Abstract
Poor inhibitory control and heightened feelings of stimulation after alcohol are two well-established risk factors for alcohol use disorder (AUD). Although these risk factors have traditionally been viewed as orthogonal, recent evidence suggests that the two are related and may share common neurobiological mechanisms. Here we examined the degree to which neural activity during inhibition was associated with subjective reports of stimulation following alcohol. To assess neural changes during inhibition, moderate alcohol drinkers performed a stop signal task during fMRI without drug. To assess subjective responses to alcohol they ingested alcohol (0.8 g/kg) or placebo beverages under double-blind conditions and provided subjective reports of stimulation and sedation. Feelings of stimulation following alcohol were inversely associated with activity in the supplementary motor area, insula, and middle frontal gyrus during inhibition (successful stop trials compared to go trials). Feelings of sedation did not correlate with brain activation. These results extend previous findings suggesting that poor inhibitory control is associated with more positive subjective responses to alcohol. These interrelated risk factors may contribute to susceptibility to future excessive alcohol use, and ultimately lead to neurobiological targets to prevent or treat AUD.
Similar content being viewed by others
Introduction
Poor inhibitory control and feelings of stimulation after alcohol are two well-established risk factors for alcohol use disorder (AUD). Poor inhibition in adolescence and young adulthood predicts the onset and severity of problematic alcohol use later in life [1,2,3,4]. Similarly, greater sensitivity to the positive, stimulant effects of alcohol and less sensitivity to the negative, sedative effects predict AUD severity up to ten years later [5,6,7,8]. Further, stimulant-like responses to alcohol also predict alcohol choice, suggesting they are indicators of alcohol reward [9]. Although poor inhibition and heightened stimulation following alcohol have traditionally been considered orthogonal to one another, recent evidence suggests that the two are related and share common neurobiological mechanisms. Identifying the neural mechanisms underlying this association could help to identify important neurobiological risk markers for addiction.
Behaviorally, poor inhibitory control is linked to heightened sensitivity to alcohol reward in both animal models and in humans. In laboratory animals, drug-naïve mice and rats bred to prefer alcohol exhibit poorer response inhibition compared with non-alcohol preferring lines [10,11,12,13]. In humans, individuals with poor inhibitory control as assessed by performance on the stop signal task report greater stimulation and less sedation following alcohol [14]. Individuals who score high on self-reported impulsivity also report greater subjective alcohol reward [15,16,17]. Self-reported impulsivity has also been linked to objective measures of alcohol reward, including greater alcohol-induced dopamine release in the striatum [18] and greater increase in heart rate following alcohol [19]. In addition, similar associations between poor inhibition and subjective reward have been reported with another drug of abuse, amphetamine [20, 21].
In addition to behavioral links between inhibitory function and drug reward, there is also preliminary evidence of shared neural circuits. Response inhibition circuitry involves right frontal regions, including the supplementary motor area (SMA; now called the juxtapositional lobule cortex), inferior frontal gyrus (IFG), insula, anterior cingulate cortex (ACC), and dorsolateral prefrontal cortex (DLPFC) [22,23,24]. These regions form an inhibitory network in conjunction with the basal ganglia, including the dorsal striatum, that allows for the ‘top-down’ cortical inhibition of subcortical motor responses [25,26,27]. Although the neural correlates of the subjective rewarding effects of drugs are less well-characterized, there is evidence that positive subjective drug responses are mediated by activity in frontal and striatal regions, including ventromedial prefrontal cortex (VMPFC), ACC, and the ventral and dorsal striatum [28,29,30,31]. Thus, both inhibitory control and drug reward sensitivity are linked to activity within fronto-striatal circuitry.
We have examined associations between neural inhibitory function and sensitivity to both drug rewards and monetary reward. First, we tested relationships between neural inhibitory function and subjective responses to amphetamine. Individuals who displayed less right frontal activity during inhibition reported greater euphoria and arousal following amphetamine [21]. Next, we assessed correlations between neural inhibitory function and activity in striatal reward regions during receipt of monetary reward. In that study, individuals who displayed less right frontal activity during inhibition displayed greater striatal activity during monetary reward [32]. Taken together, these studies provide initial evidence that less neural activity related to behavioral inhibition is associated with greater sensitivity to rewards, whether they are related to administration of a drug or receipt of money. Here, we extended these findings to examine neural inhibitory function as a predictor of sensitivity to the positive subjective effects of alcohol.
The current study assessed associations between neural activity related to behavioral inhibition and subjective reports of stimulation following alcohol in healthy young adult moderate drinkers. Brain activity during response inhibition was assessed by examining regional neural activation while subjects performed the stop signal task. Subjective alcohol response was assessed on separate sessions, during which subjects consumed a dose of alcohol (0.8 g/kg) or placebo and reported their subjective stimulation. We also assessed subjective reports of sedation as a comparison. We hypothesized that individuals with less right frontal activity during response inhibition would report greater stimulation following alcohol.
Materials and methods
Design
Healthy volunteers (a subset of the sample reported in Weafer et al. [14] who were eligible for MRI) performed the stop signal task during fMRI to assess neural correlates of inhibitory control. They also completed a four-session alcohol challenge procedure to assess their subjective responses to oral ethanol (0.8 g/kg) or placebo (see Procedure below). Participants received both ethanol and placebo twice, in alternating order, with drug administered first (ethanol or placebo) randomly assigned, under double-blind conditions. We examined neural correlates of response inhibition in the drug-free state in relation to ratings of stimulation and sedation on the alcohol vs placebo sessions. The alcohol challenge sessions were conducted at the University of Chicago and the brain imaging was conducted at the University of Illinois at Chicago. The Institutional Review Boards of both universities approved the study, which was carried out in accordance with the Declaration of Helsinki. All participants provided written informed consent for participation. Upon completion of all sessions, participants were debriefed and paid for their time.
Participants
Male and female moderate social drinkers (n = 76) were recruited through online and printed advertisements. Volunteers were eligible to participate if they consumed an average of 7–30 standard drinks per week (e.g., 12 oz beer or 1.5 oz liquor), with at least one heavy drinking episode (i.e., 4 or more drinks in one sitting for women, 5 or more for men) in the past month. These drinking criteria ensured that participants could tolerate the alcohol dose, and their habitual consumption put them at risk for developing AUD. Additional inclusion criteria were age 21–29, BMI 19–26, at least a high school education, and fluency in English. Exclusion criteria were past year DSM-5 diagnosis, lifetime history of substance use disorder (including AUD) or ADHD, serious medical conditions, night shift work, smoking >5 cigarettes/day, contraindications for MRI, left-handedness, use of medications other than birth control, or pregnancy, lactation, or plans to become pregnant in the next 3 months.
Measures
Stop signal task. To assess neural correlates of response inhibition during blood oxygenation level dependent (BOLD) fMRI, participants performed a previously adapted version [33] of the stop signal task, programmed in E-Prime software (Psychology Software Tools, Sharpsburg, PA), and based on a prior variant by Rubia et al [34]. Participants were instructed to respond as quickly as possible to go signals (left- or right-pointing blue arrows), and to inhibit responses on trials in which a stop signal (up-pointing red arrow superimposed on the go arrow) occurred. An algorithm adjusted the delay duration between the go and stop signals (i.e., the stop signal delay) to target a 50% successful inhibition rate. Stop signal reaction time (SSRT) was calculated by subtracting the mean stop signal delay from participants’ mean go reaction time. Longer SSRT indicates greater time needed to inhibit a response, and thus poorer inhibitory control. We calculated SSRT to provide a behavioral measure of participants’ inhibitory control and a means for comparison with other samples. Behavioral task performance was considered valid if participants had a mean Go reaction time of 900 ms or less, Go accuracy of at least 70%, and Stop accuracy between 30 and 70%. These validity criteria are based on our previous publication with this sample [14] and have been adjusted for the in-scanner environment. Participants completed three runs (80 go and 40 stop trials each), and each run required ~6 min to complete.
Biphasic Alcohol Effects Scale (BAES) [35]. The BAES is a measure of subjective stimulation (e.g., talkative, elated) and sedation (e.g., sedated, sluggish) responses to alcohol. Responses for the 7 individual stimulation items and 7 individual sedation items are reported on a Likert scale (0–10), with stimulation and sedation items summed separately to provide a total score for each (score range = 0–70). The BAES data reported here are from a subset of BAES data presented in our previous report [14].
Procedure
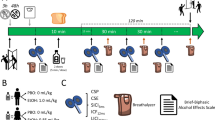
Participants abstained from drugs, including alcohol, for 24 h prior to each session, as verified by self-report, breath alcohol, and urine screens for benzodiazepines, barbiturates, cocaine, THC, opioids, MDMA, PCP, and amphetamine. Breath alcohol concentration (BrAC) was measured via a hand-held breathalyzer (Intoximeters Inc., St. Louis, MO). Participants first attended an orientation session in which they provided informed consent and were familiarized with laboratory procedures and the study protocol.
Beverage administration sessions. Participants completed four beverage administration sessions, described in detail in Weafer et al. [14]. The four beverage sessions (two alcohol-placebo session pairs) allowed us to examine the reliability of associations between neural correlates of inhibition and alcohol effects across multiple dose administrations. Briefly, ethanol and placebo were administered twice in alternating order. Sessions took place from 3:00 pm to 8:00 pm and were separated by 2–7 days. Participants were tested individually. They were instructed not to eat for 4 h prior to each session. To minimize drug expectancies, participants were told they could receive one of the following: stimulant, sedative, alcohol, or placebo. The ethanol dose was 0.8 g/kg for men and 0.7 g/kg for women to achieve equivalent BrACs across sex [36, 37]. This dose was chosen to produce peak BrACs of 80 mg/100 ml. This BrAC models a binge level of intoxication and has been shown to produce reliable increases in stimulation and sedation [38]. Each dose was split into four equal servings and consumed orally. Ethanol beverages were served in a 10% solution by volume with the subject’s preferred fruit juice flavor. The placebo beverage consisted of the fruit juice plus 3 ml ethanol added as a taste mask. All beverages were sprayed with an alcoholic mist to provide a strong alcoholic scent. Beverages were served in opaque cups, and participants were given a total of 15 min to consume the four servings. They completed the BAES immediately before and every 30 min after dose administration for 2.5 h. Between assessments, participants were allowed to read or watch movies, but they were not allowed to work, access the internet, use their phones, or sleep. Sessions ended at 8 pm, after confirmation that BrAC had fallen below 40 mg/100 ml.
Imaging session. On a separate session conducted at least two days after the last beverage administration session, participants completed the fMRI session in the drug-free state. They were first tested for recent drug and alcohol use. Subjects completed a practice block of the stop signal task outside of the scanner and then performed the stop signal task during fMRI.
Imaging acquisition and processing. Participants were imaged using a 3 T GE scanner with an 8-channel head coil array at the UIC Center for Magnetic Resonance Research. Whole-brain functional imaging was performed with a standard T2*-sensitive echo planar imaging sequence (gradient-echo; repetition time, 2000 ms; echo time, 22.2 ms; 64 × 64 matrix; 220 × 220 mm field of view; flip angle, 90°; 3 mm slice thickness with no gap, 44 axial slices). T1-weighted high-resolution anatomical images (BRAVO sequence with 1 × 1 × 1 mm3 voxels; repetition time, 9.3 ms; echo time, 3.8 ms; 220 × 220 mm field of view; flip angle, 13°) were acquired for co-registration and normalization to the MNI coordinate system.
Functional data for each scan were preprocessed using FSL (FMRIB’s Software Library, Oxford, UK), which included motion correction with mcflirt [39], non-brain removal with bet [40], spatial smoothing with a 6.0 mm FWHM Gaussian kernel, and mean intensity normalization of the volume at each timepoint. Linear registration to high resolution structural and standard space images was carried out using flirt [39, 41] and then followed up by fnirt nonlinear registration [42]. Single-session independent component analysis (ICA) with automatic dimensionality estimation using probabilistic ICA [43] available in FSL’s MELODIC version 3.15 generated filtered data for denoising with ICA-AROMA [44]. The denoised functional data were then projected in the common space, interpolated to 2 mm isotropic voxels, and used in subsequent statistical analyses in SPM12 (Wellcome Trust Center for Neuroimaging, London, UK). Within SPM12, the general linear model was applied to the time series of each voxel, convolved with the canonical hemodynamic response function and included a 128 s high-pass filter. Condition effects were modeled by specifying event-related onsets (and zero duration) for: correct go (Go) and stop (StopInh) trials, and incorrect go and stop (StopFail) trials. Effects were estimated at each voxel and for each subject, and individual participant contrast maps for StopInh>Go were created [33]. Six head motion parameters from the SPM realignment were included as regressors.
Data analyses
Stop signal task. We conducted a second-level, random effects one sample t-test for StopInh>Go. We focused on the StopInh>Go contrast, as this most directly indexes inhibitory control according to the stop signal race model of inhibition [24]. Specifically, the race model states that inhibition is a “race” between response inhibition and response execution processes, and successful inhibition occurs when the inhibition process is faster than the go process [45]. The StopInh>Go contrast directly compares brain activity during inhibition and execution, and thus is thought to model this race. In line with this, greater brain activity as indexed by the StopInh>Go contrast is significantly correlated with shorter SSRT (i.e., better response inhibition) [24]. As our hypotheses were specific to frontal regions, statistical inferences were made based on peak voxel significance corrected for family-wise error (p FWE < 0.05) within a frontal-insular-subcortical (FIS) mask previously used for analyses with this task [21, 32]. This 382,584 mm3 (47,823 voxels) mask included the following structural regions from AAL library [46] available in MarsBar: medial and lateral frontal and orbital regions, bilateral precentral gyri, anterior and middle cingulate cortex, anterior insula, and subcortical motor regions consisting of bilateral putamen, pallidum, and caudate. Analyses were restricted to this mask as it encompasses brain regions that have been previously implicated in drug reward and/or stop signal task performance [27, 47, 48].
Associations between brain activity during response inhibition and subjective response to alcohol. We extracted parameter estimates/β-weights representing BOLD response activation in arbitrary units averaged across all voxels within a 10-mm radius sphere surrounding peak areas of activation (p FWE < 0.05) for the stop signal task. We focused our analyses on significant peaks within well-established inhibitory control regions, including right SMA, IFG, anterior insula, and middle frontal gyrus (MFG). We then entered these parameter estimates as fixed effects in linear mixed effects models for repeated measures [49] in SPSS 26 to examine the degree to which brain activity during response inhibition interacted with beverage (ethanol vs placebo) and time to predict measures of stimulation and sedation. Session was also included in the model to determine whether alcohol effects on stimulation and sedation differed across the two alcohol-placebo sessions, as well as whether interactions between brain activity and alcohol effects on subjective response differed across sessions. The models included random intercept, beverage, and time effects to allow for individual differences in alcohol response and time trends, and to account for correlation between repeated measurements. Beverage order (ethanol or placebo administered first), sex, and drinks per week were included as model covariates. The effects of interest were the three-way interactions among brain activity, beverage (ethanol vs. placebo), and time.
Results
Sample characteristics. Of the 76 participants who completed this study, behavioral data were lost for 5 participants due to computer error, one participant ended the fMRI session early due to discomfort in the scanner, and 10 participants had stop signal behavioral data that did not meet the validity criteria. Table 1 presents demographic and alcohol use data for the final sample of n = 60.
Breath alcohol concentration (BrAC). Mean peak BrAC for the sample was 79 (SD = 12.0) mg/100 ml at 90 min. Men and women did not differ in peak BrAC (p = 0.34).
Brain activation during response inhibition. Behavioral responses on the Stop Task were as follows: mean SSRT = 325.8 ms (80.7), mean inhibition rate = 54.7% (sd = 0.05), mean go accuracy = 94.6% (sd = 6%), and mean go RT = 494.4 ms (117.3). Neural responses during the task were as follows: The StopInh>Go contrast showed brain activity in predominately right-lateralized inhibitory control regions previously reported using this task, including the IFG, insula, SMA, MFG, and precentral gyrus [21, 24, 33] (Fig. 1; Supplementary Table 1).
Significant (pFWE < 0.05) peak activation was observed in right prefrontal regions including the inferior frontal gyrus, insula, middle frontal gyrus, supplementary motor area (right panel sagittal view), precentral gyrus, and superior frontal gyrus. Smaller middle and inferior frontal gyri and insular activations were seen in the left hemisphere (Supplementary Table S1).
Associations between brain activation during response inhibition and feeling stimulated after alcohol. We focused our analyses on significant peaks (p FWE < 0.05) in regions that are within fronto-striatal pathways and well-documented to be involved in inhibitory control. Specifically, we extracted parameter estimates (β weights) from 10 mm radius spheres surrounding significant peaks in the SMA [14 16 66] and [4 24 52], IFG [50 16 30], dorsal anterior insula [34 24 6], and MFG [28 48 24] and entered these values into linear mixed effects models testing the degree to which brain activity during inhibition interacted with alcohol and time to predict subjective reports of stimulation. Table 2 shows that, as previously reported in this sample [14, 50], alcohol significantly increased ratings of stimulation relative to placebo (significant time × beverage interactions; ps < 0.01). Moreover, significant brain activity × time × beverage interactions were observed for the SMA [4 24 52], insula [34 24 6], and MFG [28 48 24], such that less [StopInh > Go] activity in these regions was associated with greater alcohol-induced stimulation. Figure 2 displays these interactions for the SMA and insula. The figure plots the linear mixed effects models’ estimates of the effect of alcohol relative to placebo on stimulation at each time point across the session for high (1 sd above the mean) and low (1sd below the mean) levels of SMA and insula activity, and shows that less brain activity during motor inhibition is associated with a larger increase in stimulation following alcohol compared to placebo. For ease of interpretation, we also present raw Stimulation scores for high and low brain activity in the sample as divided by median split (Fig. S1).
Significant (pFWE < 0.05) peak activation in the supplementary motor area (SMA) and anterior insula during successful response inhibition (StopInh > Go). b, c Estimates of alcohol effects (relative to placebo) on BAES Stimulation derived from linear mixed effects models testing associations between brain activation during response inhibition [i.e., extracted BOLD signal from a 10 mm radius spherical region centered at the [4 24 52] peak in the SMA (2b) and the [34 24 6] peak in the insula (2c)] and subjective response to alcohol. Solid circles represent estimates of alcohol effects at high levels of activation (1 SD above the mean) and open circles represent estimates of alcohol effects at low levels of activation (1 SD below the mean). As hypothesized, less frontal activation during response inhibition was associated with greater magnitude of alcohol effect on ratings of stimulation. Capped vertical lines indicate standard error of the mean.
Associations between brain activity during response inhibition and feelings of sedation after alcohol. We performed the same linear mixed effects models described above to test the degree to which brain activity in the same regions interacted with alcohol and time to predict alcohol-induced sedation (Table 3). As previously reported [14, 50], alcohol increased sedation relative to placebo (significant time × beverage interaction; p = 0.01). The only brain region where activity during inhibition was significantly associated with sedation ratings was the IFG (p = 0.01). However, this interaction appears to be due to associations between IFG activity and placebo responses, as opposed to alcohol responses. Specifically, as beverage was coded with placebo as the reference condition, the IFG × time interaction tested the degree to which IFG activity predicted sedation over time in the placebo (reference) condition only. The significant interaction (p < 0.01) indicates that activity in the IFG was related to greater sedation across the session following placebo. We recoded the beverage variable using alcohol as the reference condition to test the degree to which IFG predicted sedation ratings across time following alcohol. Here, the interaction was not significant (p = 0.37), indicating that activity in the IFG was not associated with sedation following alcohol.
Reliability of subjective responses across sessions. The linear mixed effects models described above included interactions between session and beverage, time, and brain activity to determine the degree to which effects differed across alcohol-placebo session pairs. No significant interactions were observed involving session, time, and beverage, indicating that alcohol effects on stimulation and sedation were consistent across alcohol-placebo session pairs. Furthermore, no four-way interactions were observed between session, beverage, time, and brain activity during inhibition, indicating that the relationships between brain activity and alcohol effects did not differ across session pairs. Finally, we calculated alcohol effect scores for each session pair by subtracting the area under the curve (AUC) following placebo from the AUC following alcohol and analyzed correlations between the first and second session pairs. Both stimulation (r = 0.31, p = 0.02) and sedation (r = 0.35, p < 0.01) showed good within-person reliability across sessions.
Association between neural and behavioral measures of inhibitory control. We analyzed correlations between the extracted β weights and SSRT obtained during task performance. No significant correlations were observed (ps > 0.22).
Associations between SSRT and feelings of stimulation and sedation after alcohol. Table S2 and Figure S2 present the results from the linear mixed effects models testing the degree to which SSRT interacted with alcohol and time to predict stimulation and sedation. Results showed a significant SSRT × time × beverage interaction for stimulation (p = 0.01) and a trend for sedation (p = 0.07), such that longer SSRT (i.e., poorer behavioral inhibitory control) was associated with greater stimulation and lesser sedation following alcohol relative to placebo.
Discussion
In this study we examined associations between neural correlates of inhibitory control and feelings of stimulation (and as a comparison, sedation) following alcohol in a sample of young adult moderate drinkers. As hypothesized, less right frontal activity during inhibition was associated with greater alcohol-induced stimulation. Specifically, individuals who displayed less activity in the right SMA and MFG, as well as dorsal anterior insula, reported greater stimulation following alcohol compared to placebo. By contrast, no brain regions showed a significant association with feelings of sedation. In addition, poor inhibitory control as indexed by the behavioral measure of SSRT was associated with greater stimulation and less sedation following alcohol. These results replicate previous findings linking behavioral and self-report measures of inhibitory control to stimulation following alcohol [14,15,16,17], and provide the first evidence of a link between neural correlates of inhibition and positive stimulant responses to alcohol.
The association between activity in the right SMA and subjective reports of stimulation could be due in part to fronto-striatal connectivity between the SMA and the striatum. Functional connectivity between these two regions is implicated in response inhibition, as the SMA is thought to exert top-down regulation of the striatum to inhibit motor output signals [23, 26]. Interestingly, this pathway is also involved in processing of rewarding stimuli, including both monetary and social reward [51]. Here, the SMA regulates reward responses via inhibitory projections to the ventral striatum [52]. As such, it is possible that individuals who display less top-down regulation of striatal motor responses by the SMA during inhibition may also display less regulation of striatal responses to reward, including the positive, stimulant-like effects of alcohol. If so, the observed association between SMA activity and stimulation may be due to less top-down SMA regulation of striatally-mediated stimulant responses to alcohol (i.e., less inhibition of striatal activity following acute alcohol consumption).
The association between insula activity during inhibition and alcohol-induced stimulation is in line with theories suggesting the critical role of this brain structure in addiction [53, 54] and is likely due to the role of the insula in the larger salience network. As part of this network, the insula is responsive to motivationally and emotionally significant stimuli or events. In regard to inhibitory control, the dorsal anterior insula responds to salient cues indicating that behavioral inhibition is required [55,56,57]. In regard to subjective drug responses, the ventral anterior insula responds to salient and motivationally significant interoceptive drug effects [56]. Indeed, the insula plays a key role in interoception and awareness of hedonic conditions [58,59,60] and the role of the insula in addiction is thought to involve the conscious experience of pleasure derived from interoceptive drug effects [61]. In line with this, alcohol increases glucose metabolism in the insula [62], and we have shown that alcohol-induced increase in insula activity correlates with ratings of stimulation [31]. Similarly, Smith et al. [63] showed that amphetamine-induced DA release in the insula correlates with wanting more amphetamine. Finally, preclinical studies show that a history of alcohol dependence in rats is associated with weaker connectivity between the insula and key constituents of the salience network [64], and excitatory insula stimulation (via designer receptors, i.e., DREADDs) reduces alcohol consumption in alcohol-preferring rats [65]. Taken together, the current findings add to a growing body of literature linking insula activity to positive subjective responses to alcohol and other drugs, and they suggest that dorsal anterior insula responses to external inhibitory signals may be inversely related to ventral anterior insula responses to interoceptive drug effects.
Our finding that less activity in the MFG during inhibition was associated with greater stimulation following alcohol replicates and extends our previous finding relating activity in this region to greater subjective euphoria following amphetamine [21]. Interestingly, the MFG peaks from the two studies were essentially overlapping (MFG peak associated with alcohol response: [28 48 24] and MFG peak associated with amphetamine response: [32 48 26]). Like the SMA, the MFG is thought to exert top-down executive control over subcortical regions, including the striatum, during inhibition [23], and it is possible that such top-down control extends to regulation of striatally-mediated subjective responses to drugs. In line with this, less cortical thickness in the MFG has been linked to greater amphetamine-induced dopamine release in the striatum [66]. It will be important for future studies to directly test the degree to which activity in the MFG influences alcohol or stimulant-induced striatal activity and associated subjective responses.
These findings raise the exciting possibility that enhancing activity in right frontal inhibitory regions might also serve to dampen positive subjective responses to alcohol. It will be important for future studies to directly test this possibility by manipulating neural inhibitory function and assessing the effects on subjective alcohol responses. Two potential means through which neural inhibitory circuitry can be manipulated are transcranial magnetic stimulation (TMS) and pharmacotherapy. Previous work has shown that TMS over the SMA effectively increases neural inhibitory function and corresponding behavioral measures of inhibitory control [67, 68]. Similarly, modafinil has been shown to enhance inhibitory control via improved SMA activity in individuals with AUD [69]. If this increase in neural inhibitory function does in fact exert downstream effects on drug-induced striatal (or insular) activity and corresponding subjective responses to alcohol, such findings would identify potential targets for novel brain-based prevention and treatment efforts for AUD.
Interestingly, we did not detect an association between neural activity during inhibition and sedative-like responses to alcohol. First, this indicates that the correlation is specific to stimulant effects and not general to magnitude of overall subjective response to alcohol. However, it is not consistent with our current and previous [14] finding that poor behavioral inhibition on the stop task is related to less sensitivity to the sedative effects of alcohol. Why an association was observed with behavioral performance of the stop task [14] and not with brain activity during the stop task is unclear. It is possible that sedative effects of alcohol are not localized to specific brain regions or networks of regions, but rather may reflect a general decrease of activity throughout the brain. This finding is consistent with a previous study [31], where we also did not find correlations between ratings of sedation and alcohol effects on regional brain activity.
Our four-session design allowed us to assess the reliability of the observed associations over two pairs of alcohol-placebo administration sessions. Consistent with our prior reports showing strong reliability of subjective responses to both alcohol and amphetamine [70], alcohol effects on stimulation and sedation were consistent across sessions and within-subject consistency was high. Moreover, the relationships between brain activity and alcohol effects on stimulation were consistent across both session pairs, providing good support for the reliability of the association between neural correlates of inhibition and greater sensitivity to the stimulant effects of alcohol.
This study had some limitations. First, we did not assess acute effects of alcohol on neural function underlying subjective responses. We chose to focus on subjective reports of stimulation and sedation in order to first establish the association between neural correlates of inhibition and participants’ subjective responses. This design choice was also intended to be consistent with our previous studies linking behavioral and neural correlates of inhibition to participants’ subjective reports following alcohol and amphetamine [14, 20, 21]. We plan to follow this study with a direct test of the relationship between activity in right frontal regions during inhibition and activity in striatal and insular regions in response to alcohol. Second, we did not observe a significant association between behavioral (SSRT) and neural correlates of inhibition. This is consistent with previous reports from our lab and others [21, 24], and is likely due to the need for very large samples and sufficient power for voxel-wise analyses to observe associations between SSRT and brain activity [24]. Third, we only tested one dose of alcohol. It will be important to assess the degree to which associations between neural correlates of inhibition and subjective responses to alcohol vary according to dose. Finally, we did not assess acute alcohol effects on brain activity during inhibition. Alcohol decreases brain activity in right frontal regions during inhibition [33, 71]. As such, individual differences in neural inhibitory function following alcohol may be an even stronger predictor of subjective responses than neural inhibitory function while sober.
In sum, the current study is the first to demonstrate a relationship between right frontal brain activity during inhibition and stimulation following an acute dose of alcohol. The findings have important implications for understanding risk for AUD and they add to a growing body of literature relating neurobehavioral measures of inhibition to drug reward. Future studies should extend these findings to directly test the degree to which improving neural inhibitory function dampens subjective stimulation following alcohol. This line of research will provide important information for understanding and treating AUD in individuals with poor inhibitory control.
Funding and disclosures
This research was supported by National Institute on Drug Abuse Grants R01 DA002812 (HdW, KLP) and R21 DA037642 (HdW). JW was supported by National Institute on Alcohol Abuse and Alcoholism grant K01 AA024519. SMG was supported by National Institute on Alcohol Abuse and Alcoholism grant K01 AA025111. DAK and MD were supported by National Institute on Alcohol Abuse and Alcoholism grant P60 AA007611.
References
Heitzeg MM, Cope LM, Martz ME, Hardee JE. Neuroimaging risk markers for substance abuse: recent findings on inhibitory control and reward system functioning. Curr Addict Rep. 2015;2:91–103.
Rubio G, Jiménez M, Rodríguez‐Jiménez R, Martínez I, Ávila C, Ferre F, et al. The role of behavioral impulsivity in the development of alcohol dependence: a 4‐year follow‐up study. Alcohol Clin Exp Res. 2008;32:1681–7.
Fernie G, Peeters M, Gullo MJ, Christiansen P, Cole JC, Sumnall H, et al. Multiple behavioural impulsivity tasks predict prospective alcohol involvement in adolescents. Addiction. 2013;108:1916–23.
Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–9.
King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–99.
King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry. 2014;75:798–806.
King AC, Hasin D, O’Connor SJ, McNamara PJ, Cao D. A prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol Psychiatry. 2016;79:489–98.
King AC, Vena A, Hasin D, de Wit H, O’Connor SJ, Cao D Subjective responses to alcohol in the development and maintenance of AUD. Am J Psychiatry. In press.
Chutuape MA, De Wit H. Relationship between subjective effects and drug preferences: ethanol and diazepam. Drug Alcohol Depend. 1994;34:243–51.
Beckwith SW, Czachowski CL. Alcohol‐preferring P rats exhibit elevated motor impulsivity concomitant with operant responding and self‐administration of alcohol. Alcohol Clin Exp Res. 2016;40:1100–10.
Bowers BJ, Wehner JM. Ethanol consumption and behavioral impulsivity are increased in protein kinase Cγ null mutant mice. J Neurosci. 2001;21:RC180.
Logue SF, Swartz RJ, Wehner JM. Genetic correlation between performance on an appetitive‐signaled nosepoke task and voluntary ethanol consumption. Alcohol Clin Exp Res. 1998;22:1912–20.
Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH. Mouse lines selected for alcohol consumption differ on certain measures of impulsivity. Alcohol Clin Exp Res. 2007;31:1839–45.
Weafer J, Phan KL, De Wit H. Poor inhibitory control is associated with greater stimulation and less sedation following alcohol. Psychopharmacology. 2020;237:825–32.
Berey BL, Leeman RF, Pittman B, O’Malley SS. Relationships of impulsivity and subjective response to alcohol use and related problems. J Stud Alcohol Drugs. 2017;78:835–43.
Berey BL, Leeman RF, Chavarria J, King AC. Relationships between generalized impulsivity and subjective stimulant and sedative responses following alcohol administration. Psychol Addict Behav. 2019;33:616.
Leeman RF, Ralevski E, Limoncelli D, Pittman B, O’Malley SS, Petrakis IL. Relationships between impulsivity and subjective response in an IV ethanol paradigm. Psychopharmacology. 2014;231:2867–76.
Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–31.
Brunelle C, Assaad JM, Barrett SP, Ávila C, Conrod PJ, Tremblay RE, et al. Heightened heart rate response to alcohol intoxication is associated with a reward‐seeking personality profile. Alcohol: Clin Exp Res. 2004;28:394–401.
Weafer J, de Wit H. Inattention, impulsive action, and subjective response to d-amphetamine. Drug Alcohol Depend. 2013;133:127–33.
Weafer J, Gorka SM, Hedeker D, Dzemidzic M, Kareken DA, Phan KL, et al. Associations between behavioral and neural correlates of inhibitory control and amphetamine reward sensitivity. Neuropsychopharmacology 2017;42:1905–13.
Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7.
Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79.
Congdon E, Mumford JA, Cohen JR, Galvan A, Aron AR, Xue G, et al. Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage. 2010;53:653–63.
Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain—conjunction analyses of the stop-signal task. Neuroimage. 2010;52:1621–32.
Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–9.
Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, et al. Striatal dopamine D2/D3 receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci. 2012;32:7316–24.
Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37:467–77.
Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–91.
Völlm BA, De Araujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, et al. Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology. 2004;29:1715–22.
Weafer J, Ross TJ, O’Connor S, Stein EA, de Wit H, Childs E. Striatal activity correlates with stimulant-like effects of alcohol in healthy volunteers. Neuropsychopharmacology. 2018;43:2532–8.
Weafer J, Crane NA, Gorka SM, Phan KL, de Wit H. Neural correlates of inhibition and reward are negatively associated. Neuroimage. 2019;196:188–94.
Kareken DA, Dzemidzic M, Wetherill L, Eiler W, Oberlin BG, Harezlak J, et al. Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology. 2013;228:335–45.
Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–8.
Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res. 1993;17:140–6.
Fillmore MT. Cognitive preoccupation with alcohol and binge drinking in college students: alcohol-induced priming of the motivation to drink. Psychol Addict Behav. 2001;15:325.
Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol. 1997;58:600–5.
Weafer J, Gallo DA, de Wit H. Effect of alcohol on encoding and consolidation of memory for alcohol‐related images. Alcohol Clin Exp Res. 2016;40:1540–7.
Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41.
Smith SM. Fast robust automated brain extraction. Hum brain Mapp. 2002;17:143–55.
Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med image Anal. 2001;5:143–56.
Andersson JL, Jenkinson M, Smith S Non-linear registration, aka spatial normalisation. FMRIB Technical Report TR07JA2. FMRIB Analysis Group of the University of Oxford. 2007.
Beckman I, Richard D Rutgers University-Mason Gross School of the Arts. 2014
Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–77.
Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Sci. 1997;8:60–64.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, et al. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–32.
Courtney KE, Ghahremani DG, Ray LA. Fronto‐striatal functional connectivity during response inhibition in alcohol dependence. Addict Biol. 2013;18:593–604.
Hedeker D, Gibbons RD Longitudinal data analysis. John Wiley & Sons, Hoboken, NJ; 2006.
Radoman M, Crane NA, Gorka SM, Weafer J, Langenecker SA, de Wit H, et al. Striatal activation to monetary reward is associated with alcohol reward sensitivity. Neuropsychopharmacology. 2021;46:343–50.
Gu R, Huang W, Camilleri J, Xu P, Wei P, Eickhoff SB, et al. Love is analogous to money in human brain: coordinate-based and functional connectivity meta-analyses of social and monetary reward anticipation. Neurosci Biobehav Rev. 2019;100:108–28.
Plichta MM, Wolf I, Hohmann S, Baumeister S, Boecker R, Schwarz AJ, et al. Simultaneous EEG and fMRI reveals a causally connected subcortical-cortical network during reward anticipation. J Neurosci. 2013;33:14526–33.
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiat. 2016;3:760–73.
Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76:342–50.
Peters SK, Dunlop K, Downar J. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front Syst Neurosci. 2016;27:104. 10
Droutman V, Read SJ, Bechara A. Revisiting the role of the insula in addiction. Trends Cogn Sci. 2015;19:414–20.
Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–49.
Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann NY Acad Sci. 2011;1225:72–82.
Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70.
Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95.
Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–50.
Zhu W, Volkow ND, Ma Y, Fowler JS, Wang GJ. Relationship between ethanol-induced changes in brain regional metabolism and its motor, behavioural and cognitive effects. Alcohol Alcohol. 2004;39:53–58.
Smith CT, Dang LC, Cowan RL, Kessler RM, Zald DH. Variability in paralimbic dopamine signaling correlates with subjective responses to d-amphetamine. Neuropharmacology. 2016;108:394–402.
Scuppa G, Tambalo S, Pfarr S, Sommer WH, Bifone A. Aberrant insular cortex connectivity in abstinent alcohol‐dependent rats is reversed by dopamine D3 receptor blockade. Addiction Biol. 2020;25:e12744.
Haaranen M, Scuppa G, Tambalo S, Järvi V, Bertozzi SM, Armirotti A, et al. Anterior insula stimulation suppresses appetitive behavior while inducing forebrain activation in alcohol-preferring rats. Transl Psychiatry. 2020;10:1–11.
Casey KF, Cherkasova MV, Larcher K, Evans AC, Baker GB, Dagher A, et al. Individual differences in frontal cortical thickness correlate with the d-amphetamine-induced striatal dopamine response in humans. J Neurosci. 2013;33:15285–94.
Watanabe T, Hanajima R, Shirota Y, Tsutsumi R, Shimizu T, Hayashi T, et al. Effects of rTMS of pre-supplementary motor area on fronto basal ganglia network activity during stop-signal task. J Neurosci. 2015;35:4813–23.
Xu B, Sandrini M, Wang WT, Smith JF, Sarlls JE, Awosika O, et al. PreSMA stimulation changes task‐free functional connectivity in the fronto‐basal‐ganglia that correlates with response inhibition efficiency. Hum Brain Mapp. 2016;37:3236–49.
Schmaal L, Joos L, Koeleman M, Veltman DJ, van den Brink W, Goudriaan AE. Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients. Biol Psychiatry. 2013;73:211–8.
Murray CH, Weafer J, de Wit H. Stability of acute responses to drugs in humans across repeated testing: Findings with alcohol and amphetamine. Drug Alcohol Depend. 2020;212:107989.
Gan G, Guevara A, Marxen M, Neumann M, Jünger E, Kobiella A, et al. Alcohol-induced impairment of inhibitory control is linked to attenuated brain responses in right fronto-temporal cortex. Biol Psychiatry. 2014;76:698–707.
Author information
Authors and Affiliations
Contributions
JW, HdW, and KLP designed the study. JW analyzed the data and wrote the first draft of the manuscript. All authors revised the manuscript critically for intellectual content and have approved the final version.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Weafer, J., Gorka, S.M., Dzemidzic, M. et al. Neural correlates of inhibitory control are associated with stimulant-like effects of alcohol. Neuropsychopharmacol. 46, 1442–1450 (2021). https://doi.org/10.1038/s41386-021-01014-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01014-5
This article is cited by
-
Change in brain asymmetry reflects level of acute alcohol intoxication and impacts on inhibitory control
Scientific Reports (2023)
-
Interoception Within the Context of Impulsivity and Addiction
Current Addiction Reports (2023)
-
Patterns of functional connectivity alterations induced by alcohol reflect somatostatin interneuron expression in the human cerebral cortex
Scientific Reports (2022)