Abstract
Neural signatures of suicide risk likely reflect a combination of specific and non-specific factors, and clarifying specific factors may facilitate development of novel treatments. Previously, we demonstrated an altered pattern of resting state connectivity between the dorsal and ventral posterior cingulate cortex (d/vPCC) and the dorsal anterior cingulate cortex (dACC), as well as altered low frequency oscillations in these regions, in individuals with a history of suicidal thoughts and behaviors (STBs) compared to healthy controls. It remains uncertain, however, whether these markers were directly related to STBs or, more generally, reflect a trait-level risk factor for depression. Here, we examined data from a 3-generational longitudinal study of depression where resting state fMRI data were analyzed from 2nd and 3rd generation offspring of probands with (FH+ = 44: STB+ = 32, STB− = 12) and without (FH− = 25: STB+ = 15, STB− = 10) a family history of major depressive disorder (MDD). Standard seed-based methods and a frequency-based analysis of intrinsic neural activity (ALFF/fALFF) were employed. FH of MDD, but not a personal history of STBs or MDD, was associated with relatively reduced dPCC-dACC, and enhanced vPCC-dACC functional connectivity. FH of MDD showed a pattern of reduced ALFF in the dPCC whereas an STB history was associated with an increase. All findings were invariant to confounding by lifetime MDD and current depression severity. Overall, contrary to predictions, resting state functional connectivity within the default mode network (DMN) was associated with FH of depression rather than STBs. These findings confirm the relevance of DMN functional connectivity for mood disorders and underscore the importance of disambiguating biological factors that differentially relate to mental disorders versus STBs.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is prevalent world-wide at around 3–5% [1] and is a significant risk factor for suicide [2, 3]. Although promising neural markers of MDD have been identified [4], biological markers specifically associated with suicidal thoughts and behaviors (STBs) remain unclear. Recent comprehensive reviews in youth [5] and adults [6, 7] highlight frontal and limbic alterations in STBs, but many of these effects are non-specific, perhaps reflecting the etiopathophysiology of the underlying mood disorders [8].
One approach to tackling the complexity of the neural systems underlying STBs is to perform hypothesis-driven analyses, focusing on particular neural circuits implicated in prior work [9,10,11]. This may complement data-driven strategies (e.g., [12]), in which neural alterations are identified via unbiased multivariate statistical methods. A neural system consistently implicated in MDD [13] and STBs [5,6,7], and thus a focus for a hypothesis-driven analysis in the present study, is the default mode network (DMN). The DMN is a network of anatomically and functionally connected regions that include posterior cingulate cortex, medial prefrontal cortex, inferior parietal cortex, and hippocampus [14], which typically de-activate, rather than activate, during difficult cognitive tasks. Hyper-connectivity within the DMN has been observed for MDD [15], as well as those at-risk for MDD [16, 17], which may underlie processes related to rumination or negatively-biased cognition [18]. In addition, previous studies have demonstrated alterations in DMN functional connectivity at rest in individuals with STBs [10, 11, 19]. Taken together, these findings suggest that DMN alterations may serve as stable and transmittable biomarker for depressive illness.
We recently evaluated DMN resting state functional connectivity related to STBs [9], given that suicidal ideation is strongly associated with rumination and brooding [20]. As there is increasing evidence for dissociable components within the DMN (e.g., [21]), we employed distinct seed regions for dorsal and ventral posterior cingulate cortex (dPCC/vPCC) which would capture more fine-grained connectivity patterns than a unitary region of interest. The ventral PCC is better associated with classical DMN regions, while the dorsal region shows slightly enhanced functional connectivity with central executive and salience network regions. We observed different patterns of functional connectivity between these seed regions and the dorsal anterior cingulate cortices (dACC). Whereas healthy adults showed greater functional connectivity between the dorsal PCC and dACC, STB+ individuals exhibiting current suicidal ideation (including a subset with a suicide attempt history) showed greater dACC connectivity from the ventral PCC. Further, the frequency spectrum of all three regions (vPCC, dPCC, dACC) was altered in the STB+ group, with a relative reduction of low frequency oscillatory activity compared to healthy adults. However, the STB+ group also reported greater depressive symptom severity and higher rates of lifetime trauma. Thus, although promising, it is unclear whether these neural correlates directly relate to STBs, or rather, reflect a risk factor associated with depression.
There are two core methodological considerations to consider when probing the DMN, which may impact the interpretation of DMN-STB related findings. First, the dACC region that was identified in prior research [9] was placed at a possible transitional zone between the DMN and salience network [22]. In this light, it is notable that the distinct PCC regions we employed as ROIs were originally defined on the basis of their interactions with ‘task-positive’ regions under different cognitive loads [21]. Thus, these findings might point to altered interactions between the DMN and salience networks, the latter of which also is implicated in STBs [23]. Second, contrary to expectations, low frequency oscillatory activity in the DMN was not clearly associated with altered functional connectivity across individuals. Such activity shows good psychometric properties [24] and distinguishes individual differences [25], suggestive of trait-like properties. However, although low frequency BOLD is associated with other measures of neural activity, it is difficult to decisively distinguish neural activity from physiological noise or variation [26]. In practice, related metrics often correlate with a region’s anatomical and functional properties, particularly the position of the region in the neural processing hierarchy, and can show task modulation [27]. In the absence of a complete neural model of the DMN’s intrinsic activity and functional connectivity, these two classes of metrics may be used to provide complementary information into the network’s functional properties.
In the present study, we addressed the limitation of the healthy control versus STB design by using an ongoing longitudinal family study of MDD, in which second (Gen 2) and third (Gen 3) generation offspring of first generation (Gen 1) probands with and without MDD were followed prospectively over time to understand the course of depression within families [28, 29]. We have shown over the years that Gen 2 and 3 offspring at high familial risk for depression (based on the MDD status of the first generation) had higher rates of anxiety and depressive disorders, and greater functional impairment [30, 31]. As suicidal ideation and attempts also were assessed at each time point, blind to the individual’s family history of depression [28, 29], we were afforded greater discrimination in comparing the effects of personal history of STBs versus familial predisposition to mood disorders on default mode function.
Building on our prior work [9], we hypothesized that individuals with a lifetime history of STBs (STB+) would demonstrate stronger vPCC-dACC over dPCC-dACC functional connectivity, and individuals without a clinical history of STBs (STB−), would exhibit the opposite pattern of activity (dPCC-dACC>vPCC-dACC), independent of their family history (FH), lifetime MDD, and current depression symptoms. Similarly, we hypothesized that low frequency intrinsic activity in these same regions of interest (dPCC, vPCC and dACC) would be reduced in STB+ compared to STB− individuals, accounting for FH, lifetime MDD, and current depression symptoms. As the sample was optimized for evaluating the effects of FH of depression on neural function, we also considered the hypothesis that a positive FH of MDD would be associated with the vPCC-dACC>dPCC-dACC pattern of functional connectivity described previously. Together, this directly addresses the key ambiguity in our previous work; namely, the confounding of STBs with trait-level risk factors for MDD.
Methods
Description of cohort
The study began in 1982 with the recruitment of two groups of first generation probands (G1). The first group was recruited from outpatient clinics and included probands with moderate to severely impairing major depressive disorder (MDD), but no history of schizophrenia, antisocial personality disorder, bipolar disorder, or primary substance use disorder. The second group included non-depressed probands, selected from an epidemiologic sample in the same community, who had no lifetime history of psychiatric illness, as confirmed through several interviews. The second (Gen 2) and third (Gen 3) generation offspring of these probands form the cohort for this study. Offspring of probands with MDD constitute the high-risk (family history, FH+) group, and those of probands without depression, the low-risk (FH−) group.
There were six waves to the study at the timepoint of this analysis, corresponding to baseline, time (~year) 2, 10, 20, 25 and 30. G2 entered the study at baseline or time 2; G3 offspring were enrolled at time 10 or 20 as they aged in. The average number of interviews was 4.6 for 2nd generation, and 2.0 for 3rd generation offspring. However, as each interview assessed the complete time period subsequent to the preceding interview, total follow up was from birth to current age. The overall design of the study has been detailed in several previous publications [28, 29].
Informed consent was obtained from adults for themselves and for their minor children; verbal assent was also obtained from minors. All participants provided consent and all procedures were approved by the New York State Psychiatric Institute’s IRB. One hundred and twenty G2 and G3 biological offspring of G1 probands underwent an MRI scan at time 30 of the study. We excluded five participants with high motion (>3 mm). All included participants showed a framewise displacement of between 0.05 and 0.56 (see Section 2.3 for further details; see Supplement for additional tests of movement parameters). Forty-six individuals under the age of 25 were removed to mitigate the statistical impact of confounding related to age, depression, and suicide symptoms. An age 25 was selected as a cutoff because this often defines a transitional period from adolescence to early adulthood (i.e., 18−25) [32]. In our own sample, this reduced the relationship between STBs and age considerably (partial η2 reduced from 0.66 to 0.41). Thus, the final sample included 69 participants, after excluding individuals with excessive head motion (n = 5) and who were under age 25 (n = 46) (note: sixty-two of these individuals also are included in a prior publication [16] examining the role of the DMN in family history of depression; that study, however, did not test PCC subregions).
Clinical interviews
Clinical interviews were conducted at each wave using the semi-structured Schedule for Affective Disorders and Schizophrenia, lifetime version [33] (the child/adolescent version was used for participants when they were 6–17 years old [34]). Each family member was interviewed blind to the clinical status of other family members by trained doctoral- and/or master-level mental health professionals, allowing us to generate independently derived measures of FH and STBs. Interview training and reliability have been documented elsewhere; reliability ranged from good to excellent. Final diagnoses were confirmed by an M.D. or Ph.D. using the best-estimate procedure [35].
As part of the interview, a gate question (Have you ever wished you were dead or thought about dying or killing yourself?”) prompted entry into a suicide module, where the frequency and severity of STBs were probed. For individuals who reported suicide attempts, number and age at each attempt was recorded along with severity of suicidal intent and medical threat. For minors, both parent and child reports were obtained, and when reports were discrepant, a clinician assigned a summary score based on all available information. To rule out spurious ideation reports, we required moderate-to-higher severity for inclusion into STB+. A subgroup of 8 individuals reported having made a suicide attempt (7 of whom reported significant ideation). We defined the STB+ group as individuals who reached the suicidal ideation criterion and/or had a history of suicide attempt, and the STB- group as individuals with no lifetime history (see Table 1). To control for the effects of current mood on DMN connectivity, current depressive symptoms were assessed using the 17-item Hamilton Depression Rating Scale (HDRS17), a standard instrument used to assess clinical depression in the last two weeks [36].
Image acquisition and parameters
Neuroimaging data were acquired at New York State Psychiatric Institute using a GE Signa 3.0 T scanner with an 8-channel head coil. During the resting state acquisition, participants were instructed to remain still, with their eyes closed, and allow their minds to wander freely. Two runs, 8 min 50 s each, were collected per participant. Only the first run was used, unless maximum motion in any direction during the scan was greater than 3 mm, in which case the second was used, provided it too had a maximum motion of <3 mm [37]. Including both runs would have led to inconsistencies regarding numbers of runs used per participant and/or fewer subjects given our motion criterion. Resting state images were acquired using an axial echoplanar imaging (EPI) sequence (TE/TR = 23.6/2800ms, 90° flip angle, receiver bandwidth = 62.5 kHz, single excitation per image, slice thickness = 3.0 mm, 0.5 mm spacing, 43 slices, 24 × 24 cm field of view, 64 × 64 matrix; effective resolution of 3.75 × 3.75 × 3.0 mm). A structural FSPGR scan, which was used for normalization, was acquired using the following parameters (TE/TR = 2.39/6.036ms, slice thickness = 1.0 mm, 162 slices, 25 × 25 cm field of view, 256 × 256 matrix).
fMRI resting state data preprocessing
Data were analyzed using a combination of SPM, FSL and AFNI scripts implemented in Nipype [38]. Preprocessing involved co-registration, slice time correction, normalization (SPM - DARTEL), despiking (AFNI), rescaling following linear detrending, and smoothing using SUSAN (FSL: 6 mm kernel). Seed-based and (fractional) amplitude of low frequency fluctuation ((f)ALFF) analyses were conducted using routines in the C-PAC software (https://fcp-indi.github.io). Seed-based analysis employed the 8 mm diameter spheres centered on dorsal (x = 0, y = −58, z = 28) and ventral (x = 0, y = −58, z = 28) PCC coordinates defined by Leech and colleagues [21], and employed in our previous study. Following preprocessing, nuisance parameters included a global signal timeseries, five a/tCompCor timeseries obtained from white matter/cerebrospinal fluid and high temporal standard deviation voxels [9, 39] and 6 motion parameters, as well as the derivatives of all 12 timeseries and a linear trend. Following nuisance regression, bandpass filtering was employed (42.56/4.26 s). Finally, Pearson’s correlation was performed between the seed region and each voxel in the brain, which were then converted into z statistics. These seed-based analyses were supplemented by an analysis—analogous to one performed in previous study [9]—using unfiltered data. Unfiltered time series were extracted from preprocessed, nuisance corrected images using the dorsal and ventral PCC seeds, and a dorsal anterior cingulate seed defined by [40], as employed by the previous study [9] (ROIs displayed in Supplementary Fig. 1). Our reasoning for examining both filtered and unfiltered timeseries here was to ensure the generalizability across different methods, similar to our previous work [9]. Correlation coefficients between the regions’ timeseries were computed, which were then z transformed.
The Amplitude of Low Frequency Fluctuations (ALFF) method [24] was implemented using C-PAC. Briefly, this method involves computing the power in each voxel time series between 0.1 and 0.01 Hz. This metric is then Z-transformed relative to the mean and standard deviation of every voxel in the individual’s brain, and this measure is used for further analysis. The fractional ALFF method (fALFF) uses the same calculation of low frequency power, but relative to the total power across all frequencies within the voxel, which can provide some correction for arbitrary physiological confounding [24]. As with ALFF, the voxel-wise measure is Z-transformed relative to the mean and standard deviation across the whole brain. Broadly, the goal of employing the methods was to obtain an estimate of the power of low frequency oscillations in the brain, relative to the rest of the individual’s brain activity. Of the two methods, fALFF is closest conceptually to the method employed in our previous work [9], but a variety of arbitrary differences – especially the different scan duration and temporal resolution of image acquisition—might influence the capacity to identify differences in oscillatory power, so both approaches were adopted. In general terms, the (f)ALFF technique was employed in this study as it is both widely used and affords exploratory whole brain analysis.
Hypothesis testing and data analysis
In line with our previous work [9], we focused on the difference between dPCC-dACC and vPCC-dACC connectivity. Our strategy employed region of interest (ROI) analyses using the same ROIs (dPCC, vPCC, dACC) used in that study (Supplementary Fig. 1: [9]. For functional connectivity analyses, ROI analyses were performed using generalized estimating equations (GEE) with a single dependent measure (dPCC-dACC minus vPCC-dACC) and between-subject effects of STBs and FH. Sequential testing of factors was performed, assessing the effects of the predictors independently and together. In addition, follow up regression analyses were performed with the connectivity measures, assessing the effects of lifetime MDD, current depression symptoms, and medication (i.e., coded as present or absent). Final models were performed using a generalized estimating equation (GEE) approach (PROC GENMOD in SAS 9.4, Cary, N.C) to account for potential non-independence of outcomes for offspring from the same family; age, sex and motion (framewise displacement) also were included in all models.
For each dependent measure, a finding was considered significant if it reached a corrected significance threshold of p < 0.025 (0.05/2 for STB/FH), for all regression models examined. To ensure comparability with our previous study [9], we examined the effects of bandpass filtering by comparing the results of filtered and unfiltered timeseries. For ALFF/fALFF analyses, a similar GEE model was used, but including ALFF/fALFF measures from dPCC/vPCC/dACC as dependent measures in separate models. As a post-hoc analysis to establish whether functional connectivity and intrinsic activity dependent measures predict FH, we examined the inter-relationship between d/vPCC-dACC connectivity and dPCC ALFF scores and used logistic regression (modeling of familial relationships was not performed for this analysis). We estimated joint and separate models, reporting Wald scores as well as classification accuracy, sensitivity, and specificity.
Supplementary whole brain analyses explored (filtered) dPCC versus vPCC maps, and mean dPCC and vPCC maps, and ALFF and fALFF measures (see Supplement). Statistical analyses included whole brain corrected analyses (p < 0.001 uncorrected, pFWE<0.05 cluster corrected). The independent measures (between-subject predictors) were the same as those used in the ROI analyses (i.e., STB, FH and lifetime MDD with age, sex and motion as covariates). Neither familial relationships nor current depression symptoms were modeled in these exploratory analyses.
Results
Demographics
Participant demographic and clinical information are summarized in Table 1. Relative to STB−, STB+ individuals were older (t(67) = 6.60, p < 0.001), but there were no significant age-related differences regarding FH (see Table 1). Consistent with prior research (e.g., [41, 42]), FH+ individuals were more likely to have experienced lifetime MDD (χ2 = 9.59, p = 0.002), but no significant relationship between lifetime MDD and STBs was observed (χ2 = 1.61, p = 0.20).
Dissociation of dorsal versus ventral PCC connectivity with dACC
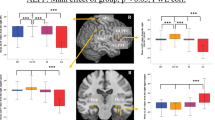
We first tested whether differential dPCC/vPCC to dACC functional connectivity was related to STBs or FH individually. No significant differences emerged when comparing STB+ and STB−; however, it was related to FH (b = −0.147, 95% CI [−0.267, −0.028], p = 0.0156; Table 2, Model A). Further examination of mean connectivity values for each FH group and each PCC region (plotted in Fig. 1A) showed overall differences between dPCC and vPCC in the high-risk group; namely, greater ventral, relative to dorsal, PCC connectivity to the dACC (beta = −0.1464 [95% CI: −0.274, −0.018], p = 0.0247) but not in the low-risk group, where dPCC and vPCC connectivity to the dACC were similar (beta = 0.033 [95% CI: −0.1092, 0.174], p = 0.653).
The overall FH effect (Model A) remained significant (p < 0.025 in all cases) when accounting for STBs (Table 2, Model B), lifetime MDD history (Table 2, Model C), and current depression symptom severity and medication usage (Table 2, Model D). As a test of sensitivity, we re-ran the model after excluding the 8 suicide attempters from the STB group, and the model remained significant, b = −0.10728 [−0.39, −0.05], p = 0.0057. Finally, there also were no differences by generation (generation-by-risk interaction, p = 0.595; see also Table S1).
To ensure consistency with our previous work [9], which employed unfiltered data for post-hoc analysis, we also performed the same analysis using unfiltered data. We observed a similar pattern of findings with FH as a predictor of d/vPCC-dACC connectivity (Fig. 1B; Table S2)—specifically, the dPCC-dACC>vPCC-dACC pattern of functional connectivity in FH− individuals, but the reverse pattern (vPCC-dACC>dPCC-dACC) in FH+ individuals.
Oscillations within the anterior and posterior cingulate cortex
We next tested whether STB or FH were associated with overall oscillatory power in the three cingulate regions of interest using ALFF and fALFF. No findings which consistently reached our corrected significance threshold (p < 0.025) across models were observed, although several findings emerged at uncorrected levels (p < 0.05) which were generally dependent on the regression model employed. Using ALFF, FH+ was associated with reduced low frequency power in the dPCC in the combined models (Table 3, Fig. 2; Models C and D), but STB+ (Models A, B, and C) and the presence of lifetime MDD (Models C and D) were associated with increased power (Table 3). STB+ individuals showed increased oscillations in the dorsal ACC (Table 3, Models A, B, and C). For fALFF, STB+ was associated with increased low frequency oscillations in the dPCC (Table S3, Models A, B, C and D). To assess potential confounding of STBs with age, we plotted ALFF/fALFF against age (Fig. S2), observing no relationships that might account for our findings.
Combined effects of functional connectivity and ALFF
The above analyses showed that FH is associated with both reduced ALFF in the dPCC and an increase from dorsal to ventral PCC functional connectivity to the dACC. However, further zero-order correlations revealed that these two measures were not significantly related to each other (rs < 0.19, ps > 0.13). Thus, these measures may reflect complementary manifestations of FH. Accordingly, we performed a post-hoc analysis using logistic regression, in which we attempted to classify FH using both measures. A logistic regression model including both d/vPCC-dACC (filtered: Wald = 6.11, p = 0.013) and dPCC ALFF (Wald = 4.26, p = 0.039: the region evincing the largest group difference), as well as motion/age/sex covariates, accurately classified 73.9% of FH+/FH− cases (sensitivity: 86.4%; specificity: 52.0%). Both metrics could independently predict FH within similar logistic model, but with a somewhat diminished capacity to classify FH (d/vPCC-dACC: Wald = 6.10, p = 0.014; classification 66.7%; sensitivity 84.1%; specificity 36.0%; dPCC ALFF: Wald = 4.34, p = 0.037, overall classification 65.2%; sensitivity 86.4%; specificity 28.0%).
Discussion
In the present study, we evaluated resting state functional connectivity within the DMN to clarify whether previously identified neural correlates of STBs may reflect an MDD risk factor. There were several notable findings. Contrary to our original hypotheses, stronger functional connectivity between the dorsal PCC and the dACC versus the ventral PCC and the dACC was related to FH but not lifetime history of STBs. Specifically, FH+ individuals showed greater vPCC-dACC than dPCC-dACC functional connectivity, whereas FH− individuals showed the reverse pattern. This pattern of altered functional connectivity remained significant when accounting for lifetime MDD history, current depression symptom severity, and psychotropic medication use, suggestive of a stable trait marker rather than a consequence of having the disorder.
In line with our prior work [9], we hypothesized that low frequency oscillations of BOLD activity would be reduced in the cingulate cortex in STB+ individuals compared to STB−. However, STB+ was associated with a numerically heightened ALFF and fALFF, particularly in the dorsal PCC and dorsal ACC, which reached uncorrected thresholds across several of the models tested. In addition, there was evidence that FH+ was associated with numerically reduced ALFF signal, particularly in the dPCC. In general, the (f)ALFF findings were more dependent on the regression model employed (i.e., separate versus combined), suggesting that confounding within a simple patient vs control design might potentially suppress effects of different factors (e.g., FH, STB, lifetime MDD), which move in opposing directions [43]. One interesting point to note is that whole brain analysis suggested that STBs were more strongly reflected in fALFF, while FH was more strongly reflected in ALFF. Such a pattern might be observed if FH had a broadband effect—oscillatory power was reduced across the spectrum, while the STB effect was highly restricted to a particular frequency band within the 0.01–0.1 Hz window. If these phenomena are spectrally independent, both could co-exist but would be difficult to differentiate statistically unless focused analysis were performed on crucial frequencies. Overall, our current findings suggest that our prior research [9] might have been unduly influenced by FH effects, given that low frequency oscillations were lower in STB than HC. More importantly, if suppression is important in this context, it requires that both STB and FH are concurrently measured in future studies to determine their relative contribution accurately.
Although both frequency (ALFF) and connectivity (d/vPCC-dACC) connectivity were related to FH, the two measures were not correlated with one another and are independently associated with family history. This is interesting to consider, as a re-analysis of our prior work [9] shows a similar level of classification (~70%) of STBs versus HC using equivalent metrics (d/vPCC-dACC connectivity; dPCC low frequency signal; age/sex/motion covariates) obtained within that study. Together, these findings suggest that d/vPCC-dACC functional connectivity is a sensitive marker of depression-related individual differences, rather than being specific to STBs, again underscoring the importance of disentangling neural factors related to MDD versus STBs [5, 44].
The three generation family study provided important insights into underlying trait-level risk factors of MDD [45, 46], which are distinct from the standard case-controlled design of a depressed or suicidal cohort versus a healthy control group. Indeed, many prior studies examining relationships between the DMN connectivity or low frequency BOLD and rumination [18, 47, 48] or suicide [10, 11, 49] have adopted this type of cross-sectional approach. The contribution of our findings, however, is to suggest that DMN functional connectivity may be influenced by a familial predisposition to depression, and interestingly, a recent study examining reliable, trait-like functional connectivity markers [50] showed that these factors were often located in the DMN. This further supports our contention that d/vPCC-dACC connectivity may reflect a trait-like marker of MDD and confirms the importance of delineation of subregions within the DMN [51, 52] for identifying reliable trait-like patterns of DMN functional connectivity. Together, such findings may offer potential to describe objective and reliably predictive markers of major depression, as well as offering potential to provide targets for neuromodulation-based interventions [53].
Furthermore, the findings suggest a way to reconsider the complex, emerging literature examining neuroimaging measures of suicide [7]; namely, many of the findings identified within prior studies employing healthy controls may be confounded by correlates of general illness severity or risk, as well as state confounds such as mood. One strategy to address this, should the neural correlates of such confounds be relatively reliable, would be to use these correlates to stratify patients, and determine whether the proposed neural correlate of suicide was broadly independent of this stratification. Within the resting state field, such a strategy may help to parse the already complex literature that is rapidly emerging [10, 11, 19, 23].
Limitations
The study has several limitations. First, the presence of suicidal ideation was assessed within the context of a diagnostic interview, rather than self-report measure (e.g., [54,55,56]). Self-report measures can be sensitive to different aspects of suicidal ideation, including its severity, frequency, content and duration, and these dimensions may be important for more detailed understanding of the neural basis of STBs. Additionally, the STB group is heterogenous with regards to the inclusion of ideators and attempters, and more broadly, differences in the severity and persistence of suicidal thinking may unduly influence our capacity to detect unique effects related to FH versus STBs. Relatedly, we could not test the associations between family history for STBs and DMN outcomes, as families with multiple generations of STBs were embedded within those with family history for depression. Second, the overall sample size was small and thus, limited the power for detecting interaction effects. Moreover, data collected per participant were relatively modest, in light of calls to increase resting state data acquisition to ~12–13 min [57] to enhance reliability. Third, the confounding of STBs by age may have reduced statistical power to detect STB related effects in the presence of age covariates. Nevertheless, there was no evidence that such confounding was related to our findings. Last, the sample was primarily of European ancestry, as was the standard at the time when the study originated in 1982, and it is unclear whether findings would generalize to other population sub-groups.
Summary
Systematic study of the neural correlates of suicide is an emerging field. We used a hypothesis-based approach, building on previous work examining DMN functional activity in STBs [9]. Contrary to our predictions, an altered pattern of posterior and anterior cingulate functional connectivity was associated with FH but not STBs. Likewise, low frequency BOLD oscillations, which were previously shown to be reduced in STBs, were generally reduced in individuals with a FH+ but increased in STB+. Together, in line with considerable prior research, the findings confirm the importance of the DMN as a key neural correlate of mood disorders, and suggest that trait-level, familial influences are important. They also indicate that identification of neural markers of STBs will require careful modeling of state- and trait-level factors that account for MDD.
Funding and disclosures
This project was supported by the National Institute of Mental Health R01 MH-036197 (MMW, JP) and through an American Foundation for Suicide Prevention grant awarded to AT (SRG-0-130-16). RPA was partially supported by U01 MH116923, R01 MH119771, and R56 MH121426. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or of any other sponsor.
In the last three years, Dr. Weissman has reported receiving royalties from Oxford University Press, Perseus Books Group, American Psychiatric Association Publishing, and Multi-Health Systems. JP has received funding from Takeda (formerly Shire) and Aevi Genomics. None of these present any conflict with the present work, and no other authors report any disclosures.
References
Ferrari AJ, Somerville AJ, Baxter AJ, Norman R, Patten SB, Vos T, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013;43:471–81.
Mykletun A, Bjerkeset O, Dewey M, Prince M, Overland S, Stewart R. Anxiety, depression, and cause-specific mortality: the HUNT study. Psychosom Med. 2007;69:323–31.
Hawton K, Casanas ICC, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord. 2013;147:17–28.
Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry. 2018;175:1111–20.
Auerbach RP, Pagliaccio D, Allison GO, Alqueza KL, Alonso MF. Neural Correlates Associated With Suicide and Nonsuicidal Self-injury in Youth. Biol Psychiatry. 2021;89:119–33.
Schmaal L, van Harmelen AL, Chatzi V, Lippard ETC, Toenders YJ, Averill LA, et al. Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Mol Psychiatry. 2020;25:408–27.
Huang X, Rootes-Murdy K, Bastidas DM, Nee DE, Franklin JC. Brain differences associated with self-injurious thoughts and behaviors: a meta-analysis of neuroimaging studies. Sci Rep. 2020;10:2404.
Janiri D, Moser DA, Doucet GE, Luber MJ, Rasgon A, Lee WH, et al. Shared neural phenotypes for mood and anxiety disorders: a meta-analysis of 226 task-related functional imaging studies. JAMA Psychiatry. 2020;77:172–9.
Chase HW, Segreti AM, Keller TA, Cherkassky VL, Just MA, Pan LA, et al. Alterations of functional connectivity and intrinsic activity within the cingulate cortex of suicidal ideators. J Affect Disord. 2017;212:78–85.
Malhi GS, Das P, Outhred T, Bryant RA, Calhoun V, Mann JJ. Default mode dysfunction underpins suicidal activity in mood disorders. Psychol Med. 2020;50:1214–23.
Ordaz SJ, Goyer MS, Ho TC, Singh MK, Gotlib IH. Network basis of suicidal ideation in depressed adolescents. J Affect Disord. 2018;226:92–99.
Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38.
Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci Biobehav Rev. 2015;56:330–44.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82.
Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78:224–30.
Posner J, Cha J, Wang Z, Talati A, Warner V, Gerber A, et al. Increased default mode network connectivity in individuals at high familial risk for depression. Neuropsychopharmacology. 2016;41:1759–67.
Chai XJ, Hirshfeld-Becker D, Biederman J, Uchida M, Doehrmann O, Leonard JA, et al. Altered intrinsic functional brain architecture in children at familial risk of major depression. Biol Psychiatry. 2016;80:849–58.
Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548–55.
Zhang S, Chen JM, Kuang L, Cao J, Zhang H, Ai M, et al. Association between abnormal default mode network activity and suicidality in depressed adolescents. BMC Psychiatry. 2016;16:337.
Miranda R, Nolen-Hoeksema S. Brooding and reflection: rumination predicts suicidal ideation at 1-year follow-up in a community sample. Behav Res Ther. 2007;45:3088–95.
Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–24.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506.
Schwartz J, Ordaz SJ, Ho TC, Gotlib IH. Longitudinal decreases in suicidal ideation are associated with increases in salience network coherence in depressed adolescents. J Affect Disord. 2019;245:545–52.
Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49:1432–45.
Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B, et al. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage. 2011;54:2950–9.
Auer DP. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the ‘resting’ brain. Magn Reson Imaging. 2008;26:1055–64.
Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci. 2011;31:7910–9.
Weissman MM, Wickramaratne P, Gameroff MJ, Warner V, Pilowsky D, Kohad RG, et al. Offspring of depressed parents: 30 years later. Am J Psychiatry. 2016;173:1024–32.
Weissman MM, Berry OO, Warner V, Gameroff MJ, Skipper J, Talati A, et al. A 30-year study of 3 generations at high risk and low risk for depression. JAMA Psychiatry. 2016;73:970–7.
Warner V, Wickramaratne P, Weissman MM. The role of fear and anxiety in the familial risk for major depression: a three-generation study. Psychol Med. 2008;38:1543–56.
Weissman MM, Wickramaratne P, Nomura Y, Warner V, Verdeli H, Pilowsky DJ, et al. Families at high and low risk for depression: a 3-generation study. Arch Gen Psychiatry. 2005;62:29–36.
Arnett JJ. Emerging adulthood: the winding road from the late teens through the twenties. Oxford University Press; 2014.
Mannuzza S, Fyer AJ, Klein DF, Endicott J. Schedule for affective disorders and schizophrenia-lifetime version modified for the study of anxiety disorders (SADS-LA): rationale and conceptual development. J Psychiatr Res. 1986;20:317–25.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8.
Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–83.
Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96.
Belleau EL, Kremens R, Ang YS, Pisoni A, Bondy E, Durham K, et al. Reward functioning abnormalities in adolescents at high familial risk for depressive disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:270–9.
Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform. 2011;5:13.
Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101.
Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–90.
Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, et al. Development and natural history of mood disorders. Biol Psychiatry. 2002;52:529–42.
Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. Am J Psychiatry. 2006;163:1001–8.
Watson D, Clark LA, Chmielewski M, Kotov R. The value of suppressor effects in explicating the construct validity of symptom measures. Psychol Assess. 2013;25:929–41.
Milne BJ, Caspi A, Harrington H, Poulton R, Rutter M, Moffitt TE. Predictive value of family history on severity of illness: the case for depression, anxiety, alcohol dependence, and drug dependence. Arch Gen Psychiatry. 2009;66:738–47.
Talati A, Weissman MM, Hamilton SP. Using the high-risk family design to identify biomarkers for major depression. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120129.
Hao X, Talati A, Shankman SA, Liu J, Kaiser J, Tenke CE, et al. Stability of cortical thinning in persons at increased familial risk for major depressive disorder across 8 years. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:619–25.
Kuhn S, Vanderhasselt MA, De Raedt R, Gallinat J. Why ruminators won’t stop: the structural and resting state correlates of rumination and its relation to depression. J Affect Disord. 2012;141:352–60.
Makovac E, Fagioli S, Rae CL, Critchley HD, Ottaviani C. Can’t get it off my brain: meta-analysis of neuroimaging studies on perseverative cognition. Psychiatry Res Neuroimaging. 2020;295:111020.
Lan MJ, Rizk MM, Pantazatos SP, Rubin-Falcone H, Miller JM, Sublette ME, et al. Resting-state amplitude of low-frequency fluctuation is associated with suicidal ideation. Depress Anxiety. 2019;36:433–41.
Seitzman BA, Gratton C, Laumann TO, Gordon EM, Adeyemo B, Dworetsky A, et al. Trait-like variants in human functional brain networks. Proc Natl Acad Sci USA. 2019;116:22851–61.
Braga RM, Buckner RL. Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron. 2017;95:457–71. e5.
Bzdok D, Heeger A, Langner R, Laird AR, Fox PT, Palomero-Gallagher N, et al. Subspecialization in the human posterior medial cortex. Neuroimage. 2015;106:55–71.
Bauer CCC, Okano K, Ghosh SS, Lee YJ, Melero H, Angeles CL, et al. Real-time fMRI neurofeedback reduces auditory hallucinations and modulates resting state connectivity of involved brain regions: Part 2: Default mode network -preliminary evidence. Psychiatry Res. 2020;284:112770.
Rush AJ, First MB, Blacker D, American Psychiatric Association. Task force for the handbook of psychiatric measures. Handbook of psychiatric measures. 2nd ed. Washington, DC: American Psychiatric Publication; 2008.
Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–77.
Nock MK, Holmberg EB, Photos VI, Michel BD. Self-injurious thoughts and behaviors interview: development, reliability, and validity in an adolescent sample. Psychol Assess. 2007;19:309–17.
Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chase, H.W., Auerbach, R.P., Brent, D.A. et al. Dissociating default mode network resting state markers of suicide from familial risk factors for depression. Neuropsychopharmacol. 46, 1830–1838 (2021). https://doi.org/10.1038/s41386-021-01022-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01022-5