Abstract
Although impulsive action is strongly associated with addiction, the neural underpinnings of this relationship and how they are influenced by sex have not been well characterized. Here, we used a titrating reaction time task to assess differences in impulsive action in male and female Long Evans rats both before and after short (4–6 days) or long (25–27 days) abstinence from 2 weeks of cocaine or water/saline self-administration (6 h daily access). Neural activity in the prelimbic cortex (PrL) and nucleus accumbens (NAc) core was assessed at each time point. We found that a history of cocaine self-administration increased impulsivity in all rats following short, but not long, abstinence. Furthermore, male rats with an increased ratio of excited to inhibited neurons in the PrL at the start of each trial in the task exhibited higher impulsivity in the naïve state (before self-administration). Following short abstinence from cocaine, PrL activity in males became more inhibited, and this change in activity predicted the shift in impulsivity. However, PrL activity did not track impulsivity in female rats. Additionally, although the NAc core tracked several aspects of behavior in the task, it did not track impulsivity in either sex. Together, these findings demonstrate a sex-dependent role for the PrL in impulsivity both before and after a history of cocaine.
Similar content being viewed by others
Introduction
Heightened impulsivity is often seen in individuals with substance use disorders [1, 2]. In addition, impulsivity can be fragmented into multiple subtypes [3], with one subtype being impulsive action, defined as the inability to wait when making a response. In preclinical models, impulsive action has a bilateral relationship with addiction-like behavior, particularly with cocaine. Animals with high impulsive action exhibit greater cocaine-seeking [4,5,6], while animals with a history of cocaine have increased impulsive action [7, 8]; but see [4, 9].
However, the neurocircuitry underlying the interaction between cocaine and impulsive action is poorly understood. One circuit that is well-positioned to play a role in this interaction is the projection from the prelimbic cortex (PrL) to the nucleus accumbens (NAc) core, which has been heavily implicated in cocaine-seeking behavior [10,11,12]. In addition, neural structure and function in the PrL and NAc core are altered following a history of cocaine (PrL [13,14,15]; NAc core [16,17,18]). Furthermore, cocaine-induced neural adaptations in the PrL and NAc core are associated with changes in multiple behaviors, including drug-seeking [19,20,21], sign-tracking [22], second-order conditioning [23], and delay processing [24].
In addition to their interaction with cocaine, both the PrL and NAc core have been implicated in impulsive action. Several studies have shown that pharmacological inactivation of the PrL increases impulsivity [25,26,27,28]; but see [29]. In addition, several studies suggest that dopamine in the NAc core (through its actions on D1 receptors) promotes impulsive action [30,31,32]. Furthermore, neural activity in both the PrL and NAc core tracks impulsivity [26, 28, 33,34,35,36].
Finally, several studies have noted sex differences in both drug use and impulsivity [37, 38]. Females more rapidly initiate self-administration of cocaine [39], self-administer more cocaine [40, 41] and have heightened cocaine-seeking [42]. Conversely, males tend to have heightened impulsivity in tasks measuring impulsive action [43, 44] but see [45, 46]. However, to our knowledge no studies have investigated if there are sex differences following a history of cocaine on impulsive action and its underlying neurocircuitry.
Given the role of the PrL and NAc core in impulsive action and the ability of cocaine to alter PrL and NAc core activity and associated behavior, here we determined the effect of a history of cocaine self-administration on neural activity in the PrL and NAc core during a task measuring impulsive action. In addition, we examined if sex differences exist in impulsivity and neural activity during the task both before and after a history of cocaine.
Materials and methods
Behavior
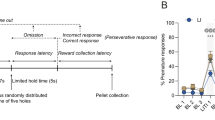
Detailed methods are described in Supplementary Methods. All experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee. Briefly, 35 male and female Long Evans rats (20 male, 15 female, 60–90 days old, ∼300–325 g; Envigo/Harlan) were trained in the titrating reaction time task (TRTT) as described previously [47] (Fig. 1A). Rats were initially trained to press a lever for a sucrose pellet (counterbalanced to be the left or right lever). After obtaining 50 reinforcers for 2 consecutive days, rats were trained to press the lever within a 5 s window (cued by the light above the lever). After obtaining 50% accuracy on this task for two consecutive days, rats began the TRTT (Fig. 1B). For each trial in this task, a lever was initially extended into the chamber. Following a VT 1 s delay (precue period; 0.5–1.5 s range), a cue light was illuminated above the lever. The cue light was on for a duration ranging from 0.03 to 5 s (depending on each animals’ ability, see below). After the cue light was extinguished, the lever remained extended for an additional 2 s (postcue period). The lever was then retracted until the beginning of the next trial with an intertrial interval of VT 4 s (1–7 s range). Presses on the lever while the cue light was on resulted in the retraction of the lever and the delivery of a sucrose pellet (correct response), while presses when the cue light was off resulted in the retraction of the lever and delivery of a 0.3 s white noise burst (incorrect response). The cue duration was titrated down by 10% if the rat got the previous response correct, and titrated up by 10% if the rat got the previous response incorrect. Thus, all rats got ~50% correct and incorrect regardless of their individual ability in the task. Rats trained on the TRTT for ~3 weeks. Upon achieving stable behavior, animals underwent catheterization and electrode array implantation surgery. Animals had 1 week of recovery time with ad libitum food and water. Next, animals were retrained in the TRTT. As soon as behavior stabilized, neural activity was recorded during a session of the TRTT (see Fig. 1A, “Naïve TRTT”). An additional task examining behavioral persistence was administered for 1 h as part of an unrelated experiment (not presented here).
A Experimental timeline. After a period of training and surgery, animals were tested in the titrating reaction time task (Naïve TRTT). They then underwent 2 weeks of self-administration for either cocaine or water/saline. Following self-administration, animals entered forced abstinence and then retested in the TRTT after 4–6 days (Short Abstinence TRTT) and 25–27 days (Long Abstinence TRTT). Neural activity was recorded during both naïve TRTT and abstinent TRTT test sessions. B Schematic of a trial in the TRTT. Responses when the cue light was on (‘Correct’) resulted in delivery of a sucrose pellet. Responses either before or after cue illumination (‘Error’) resulted in a brief white noise stimulus and no sucrose pellet. Responses during the ‘Precue’ period (‘Early Error’) were considered impulsive. C Males and females had similar levels of impulsivity at baseline. D Rats were split into ‘high’ and ‘low’ impulsive subgroups. *p < 0.05.
Subsequently, animals were trained in the self-administration task in a distinct set of operant chambers (see Fig. 1A “Self-Administration”). Here, nosepoking into a cued aperture extinguished the cue and led to an intravenous infusion of 0.33 mg of cocaine in 0.2 ml of 0.9% saline (n = 10 males, n = 8 females) or an equivalent volume of both 0.9% saline (i.v.) and water (delivered to the water receptacle; n = 10 males, n = 7 females). This infusion was paired with a 30 s tone and houselight compound stimulus, and additional nosepokes during this 30 s period did not result in further infusions. Self-administration lasted for 6 h/day for 14 days. For this period, water/saline rats were mildly water restricted to 20 ml of water/day and cocaine rats received 35 ml of water/day (extra 15 ml to account for the extra water the water/saline rats self-administered). In addition, 10 of the 17 “water/saline” rats were not patent, and thus only received water. To account for this, we initially statistically examined “water” and “water/saline” rats separately to determine if there were differences between the two. However, no significant differences were observed between the groups (Fs < 3.29, ps > 0.093) and thus all reported analyses combined the two groups. Cocaine hydrochloride was obtained from the National Institute on Drug Abuse and dissolved in 0.9 percent saline.
Following completion of the 2 weeks of self-administration, animals were entered into drug abstinence and began retraining on the TRTT. Neural activity was recorded during both naïve TRTT and during TRRT sessions conducted after two abstinence periods; 4–6 days (“Short Abstinence”) and 25–27 days (“Long Abstinence”) (see Fig. 1A). In female rats, vaginal swabs were collected shortly following each electrophysiological recording and the stage of the estrous cycle was assessed with a brightfield microscope using the criteria outlined in [48].
Data analysis
TRTT behavior
Drug naïve impulsivity
Each trial in the TRTT yielded one of four possible responses: Early errors (responses during the precue period), Correct responses (responses during the cue period), Late errors (responses during the postcue period), and Omissions (no response). Impulsivity was defined by the formula: Early errors/(Early errors + Correct responses + Late errors) * 100 to obtain a percentage score. An impulsivity score of 100% indicated maximal impulsive behavior while a score of 0% represented no impulsive action. We assessed stability in impulsive behavior before our first electrophysiological recording session (i.e., labeled “Naive TRTT” in Fig. 1A) by using a repeated-measures ANOVA across three sessions to ensure there were no trends in behavior. We discuss other measures (e.g. attention) in the Supplementary Methods.
Self-administration
To determine if a significant escalation of intake took place for either group, we ran a 2 × 2 × 14 ANOVA (Drug × Sex × Session) on the number of reinforcers obtained over the 14 days.
Abstinent impulsivity
Our primary goal was to assess the change in impulsivity following abstinence from cocaine or water/saline self-administration as a function of sex. However, we were also interested in how baseline differences in impulsivity might moderate this effect, as baseline impulsivity can impact the effect of cocaine on impulsivity during withdrawal [4, 9] and can influence neural activity in the PrL and NAc core during an impulsivity task [49,50,51,52]. Thus, we assessed the change in impulsivity following abstinence with a 2 × 3 × 2 × 2 ANOVA (Drug × Session × Sex × Baseline Impulsivity), where baseline impulsivity was determined by a median split. We also assessed if either the amount of drug administered or the rate of escalation correlated with either baseline impulsivity or the change in impulsivity using Pearson correlations. Rate of escalation was estimated using the linear slope of administration between days 1 and 14.
Electrophysiological analysis
To classify neurons according to their firing pattern, we first constructed peri-event histograms for each event as described previously [53]. Baseline for each peri-event histogram was established as the average activity in the −2 to 0 s prior to lever extension and cue and −3 to −1 s prior to lever press (to account for anticipatory changes in neural activity that occurred shortly before the lever press). We then used paired t-tests to compare baseline activity to event-related activity. Specifically, we assessed the 200 ms bin following lever extension, cue illumination, and lever press (correct and error), as well as the 200 ms bin before lever press (importantly, baselines did not overlap with these time bins). Neurons were classified as “Excited” if they significantly increased activity, “Inhibited” if they significantly decreased activity, or “Nonphasic” if there was no significant change in activity. We then used χ2 tests to assess differences in the proportions of neural subtypes across groups and conditions. To correlate neural profiles with behavior, we first calculated the weighted average of neural subtypes for each rat. Excited neurons were weighted as 1, nonphasic as 0, and inhibited as −1. For each rat, values for each neuron (1, 0, or −1) were averaged together to calculate a “Neural Profile”. Thus, rats with a value above 0 had a more excited profile, while those with a value below 0 had a more inhibited profile. This neural profile was then correlated with naïve impulsivity. Finally, we also correlated the change in the neural profile (Abstinent – Naïve) with change in impulsivity (Abstinent – Naïve).
Results
Behavior and neural signaling during the TRTT task in drug naïve state
Behavior
Animals had stable impulsivity (F(2,66) = 1.86, p = 0.163) before beginning self-administration, and there were no differences in impulsivity between sexes (t(33) = 0.65, p = 0.521; Fig. 1C). Furthermore, rats classified as high and low impulsive were significantly different from each other in their impulsivity levels (t(32) = 8.01, p < 0.001; Fig. 1D). Rats of both sexes were equally divided between the low and high impulsive groups (low impulsive: 8 females, 9 males; high impulsive: 7 females, 10 males). There were no sex differences in other behavioral measures (see Supplementary Results).
Electrophysiology
198 PrL neurons and 76 NAc core neurons were recorded during the first recording session (labeled “Naive TRTT” in Fig. 1A) prior to self-administration experience. Examples of excited and inhibited neurons are shown in Fig. 2A, B. Following lever extension, males had heavier recruitment of event responsive neurons in the PrL than females, as shown by higher percent of phasically active cells (PrL phasic/nonphasic ratio to lever extension, males vs. females: χ2 = 12.62, p < 0.001, Fig. 2Cleft). There were no such differences between sexes in the NAc core (NAc core phasic/nonphasic ratio to lever extension, males vs. females: χ2 = 0.73, p = 0.393, Fig. 2Cright). In addition, while there were no differences in the excited/inhibited ratio between sexes in either brain region (PrL excited/inhibited ratio to lever extension, males vs. females: χ2 = 1.27, p = 0.261; NAc core excited/inhibited ratio to lever extension, males vs. females: χ2 = 1.37, p = 0.242; Fig. 2D), the NAc core had a more excited profile than the PrL when collapsing across sex (excited/inhibited ratio to lever extension, PrL vs. NAc core: χ2 = 3.97, p = 0.046). There were also no significant differences between sexes for neural responses to the cue light or to lever presses (correct or error) for either brain region (χ2s < 5.50, ps > 0.064). Finally there was no effect of estrous cycle on basal firing rate (Fs < 1.65, ps > 0.236) or neural responses to events in the task (χ2 < 5.73, ps > 0.057).
A An example of an ‘excited’ neuron (aligned to the extension of the lever into the chamber). B An example of an ‘inhibited’ neuron (aligned to the extension of the lever into the chamber). C Proportion of neurons that were responsive (‘phasic’) to the extension of the lever into the chamber. In the PrL, males had significantly more phasic neurons than females (left). There were no such differences in the NAc core (right). D Proportion of phasic neurons that were excited or inhibited. There were no differences between males and females, although NAc core neurons were more excited than PrL neurons overall. *p < 0.05.
In males, the PrL neural profile following lever extension significantly predicted impulsivity. Specifically, rats with a more excited profile were more impulsive (r2 = 0.30, p = 0.024; Fig. 3A). There was no such relationship in female rats or in the NAc core (r2s < 0.19, ps > 0.248; Fig. 3B–D). In total, these data suggest that the PrL is strongly involved in tracking impulsive action in male, but not female, rats prior to cocaine experience.
Self-administration behavior
Cocaine rats significantly escalated the amount of drug self-administered across days (Session: F(5.88, 94.05) = 9.65, p < 0.001; Fig. 4A). Conversely, female (but not male) rats significantly decreased self-administration of water/saline across days (Sex x Session: F(7.27,109.10) = 2.69, p = 0.012; Fig. 4B). For both cocaine and water/saline, females self-administered more than males when adjusting for body weight (Cocaine: F(1,16) = 7.31, p = 0.016; Water/Saline: F(1,15) = 13.63, p = 0.002; Fig. 4A, B inset).
A Animals significantly escalated cocaine intake across the 14 days of self-administration. Females self-administered more cocaine than males, when adjusting for body weight (inset). B Females, but not males, significantly decreased water/saline intake across the 14 days of self-administration. Females self-administered more water/saline than males, when adjusting for body weight (inset). C Cocaine increased impulsivity in rats following abstinence from self-administration. *p < 0.05.
Behavior and neural signaling during the TRTT task following abstinence
Cocaine rats significantly increased impulsivity following abstinence from self-administration compared to water/saline rats (drug × session: F(2,48) = 3.20, p = 0.0498). Bonferonni post hoc tests showed that this effect was only significant across all cocaine rats after short abstinence (Fig. 4C). Other behavioral measures are noted in the Supplementary Results (Table S1, Fig. S1).
We saw no relationship between impulsivity at any time point or change in impulsivity with the amount of drug consumed or rate of escalation (r2s < 0.17, ps > 0.091). Additionally, estrous cycle had no impact on impulsivity, either before or after self-administration (Fs < 1.69, ps > 0.225) and self-administration had no effect on estrous cycle (χ2 = 0.87, p = 0.928). In total, these data demonstrate that a brief abstinence from cocaine increases impulsivity in all rats.
Electrophysiology
187 PrL neurons and 77 NAc core neurons were recorded during a single session following short abstinence (labeled ‘Short Abstinence TRTT’ in Fig. 1A) and 154 PrL neurons and 83 NAc neurons were recorded during a single session following long abstinence (labeled ‘Long Abstinence TRTT’ in Fig. 1A). After short abstinence, cocaine significantly shifted male PrL activity to a more inhibited profile following lever extension (PrL excited/inhibited ratio to lever extension in males, cocaine naïve vs. cocaine short abstinence: χ2 = 4.36, p = 0.037; Fig. 5Aleft); this effect diminished following long abstinence (PrL excited/inhibited ratio to lever extension in males, cocaine naïve vs. cocaine long abstinence: χ2 = 1.11, p = 0.292). No such effects were seen in females (PrL excited/inhibited ratio to lever extension in females, cocaine naïve vs. cocaine short abstinence: χ2 = 0.03, p = 0.858; Fig. 5A right) or for any group in the NAc core (χ2s < 1.22, ps > 0.270, data not shown).
A Left. Short abstinence from cocaine shifted PrL neural activity to an inhibitory profile in male rats. Right. No changes in the proportion of excited/inhibited PrL neurons were seen in females. B Left. The shift in PrL activity following short abstinence correlated with the change in impulsivity in male rats. Right. There was no relationship between the shift in PrL activity and the change in impulsivity in female rats. *p < 0.05.
Importantly, in male rats the shift in PrL neural profile following short abstinence correlated with the change in impulsivity. Male rats with a stronger shift towards an inhibited profile saw a smaller increase in impulsivity (r2 = 0.70, p = 0.005; Fig. 5B left). However, the original relationship between impulsivity and PrL activity did not reappear following long abstinence (r2 = 0.11, p = 0.386; Fig. S2), demonstrating that a return to naïve levels of impulsivity did not coincide with a restoration of the relationship between PrL activity and impulsivity. No relationships were seen in female rats (r2 = 0.02, p = 0.747; Fig. 5B right) or water/saline rats (r2s < 0.02, ps > 0.232; Fig. S3). Finally, baseline impulsivity did not interact with the change in neural profile in any group. All other electrophysiological results are noted in the Supplementary Materials (Supplementary Results, Figs. S4, S5, Tables S2, S3). In total, these results suggest that cocaine alters PrL processing of the impulsivity task in males, and that this change is associated with the change in impulsivity.
Histology
Data were only included for cells in the PrL and NAc core. The placements of these cells are depicted in Supplementary Fig. S6.
Discussion
Several studies have demonstrated a relationship between impulsive action and cocaine use [4, 7, 8, 54]. However, few have investigated the neural underpinnings of this relationship or its interaction with sex. Here, we show that a history of cocaine self-administration increased impulsivity in all rats. Furthermore, we found that neural activity in the PrL tracked impulsivity in male, but not female rats in the naïve state (before self-administration). Finally, we found that this activity in male rats changed following a history of cocaine, and that this change in activity predicted the shift in impulsivity. Together, these findings demonstrate a sex-dependent role for the PrL in impulsivity both before and after a history of extended access cocaine.
A history of cocaine increases impulsive action
Male and female rats did not differ in drug-naïve impulsive action (or cue length), replicating previous work [45, 46]. However, other studies have shown that males have higher impulsive action [43, 44]. Notably, these studies used the 5-choice serial reaction time task, whereas those that did not find sex differences (including the present study) did not. The sex differences in the 5-choice task were most pronounced following an unexpected experimenter-imposed increase in the duration of the precue period, which was not administered in the studies that found no sex differences. Therefore, it is possible that sex differences in impulsive action only manifest following an unexpected challenge in task events, particularly related to the precue period.
In addition, all rats increased impulsivity following short abstinence from self-administration of cocaine. This finding expands upon previous work in male rats by [7] which also found increased impulsive action following brief abstinence from cocaine self-administration. Conversely, Dalley et al. [4, 54] found no effect of early abstinence on impulsivity, although it is important to note that rats only self-administered for 5 days before withdrawal in their studies. Our study also found that the effects of cocaine on impulsivity were short-lived, with animals returning to baseline levels of impulsivity after 4 weeks. These findings differ from those of [8] where an increase in impulsivity was seen following 4 weeks of abstinence. However, these animals also concurrently underwent extinction training for cocaine. Thus, duration of self-administration and the method of withdrawal (abstinence vs. extinction) may play an important role in modulating the effects of cocaine self-administration on impulsive action.
Neural activity in the PrL tracks impulsivity in male, but not female, rats
Neurons in both the PrL and NAc core tracked several aspects of the behavioral task, including lever extension (the cue signaling the start of each trial), lever press, and reward outcome. In males, we found that rats exhibiting heightened activity in the PrL following lever extension had higher impulsivity. This finding fits well with previous studies in male rats demonstrating a relationship between increased excitability in the PrL and impulsivity [27, 55]. This neural activity may reflect top-down promotion of stimulus-response pathways [56], motor preparation [57], or an attention-orienting response. Notably, a dampening of either process would be expected to result in decreased impulsivity.
However, this finding did not extend to female rats. In fact, fewer neurons in the PrL were modulated by lever extension than males, suggesting that the impulsivity task engaged the PrL more in males than in females. Lower PrL activity in females has been reported for a variety of behaviors, including both controllable and uncontrollable stress [58, 59] and contextual renewal for appetitive cues [60]. In addition, a study measuring impulsive action in human subjects found that men had higher activity in Brodmann area 32 (homologous to rodent PrL [61]) during the task than women, even though there were no behavioral differences between men and women [62, 63]. Interestingly, this same study found that females had heightened activity in the caudate tail, suggesting that the caudal portion of the dorsomedial striatum in rodents may be an important region for future studies examining the neural underpinnings of impulsive action in females.
We also found that neural activity in the NAc core did not predict individual differences in impulsivity. This suggests that the PrL may influence impulsivity through a pathway other than its projection to the NAc core. Other output regions of the PrL [64] that are implicated in impulsive action include the dorsomedial striatum [65], anterior insula [66], and nucleus reuniens of the thalamus [67], and these pathways may prove fruitful for future studies investigating the neural underpinnings of impulsive action.
Neural activity in the PrL shifts following a history of cocaine in male rats
In males, PrL activity following lever extension (the cue signaling the start of the trial) became more inhibited following a history of cocaine. This pattern of activity in the PrL in response to food cues is distinct from those that have been seen following cocaine cues, which tend elicit an increase in PrL activity in rodents [68,69,70]; but see [71]; and elicit an increase in homologous anterior cingulate activity in humans [72, 73]; but see [74, 75]. However, several studies have demonstrated that basal PrL activity is dampened following a history of cocaine [68, 76,77,78,79]; but see [71]. Thus, our finding suggests that PrL activity to non-cocaine cues may be more similar to the dampening of PrL activity seen under basal conditions.
We also found that male rats with a stronger shift towards PrL inhibition following a history of cocaine had a weaker increase in impulsivity. This suggests that the dampening of the PrL’s activity during the impulsivity task does not drive the increase in impulsivity itself, but instead may act as an adaptive response to counter the behavioral change. The PrL undergoes many neural adaptations following a history of cocaine [13, 80, 81], although to our knowledge none of these have been shown to act in opposition to cocaine-induced changes in behavior. Nonetheless, neural adaptations that run counter to cocaine’s effect on behavior have been seen in other brain regions, including the anterior insula in rats [53] and rostral ventral anterior cingulate cortex in humans [82]. Thus, while there is a strong focus in the literature on the role of homeostatic dysregulation in addiction [83], the current and aforementioned studies suggest that adaptive homeostatic processes may also play an important and understudied role.
Because the shift in PrL activity ran counter to the change in impulsivity, our data suggest that a region other than the PrL is driving the increased impulsive action. While there are many possible candidates for such a brain region, one strong contender is the infralimbic cortex (IL). The IL is implicated in impulsivity [29] and is known to act in opposition to the PrL in a number of behaviors, including cue and context learning [84], fear learning and expression [85], and drug-seeking [85]. Furthermore, a history of cocaine has opposing effects on the excitability of neurons in the PrL and IL [81]. Finally, the IL projects to the NAc shell [64], which itself has been strongly implicated in impulsive action [86, 87].
Conclusion
We found that a history of cocaine self-administration increased impulsive action. In additon, PrL neural activity in male rats tracked impulsive action and shifted patterns of activity following a history of cocaine. However, PrL neural activity did not track impulsivity in females, suggesting that different brain regions may underlie this activity. Future work is needed to investigate the role of PrL afferents and efferents in impulsive action as well as other brain regions that may underlie this behavior in females.
Funding and disclosure
The work was supported by National Institute on Drug Abuse grant DA045764 to T.M.M. and grants DA014339 and DA034021 to R.M.C. Cocaine used in these experiments was generously provided by the NIDA Drug Supply Program. The authors declare no competing financial interests.
References
De Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31.
Pattij T, De Vries TJ. The role of impulsivity in relapse vulnerability. Curr Opin Neurobiol. 2013;23:700–5.
Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–61.
Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70.
Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5.
Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psych. 2009;65:851–6.
Winstanley CA, Bachtell RK, Theobald DE, Laali S, Green TA, Kumar A, et al. Increased impulsivity during withdrawal from cocaine self-administration: role for ΔFosB in the orbitofrontal cortex. Cereb Cortex. 2009;19:435–44.
Broos N, van Mourik Y, Schetters D, De Vries TJ, Pattij T. Dissociable effects of cocaine and yohimbine on impulsive action and relapse to cocaine seeking. Psychopharmacology. 2017;234:3343–51.
Caprioli D, Hong YT, Sawiak SJ, Ferrari V, Williamson DJ, Jupp B, et al. Baseline-dependent effects of cocaine pre-exposure on impulsivity and D 2/3 receptor availability in the rat striatum: possible relevance to the attention-deficit hyperactivity syndrome. Neuropsychopharmacology. 2013;38:1460–71.
McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–7.
McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65.
McGlinchey EM, James MH, Mahler SV, Pantazis C, Aston-Jones G. Prelimbic to accumbens core pathway is recruited in a dopamine-dependent manner to drive cued reinstatement of cocaine seeking. J Neurosci. 2016;36:8700–11.
Hearing M, Kotecki L, de Velasco EM, Fajardo-Serrano A, Chung HJ, Luján R, et al. Repeated cocaine weakens GABAB-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron. 2013;80:159–70.
Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self‐administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–66.
Slaker ML, Jorgensen ET, Hegarty DM, Liu X, Kong Y, Zhang F, et al. Cocaine exposure modulates perineuronal nets and synaptic excitability of fast-spiking interneurons in the medial prefrontal cortex. Eneuro. 2018;5:ENEURO.0221-18.2018.
Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–83.
Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–72.
Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci. 2011;31:1895–904.
Hollander JA, Carelli RM. Abstinence from cocaine self-administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacology. 2005;30:1464–74.
Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–9.
West EA, Saddoris MP, Kerfoot EC, Carelli RM. Prelimbic and infralimbic cortical regions differentially encode cocaine‐associated stimuli and cocaine‐seeking before and following abstinence. Eur J Neurosci. 2014;39:1891–902.
Saddoris MP, Wang X, Sugam JA, Carelli RM. Cocaine self-administration experience induces pathological phasic accumbens dopamine signals and abnormal incentive behaviors in drug-abstinent rats. J Neurosci. 2016;36:235–50.
Saddoris MP, Carelli RM. Cocaine self-administration abolishes associative neural encoding in the nucleus accumbens necessary for higher-order learning. Biol Psych. 2014;75:156–64.
Burton AC, Bissonette GB, Vazquez D, Blume EM, Donnelly M, Heatley KC, et al. Previous cocaine self-administration disrupts reward expectancy encoding in ventral striatum. Neuropsychopharmacology. 2018;43:2350–60.
Izaki Y, Fujiwara SE, Akema T. Involvement of the rat prefrontal cortex in a delayed reinforcement operant task. Neuroreport. 2007;18:1687–90.
Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139:865–76.
Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–31.
Narayanan NS, Cavanagh JF, Frank MJ, Laubach M. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat Neurosci. 2013;16:1888.
Murphy ER, Fernando AB, Urcelay GP, Robinson ES, Mar AC, Theobald DE, et al. Impulsive behaviour induced by both NMDA receptor antagonism and GABA A receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology. 2012;219:401–10.
Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, Van, Gaalen MM. Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology. 2007;191:587–98.
Economidou D, Theobald DE, Robbins TW, Everitt BJ, Dalley JW. Norepinephrine and dopamine modulate impulsivity on the five-choice serial reaction time task through opponent actions in the shell and core sub-regions of the nucleus accumbens. Neuropsychopharmacology. 2012;37:2057–66.
Cheng RK, Liao RM. Regional differences in dopamine receptor blockade affect timing impulsivity that is altered by d-amphetamine on differential reinforcement of low-rate responding (DRL) behavior in rats. Behav Brain Res. 2017;331:177–87.
Narayanan NS, Laubach M. Delay activity in rodent frontal cortex during a simple reaction time task. J Neurophys. 2009;101:2859–71.
Totah NK, Jackson ME, Moghaddam B. Preparatory attention relies on dynamic interactions between prelimbic cortex and anterior cingulate cortex. Cereb Cortex. 2013;23:729–38.
Donnelly NA, Holtzman T, Rich PD, Nevado-Holgado AJ, Fernando AB, Van Dijck G, et al. Oscillatory activity in the medial prefrontal cortex and nucleus accumbens correlates with impulsivity and reward outcome. PloS ONE. 2014;9:e111300.
Donnelly NA, Paulsen O, Robbins TW, Dalley JW. Ramping single unit activity in the medial prefrontal cortex and ventral striatum reflects the onset of waiting but not imminent impulsive actions. Eur J Neurosci. 2015;41:1524–37.
Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocr. 2008;29:36–47.
Weafer J, de Wit H. Sex differences in impulsive action and impulsive choice. Addict Behav. 2014;39:1573–9.
Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82.
Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology. 2002;161:304–13.
Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long-or short-access to cocaine self-administration. Pharm Biochem Be. 2004;78:199–207.
Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology. 2000;148:196–200.
Jentsch JD, Taylor JR. Sex-related differences in spatial divided attention and motor impulsivity in rats. Behav Neurosci. 2003;117:76–83.
Bayless DW, Darling JS, Stout WJ, Daniel JM. Sex differences in attentional processes in adult rats as measured by performance on the 5-choice serial reaction time task. Behav Brain Res. 2012;235:48–54.
Anker JJ, Gliddon LA, Carroll ME. Impulsivity on a Go/No-go task for intravenous cocaine or food in male and female rats selectively bred for high and low saccharin intake. Behav Pharm. 2008;19:615–29.
Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: the role of dopamine and glutamate. Behav Brain Res. 2012;230:21–33.
Moschak TM, Terry DR, Daughters SB, Carelli RM. Low distress tolerance predicts heightened drug seeking and taking after extended abstinence from cocaine self‐administration. Addict Biol. 2018;23:130–41.
Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Bio. 2002;62:609–14.
Moschak TM, Mitchell SH. Partial inactivation of nucleus accumbens core decreases delay discounting in rats without affecting sensitivity to delay or magnitude. Behav Brain Res. 2014;268:159–68.
Moschak TM, Carelli RM. Impulsive rats exhibit blunted dopamine release dynamics during a delay discounting task independent of cocaine history. ENeuro. 2017;4:ENEURO.0119-17.2017.
Sackett DA, Moschak TM, Carelli RM. Nucleus accumbens shell dopamine mediates outcome value, but not predicted value, in a magnitude decision‐making task. Eur J Neurosci. 2020;51:1526–38.
Winstanley CA, Floresco SB. Deciphering decision making: variation in animal models of effort-and uncertainty-based choice reveals distinct neural circuitries underlying core cognitive processes. J Neurosci. 2016;36:12069–79.
Moschak TM, Wang X, Carelli RM. A neuronal ensemble in the rostral agranular insula tracks cocaine-induced devaluation of natural reward and predicts cocaine seeking. J Neurosci. 2018;38:8463–72.
Dalley JW, Lääne K, Pena Y, Theobald DE, Everitt BJ, Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology. 2005;182:579–87.
Hayton SJ, Olmstead MC, Dumont EC. Shift in the intrinsic excitability of medial prefrontal cortex neurons following training in impulse control and cued-responding tasks. PLoS One. 2011;6:e23885.
Sharpe MJ, Killcross S. Modulation of attention and action in the medial prefrontal cortex of rats. Psychological Rev. 2018;125:822–43.
Risterucci C, Terramorsi D, Nieoullon A, Amalric M. Excitotoxic lesions of the prelimbic‐infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur J Neurosc. 2003;17:1498–508.
Baratta MV, Leslie NR, Fallon IP, Dolzani SD, Chun LE, Tamalunas AM, et al. Behavioural and neural sequelae of stressor exposure are not modulated by controllability in females. Eur J Neurosci. 2018;47:959–67.
Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, Maier SF. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051:90–9.
Anderson LC, Petrovich GD. Sex specific recruitment of a medial prefrontal cortex-hippocampal-thalamic system during context-dependent renewal of responding to food cues in rats. Neurobiol Learn Mem. 2017;139:11–21.
Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN. Circuit-based corticostriatal homologies between rat and primate. Biol Psych. 2016;80:509–21.
Li CS, Huang C, Constable RT, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. Neuroimage. 2006;32:1918–29.
Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res. 2006;1105:130–42.
Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58.
Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav Neurosci. 2001;115:799.
Belin-Rauscent A, Daniel ML, Puaud M, Jupp B, Sawiak S, Howett D, et al. From impulses to maladaptive actions: the insula is a neurobiological gate for the development of compulsive behavior. Mol Psychiatr. 2016;21:491–9.
Prasad JA, Macgregor EM, Chudasama Y. Lesions of the thalamic reuniens cause impulsive but not compulsive responses. Brain Struct Funct. 2013;218:85–96.
Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8.
Zavala AR, Osredkar T, Joyce JN, Neisewander JL. Upregulation of Arc mRNA expression in the prefrontal cortex following cue‐induced reinstatement of extinguished cocaine‐seeking behavior. Synapse. 2008;62:421–31.
Hearing MC, Miller SW, See RE, McGinty JF. Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology. 2008;198:77–91.
Smith WC, Rosenberg MH, Claar LD, Chang V, Shah SN, Walwyn WM, et al. Frontostriatal circuit dynamics correlate with cocaine cue-evoked behavioral arousal during early abstinence. Eneuro. 2016;3:ENEURO.0105-16.2016.
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–8.
Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatr. 2000;157:1789–98.
Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–86.
Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115:137–44.
Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–62.
Gozzi A, Tessari M, Dacome L, Agosta F, Lepore S, Lanzoni A, et al. Neuroimaging evidence of altered fronto-cortical and striatal function after prolonged cocaine self-administration in the rat. Neuropsychopharmacology. 2011;36:2431–40.
Lu H, Zou Q, Chefer S, Ross TJ, Vaupel DB, Guillem K, et al. Abstinence from cocaine and sucrose self-administration reveals altered mesocorticolimbic circuit connectivity by resting state MRI. Brain Connect. 2014;4:499–510.
McCracken CB, Grace AA. Persistent cocaine-induced reversal learning deficits are associated with altered limbic cortico-striatal local field potential synchronization. J Neurosci. 2013;33:17469–82.
Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu XT, et al. Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J Neurosci. 2005;25:936–40.
Sepulveda-Orengo MT, Healey KL, Kim R, Auriemma AC, Rojas J, Woronoff N, et al. Riluzole impairs cocaine reinstatement and restores adaptations in intrinsic excitability and GLT-1 expression. Neuropsychopharmacology. 2018;43:1212–23.
Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, et al. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. P Natl Acad Sci. 2009;106:9453–8.
Koob GF. Hedonic homeostatic dysregulation as a driver of drug-seeking behavior. Drug Discov Today: Dis Models. 2008;5:207–15.
Hayen A, Meese-Tamuri S, Gates A, Ito R. Opposing roles of prelimbic and infralimbic dopamine in conditioned cue and place preference. Psychopharmacology. 2014;231:2483–92.
Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–88.
Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, Van, Gaalen MM. Involvement of dopamine D 1 and D 2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology. 2007;191:587–98.
Feja M, Hayn L, Koch M. Nucleus accumbens core and shell inactivation differentially affects impulsive behaviours in rats. Prog Neuro-Psychoph. 2014;54:31–42.
Acknowledgements
We thank Joey Sloand, Caitlin Nygren, Iniya Muthukumaren, and Elijah Richardson for technical assistance.
Author information
Authors and Affiliations
Contributions
TMM and RMC were responsible for the study concept and design. TMM acquired the animal data. TMM and RMC assisted with data analysis and interpretation of findings. TMM drafted the manuscript. TMM and RMC provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication. All authors agree to be accountable for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Moschak, T.M., Carelli, R.M. A sex-dependent role for the prelimbic cortex in impulsive action both before and following early cocaine abstinence. Neuropsychopharmacol. 46, 1565–1573 (2021). https://doi.org/10.1038/s41386-021-01024-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01024-3