procedures are described in the supplementary material.
Statistical map representing (A) anterior and (B) posterior default mode network (DMN) determined across all subjects. Group independent component analysis showed (C) increased functional connectivity (FC) in the anterior DMN in the depression group compared to the no depression group in subgenual anterior cingulate, and (D) decreased FC in the posterior DMN in the depression group compared to the no depression group in precuneus. Ant Anterior, Dep Depression Group, ND No Depression Group, Post Posterior.
WMH segmentation
We used the automated localization and segmentation method of Wu et al. to calculate and normalize WMH volumes [29]. For all subjects, WMH volumes were calculated using FLAIR images and were normalized relative to the overall brain volume. The method of Wu et al. uses an iterative algorithm, which involves automated selection of “seeds” of possible WMH lesions and fuzzy connectedness that clusters voxels based on their adjacency and affinity to segment WMH lesions around the seeds [30]. The automated WMH segmentation system was implemented in C++ and the Insight Toolkit (ITK).
Statistical analysis
Statistical analyses of demographic data were performed with SPSS (version 20.0; SPSS Inc., Chicago, IL). The two-sample independent t test was used to assess potential differences between the depression group and no depression group for all continuous demographic variables and clinical values. The Chi-square test was used for analysis of categorical variables. In all analyses, a two-tailed α level of 0.05 was taken to indicate statistical significance.
General linear model (GLM) was used to measure the group differences of the ICA maps for the anterior and the posterior DMNs. To examine the relationships between Aβ deposition, WMH burden, and cognitive functions and FC of the DMNs in both groups, the global mean SUVR values, log-transformed total WMH volumes, and CERAD total scores were correlated with the voxel-wise ICA maps of the anterior and posterior DMN FC using the GLM. The HAMD17 total scores of the depression group were also used to investigate relationships between severity of depression and FC of the DMNs. In addition, the GLM model with FC as the main outcome variable and global mean SUVR value, total WMH volumes, CERAD-K total scores, and group as independent variables were performed by including their interactions (Aβ retention, WMH burden and cognitive functions by group). We controlled for the effects of age, education, and sex in all GLM analyses. The threshold was set at P < 0.05 (false discovery rate (FDR)) to control for multiple comparisons [31]. To examine whether regional functional synchronization mediated the association between Aβ retention and HAMD17 total scores in the depression group, mediation analysis was performed using the PROCESS macro controlled for age, sex, and education level [32].
Results
Baseline demographic and clinical data
Supplementary Table S1 shows the baseline demographic data of the depression group and no depression group. All variables were normally distributed. There were no significant differences in sex, age, or education distribution between the two groups. The two groups also had no significant differences in amyloid positivity, APOE4 genotype, WMH burden, neuropsychological tests, or mean SUVR values.
Group difference in FC
Fig. 1A, B show statistical maps representing the anterior and posterior DMN determined across all subjects. Group ICA shows significantly increased FC in the anterior DMN in the depression group compared to the no depression group in the subgenual anterior cingulate (ACC) (Fig. 1C and Table 1, FDR corrected P < 0.05). In contrast, precuneus FC of the depression group was significantly decreased in the posterior DMN (Fig. 1D and Table 1, FDR corrected P < 0.05).
Effects of depression on relationships between Aβ retention and FCs
In the anterior DMN, there was a significant group by Aß retention interaction in the FCs of subgenual ACC (Fig. 2A and Table 1, FDR corrected p < 0.05). In terms of posterior DMN, posterior cingulate FC showed significant group by Aß retention interaction (Fig. 2B and Table 1, FDR corrected p < 0.05). As the interaction between group and Aβ retention was statistically significant, to determine the direction of the association between FC and Aβ retention, we performed separate GLMs for each group (FC as an outcome and Aβ retention as an independent variable) controlling for age, sex, and education. The global mean SUVR scores showed a positive correlation with subgenual ACC and thalamus of the anterior DMN FC, and negative correlations in the precuneus and angular gyrus of posterior DMN FC (Fig. 3, FDR corrected P < 0.05) in the depression group. However, no significant correlations between the global mean SUVR scores and the FC were observed in the no depression group.
A significant group by Aß retention interaction was not in the FC of (A) subgenual anterior cingulate cortex (ACC) for the anterior DMN and (B) posterior cingulate for the posterior DMN (all both FDR corrected p < 0.05). C In depression group, the FC of the subgenual ACC had positive correlations with the HAMD17 total scores. Aß Beta-Amyloid, Ant Anterior, Dep Depression Group, DMN Default Mode Network, ND No Depression Group, Post Posterior.
In the depression group, the global mean SUVR scores showed (A) positive correlation with the subgenual ACC and thalamus of the anterior DMN FC, and (B) negative correlations in precuneus and angular gyrus of the posterior DMN FC (FDR corrected p < 0.05). Aß Beta-Amyloid, Ant Anterior, Dep Depression Group, DMN Default Mode Network, Post Posterior.
Depression severity and FCs
Fig. 2C and Table 1 show the results of correlation analysis between the HAMD17 total scores and the FCs within anterior DMN. In the anterior DMN of the depression group, the FC of the subgenual ACC showed positive correlations with the HAMD17 total scores (FDR corrected P < 0.05). However, no significant correlations were observed between HAMD17 total scores and posterior DMN in the depression group.
Effects of depression on the relationships between cognitive functions and FCs
There were significant group by CERAD-K total scores interaction in the left and right fusiform gyrus of posterior DMN (Supplementary Fig. S1A, Table 1, FDR corrected p < 0.05). In addition, CERAD-K total scores showed significant positive correlations in the bilateral parahippocampal gyrus and right precuneus of the posterior DMN in the depression group (Supplementary Fig. S1B, Table 1, FDR corrected P < 0.05). However, no significant correlations were observed in the no depression group.
Effects of depression on the relationships between WMH burden and FCs
In the anterior DMN, there were significant group by log-transformed total WMH volumes interactions in ACC (Supplementary Fig. S2A, Supplementary Table S2, FDR corrected p < 0.05). However, no group by WMH burden interactions in the posterior DMN FC was observed. In addition, significant positive correlations between log-transformed total WMH volumes and ACC FC within the anterior DMN were observed in the depression group (Supplementary Fig. S2B, Supplementary Table S2, FDR corrected p < 0.05).
Effects of Aβ retention on depression severity
There were no significant correlations between the mean 18F-FMM SUVR values and the HAMD17 total scores. However, as subgenual ACC in the anterior DMN showed significant correlations with Aβ retention and HAMD17 total scores, we performed mediation analysis to investigate whether subgenual ACC FC mediated Aβ retention on severity of depression. Fig. 4 shows the results of mediation analysis with the global mean 18F-FMM SUVR value as an independent factor and the HAMD17 total score as a dependent factor in the depression group. The proposed mediator was the FC value of subgenual ACC within the anterior DMN, which showed significant group by Aβ retention. Mediation analysis showed that there was no significant direct effect of global mean 18F-FMM SUVR value on HAMD17 total score (β = 0.08, P = 0.44). On the other hand, the effect of global mean 18F-FMM SUVR value on HAMD17 total score was mediated by subgenual ACC FC value within the anterior DMN (β = 0.27, P = 0.002).
No significant direct effect of global mean FMM SUVR values on HAMD17 total scores (β = 0.08, p = 0.44) were noted. Effect of global mean FMM SUVR values on the HAMD17 total scores was mediated by subgenual ACC FC values within the anterior DMN (β = 0.27, p = 0.002). Ant Anterior, CERAD Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease, Dep Depression group, DMN Default Mode Network, FC Functional Connectivity, Post Posterior.
Discussion
To our knowledge, this is the first study to investigate the neurobiological mechanisms underlying the associations between cerebral Aβ burden, aberrant FC of DMN, and depressive disorder in cognitively normal older adults. We also investigated the role of WMH burden in these relationships.
Differences in patterns of FC between the depression group and no depression group
Group ICA analysis showed that, in comparison with the no depression group, the depression group had increased FC in the anterior DMN and decreased FC in the posterior DMN. Consistent with our findings, previous studies showed that patients with depression have a dissociation pattern between the anterior and the posterior parts of the DMN [23, 24]. In a study using diffusion tensor imaging, patients with LLD were shown to have reduced white matter integrity of the cingulate bundle connecting the anterior and the posterior DMN, and this reduced integrity was correlated with severity of depression [33]. This suggests that destructive white matter transformations, such as demyelination and axonal damage, in the tract connecting the anterior and posterior DMN may be responsible for the anterior-posterior DMN dissociation observed in LLD. However, there were no previous studies integrating aberrance of DMN with amyloid pathology in LLD.
Effects of depression on the relationships between Aβ retention and FCs
Earlier studies showed that global cerebral Aβ retention is negatively correlated with the posterior DMN FC and positively correlated with the anterior DMN FC in cognitively normal adults [21, 34]. We extended previous research by suggesting that the Aβ-associated anterior-posterior DMN dissociation could be more pronounced in patients with depression than in people without depression.
Since cerebral Aβ burden is known to be associated with decrement of synaptic plasticity [16], the FC aberrance could have been more predominant in areas known to be more vulnerable to early Aβ deposition, which is the posterior DMN [18]. In order to compensate for the lowered FC of the posterior DMN and associated cognitive dysfunctions, the FC of the anterior DMN could have been increased, which resulted in rumination and depressive symptoms [22]. In the other perspective, depressive symptoms might have emerged first and consequently exacerbated inflammatory cascade in patients within the AD trajectory [35]. Heightened neuroinflammatory process might have facilitated the Aβ cascade, which could have been more predominant in the more vulnerable region of the brain or the posterior DMN [18]. The anterior DMN FC might have elevated as a result of the depressive symptoms per se [23, 24], whereas the posterior FC might have lowered due to synaptic dysfunction caused by the early Aβ deposition [21]. However, longitudinal studies are needed to confirm this hypothesis.
Effects of depression on relationships between cognitive function and FC
Previous studies have suggested that failure to downregulate DMN activity normally in the resting state could be an important hallmark of depression [36]. Our results confirmed previous reports by showing that the FC of the anterior DMN was heightened in association with increased depressive symptoms, whereas the FC in the posterior DMN was reduced in association with decreased episodic memory [23, 24, 37]. We also advanced previous models by illustrating a significant group by cognitive score interaction in the left and right fusiform gyrus of posterior DMN. Similarly, correlations between cognitive function and FC of the bilateral parahippocampal gyrus and right precuneus were evident in the depression group, but not in the no depression group. Both parahippocampal gyrus and precuneus play critical roles in the formation and recall of episodic memory, both of which are well-studied brain areas in AD [38, 39]. Fusiform gyrus, a brain region mainly involved in facial recognition and lexical-semantic memory, is also known to play important roles in the onset and progression of AD [40, 41]. This suggests that the detrimental effect of the decreased posterior DMN FC and associated cognitive dysfunction could be more predominant in patients with depression.
Effects of depression on the relationships between WMH burden and FCs
According to the “vascular depression hypothesis,” cerebrovascular disease and its subsequent WMH are essential parts of the pathogenesis of LLD [42]. We showed a positive correlation between WMH burden and the FC of ACC in the depression group. An earlier study showed that older adults with greater WMH burden showed a higher DMN FC in the medial frontal gyrus, a brain region adjacent to the ACC [43]. The authors speculated further that the FC increment may reflect a compensatory recruitment or reallocation of cognitive resources. Taken together, these observations suggested that a greater WMH burden may have resulted in a compensatory increment in the FC of ACC, which culminated in worse rumination and depressive symptoms. In contrast, Wu et al. reported that there was a negative rather than a positive correlation between WMH burden with anterior DMN FC in patients with LLD [44].
The depressed patients in the study of Wu et al. had higher severity of depression than the depressed patients in the present study. We conducted subgroup analysis with patients having moderate to severe severity (HAMD17 score ≥18) only, and correlation analysis of these subjects showed significant positive correlations between log-transformed total WMH volumes and the ACC FC within the anterior DMN (table S2). This discrepancy, although still not clear, might be attributable to diverse reasons such as differences in sample size, presence of the Aß pathology, and the resting state network identification methods (ICA vs seed-based analysis). For example, previous studies showed that subtle changes in analytic approach to resting state fMRI, such as using slightly different spatial seeds or altering the model order dimensionality estimation in ICA, can have a significant impact on the spatial characteristics of the resting state networks identified [45]. However, further replication studies with larger sample sizes are needed to clarify this point.
Relationships between Aβ retention, FC, and depression
There was no significant correlation between global cerebral Aβ retention and severity of depression, but subgenual ACC FC showed significant correlations with both global cerebral Aβ retention and severity of depression. However, these simple correlation patterns were not sufficient to elucidate the role of aberrant DMN FC linking Aβ retention and depressive symptoms. Mediation analysis provided new evidence that cerebral Aβ burden did not have a direct effect on the symptoms of depression but, rather, was mediated by the increment of FC in subgenual ACC within the anterior DMN. This suggests that a high Aβ burden causes either a toxic excitatory or compensatory increment in the anterior DMN FC, or so-called frontostriatal circuitry damage, leading to LLD [6].
Strengths, limitations, and future perspectives
By including a large sample size, objective depressive symptom measurement, and the inclusion of drug-naïve patients only, we were able to integrate and investigate the effects of depression on Aβ pathology and aberrance of DMN FC in a comprehensive manner. However, this study had several limitations. This was a cross-sectional study, so the results only elucidate correlations and have limited ability to interpret causal relations. Further longitudinal studies are needed to elucidate the causal relation of cerebral Aβ with the aberrant connectivity of the DMN and development of depression in the trajectory of AD. We focused on global Aβ retention, and so were unable to investigate the relationship between regional Aβ retention and regional FC pattern of the DMN. Although previous works showed importance of other functional networks beyond DMN, such as executive control network (ECN) [46,47,48] and salience network (SN) [49,50,51], in LLD and AD pathology, we did not broaden our research to investigate their intra- and inter-network connectivity. Studies consistently reported decreased functional connectivity (FC) of ECN in patients with LLD and AD compared with healthy controls [52]. The FC of ECN was increased in whose depressive symptoms remitted [53], and its disturbance was associated with progression of dementia in patients with AD trajectory [46]. The aberrant FC of ECN was also associated with executive and cognitive dysfunction in both LLD [47] and AD [48]. In terms of SN, more studies focused on inter-network rather than within-network connectivity in LLD. The FC between SN and ECN was less decreased in patients with LLD than in normal control [51]. Patients with AD showed increased FC within the SN [49], and this higher FC of SN was reported in association with Aβ burden [50]. More studies are needed to elucidate interactive roles of multiple networks related to LLD and AD.
In conclusion, the findings of the present study suggest that anterior and posterior DMN FC dissociation may be an important neurobiological pathway underpinning Aβ pathology and depression. Cerebral Aβ burden may initiate different detrimental cascades in the anterior and posterior DMN. We also showed that higher degree of vascular pathophysiology was associated with the anterior and posterior DMN FC dissociation in patients with LLD. Finally, we elucidated the role of Aβ in the pathogenesis of depression by illustrating that the increment of the severity of depression with high cerebral Aβ burden was mediated by the heightened FC of the anterior DMN.
Funding and disclosure
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1A2C2009100). The authors have no conflicts of interest to declare.
References
Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–31.
Kessing LV. Depression and the risk for dementia. Curr Opin Psychiatry. 2012;25:457–61.
Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–8.
Peters ME, Lyketsos CG. Beyond memory: a focus on the other neuropsychiatric symptoms of dementia. Am J Geriatr Psychiatry. 2015;23:115–8.
Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–35.
Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345–57.
Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524–32.
Qiu WQ, Zhu H, Dean M, Liu Z, Vu L, Fan G, et al. Amyloid-associated depression and ApoE4 allele: longitudinal follow-up for the development of Alzheimer’s disease. Int J Geriatr Psychiatry. 2016;31:316–22.
Pomara N, Bruno D, Sarreal AS, Hernando RT, Nierenberg J, Petkova E, et al. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. Am J Psychiatry. 2012;169:523–30.
Yasuno F, Kazui H, Morita N, Kajimoto K, Ihara M, Taguchi A, et al. High amyloid-beta deposition related to depressive symptoms in older individuals with normal cognition: a pilot study. Int J Geriatr Psychiatry. 2016;31:920–8.
Blasko I, Kemmler G, Jungwirth S, Wichart I, Krampla W, Weissgram S, et al. Plasma amyloid beta-42 independently predicts both late-onset depression and Alzheimer disease. Am J Geriatr Psychiatry. 2010;18:973–82.
Donovan NJ, Locascio JJ, Marshall GA, Gatchel J, Hanseeuw BJ, Rentz DM, et al. Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am J Psychiatry. 2018;175:530–37.
Alexopoulos GS. Mechanisms and treatment of late-life depression. Transl Psychiatry. 2019;9:188.
De Winter FL, Emsell L, Bouckaert F, Claes L, Jain S, Farrar G, et al. No association of lower hippocampal volume with Alzheimer’s disease pathology in late-life depression. Am J Psychiatry. 2017;174:237–45.
McCutcheon ST, Han D, Troncoso J, Koliatsos VE, Albert M, Lyketsos CG, et al. Clinicopathological correlates of depression in early Alzheimer’s disease in the NACC. Int J Geriatr Psychiatry. 2016;31:1301–11.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92.
Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28.
Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17.
Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–94.
Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–7.
Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex. 2011;21:2399–407.
Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–62.
Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–7.
Coutinho JF, Fernandesl SV, Soares JM, Maia L, Goncalves OF, Sampaio A. Default mode network dissociation in depressive and anxiety states. Brain Imaging Behav. 2016;10:147–57.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. quiz 34-57
Thurfjell L, Lilja J, Lundqvist R, Buckley C, Smith A, Vandenberghe R, et al. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med. 2014;55:1623–8.
Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45:S163–72.
Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–65.
Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133–42.
Udupa JK, Wei L, Samarasekera S, Miki Y, van Buchem MA, Grossman RI. Multiple sclerosis lesion quantification using fuzzy-connectedness principles. IEEE Trans Med Imaging. 1997;16:598–609.
Genovese C, Lazar N, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–78.
Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–91.
Charlton RA, Lamar M, Zhang A, Yang S, Ajilore O, Kumar A. White-matter tract integrity in late-life depression: associations with severity and cognition. Psychol Med. 2014;44:1427–37.
Lim HK, Nebes R, Snitz B, Cohen A, Mathis C, Price J, et al. Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly subjects. Brain. 2014;137:3327–38.
Khemka VK, Ganguly A, Bagchi D, Ghosh A, Bir A, Biswas A, et al. Raised serum proinflammatory cytokines in Alzheimer’s disease with depression. Aging Dis. 2014;5:170–6.
Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–7.
Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci. 2013;7:666.
Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104.
Nellessen N, Rottschy C, Eickhoff SB, Ketteler ST, Kuhn H, Shah NJ, et al. Specific and disease stage-dependent episodic memory-related brain activation patterns in Alzheimer’s disease: a coordinate-based meta-analysis. Brain Struct Funct. 2015;220:1555–71.
Ma D, Fetahu IS, Wang M, Fang R, Li J, Liu H, et al. The fusiform gyrus exhibits an epigenetic signature for Alzheimer’s disease. Clin Epigenetics. 2020;12:129.
Bokde AL, Lopez-Bayo P, Meindl T, Pechler S, Born C, Faltraco F, et al. Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain. 2006;129:1113–24.
Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–22.
Chen X, Huang L, Ye Q, Yang D, Qin R, Luo C, et al. Disrupted functional and structural connectivity within default mode network contribute to WMH-related cognitive impairment. Neuroimage Clin. 2019;24:102088.
Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF 3rd, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 2011;194:39–46.
Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8.
Cai S, Peng Y, Chong T, Zhang Y, von Deneen KM, Huang L, et al. Differentiated effective connectivity patterns of the executive control network in progressive MCI: a potential biomarker for predicting AD. Curr Alzheimer Res. 2017;14:937–50.
Gandelman JA, Albert K, Boyd BD, Park JW, Riddle M, Woodward ND, et al. Intrinsic functional network connectivity is associated with clinical symptoms and cognition in late-life depression. Biol Psychiatry Cogn Neurosci Neuroimaging 2019;4:160–70.
Wang P, Zhou B, Yao H, Zhan Y, Zhang Z, Cui Y, et al. Aberrant intra- and inter-network connectivity architectures in Alzheimer’s disease and mild cognitive impairment. Sci Rep. 2015;5:14824.
Balthazar ML, Pereira FR, Lopes TM, da Silva EL, Coan AC, Campos BM, et al. Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Hum Brain Mapp. 2014;35:1237–46.
Fredericks CA, Sturm VE, Brown JA, Hua AY, Bilgel M, Wong DF, et al. Early affective changes and increased connectivity in preclinical Alzheimer’s disease. Alzheimers Dement. 2018;10:471–79.
Li W, Wang Y, Ward BD, Antuono PG, Li SJ, Goveas JS. Intrinsic inter-network brain dysfunction correlates with symptom dimensions in late-life depression. J Psychiatr Res. 2017;87:71–80.
Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65.
Karim HT, Andreescu C, Tudorascu D, Smagula SF, Butters MA, Karp JF, et al. Intrinsic functional connectivity in late-life depression: trajectories over the course of pharmacotherapy in remitters and non-remitters. Mol Psychiatry. 2017;22:450–57.
Author information
Authors and Affiliations
Contributions
S-MW and HKL drafted the manuscript and contributed to project design, data collection, management, analysis, and interpretation. N-YK and DWK contributed to project design, data collection, and management. YHU, H-RN, and CUL contributed to data management and revision of the manuscript.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, SM., Kim, NY., Um, Y.H. et al. Default mode network dissociation linking cerebral beta amyloid retention and depression in cognitively normal older adults. Neuropsychopharmacol. 46, 2180–2187 (2021). https://doi.org/10.1038/s41386-021-01072-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01072-9
This article is cited by
-
Association of amyloid-beta with depression or depressive symptoms in older adults without dementia: a systematic review and meta-analysis
Translational Psychiatry (2024)
-
Biological factors influencing depression in later life: role of aging processes and treatment implications
Translational Psychiatry (2023)
-
The heterogeneity of late-life depression and its pathobiology: a brain network dysfunction disorder
Journal of Neural Transmission (2023)