Abstract
Affective disorders (AD, including bipolar disorder, BD, and major depressive disorder) are severe recurrent illnesses. Identifying neural markers of processes underlying AD development in at-risk youth can provide objective, “early-warning” signs that may predate onset or worsening of symptoms. Using data (n = 34) from the Bipolar Offspring Study, we examined relationships between neural response in regions supporting executive function, and those supporting self-monitoring, during an emotional n-back task (focusing on the 2-back face distractor versus the 0-back no-face control conditions) and future depressive and hypo/manic symptoms across two groups of youth at familial risk for AD: Offspring of parents with BD (n = 15, age = 14.15) and offspring of parents with non-BD psychopathology (n = 19, age = 13.62). Participants were scanned and assessed twice, approximately 4 years apart. Across groups, less deactivation in the mid-cingulate cortex during emotional regulation (Rate Ratio = 3.07(95% CI:1.09–8.66), χ2(1) = 4.48, p = 0.03) at Time-1, and increases in functional connectivity from Time-1 to 2 (Rate Ratio = 1.45(95% CI:1.15–1.84), χ2(1) = 8.69, p = 0.003) between regions that showed deactivation during emotional regulation and the right caudate, predicted higher depression severity at Time-2. Both effects were robust to sensitivity analyses controlling for clinical characteristics. Decreases in deactivation between Times 1 and 2 in the right putamen tail were associated with increases in hypo/mania at Time-2, but this effect was not robust to sensitivity analyses. Our findings reflect neural mechanisms of risk for worsening affective symptoms, particularly depression, in youth across a range of familial risk for affective disorders. They may serve as potential objective, early-warning signs of AD in youth.
Similar content being viewed by others
Introduction
Affective disorders (AD; including bipolar disorder, BD, and major depressive disorder, MDD) are severe recurrent illnesses associated with high risk for suicide [1] and poor clinical and functional outcomes [2, 3]. AD develop in youth through early adulthood [4], with peak onset of BD typically between adolescence and the early 20 s [5,6,7,8]; and peak onset of MDD in the mid-20s [4]. Twin, adoption, and high-risk family studies show that BD in particular runs in families [9,10,11,12]. Specifically, the prevalence of BD in first-degree relatives of adults with BD is 8–10 times that of community samples [9, 13]. Offspring of parents with BD are also more likely to develop non-BD affective disorders [10, 14]. Furthermore, worsening depressive or hypo/manic symptoms often predate the emergence of BD [9, 10, 14]. Monitoring the development of affective symptoms and AD progression in offspring of parents with BD can help to identify those offspring at risk for future/worsening AD, and, ultimately, those at future risk of BD.
The Bipolar Offspring Study (BIOS) is the largest study examining risk and progression of AD in offspring of parents with BD [9]. BIOS and other studies have shown that rates of AD are ~32% [14] and BD ~15–20% [10, 15,16,17] in offspring of parents with BD (OBP). Notably, BIOS also recruited offspring of control parents with non-BD psychopathology [OCP; 10, 14]. These offspring are at higher risk of AD than offspring of healthy parents [18,19,20,21], although at lower risk of BD than OBP [10, 22]. This allowed BIOS to include offspring recruited across a range of familial risk for future AD. Although familial AD and presence of affective symptoms can help predict risk for future AD development [10, 23, 24], these factors do not inform understanding of the neurobiological processes underlying this risk. Identifying neural markers of these processes can provide “early-warning” signs that may predate the observable symptoms associated with the development or worsening of AD and can provide objective markers to monitor AD progression and treatment outcomes.
Given that emotional dysregulation is a key clinical characteristic of AD [25], identifying patterns of emotional regulation circuitry function that are associated with future affective symptom worsening in at-risk individuals is needed. This neural circuitry includes the amygdala and striatum, important for processing and responding to salient cues, lateral prefrontal cortical regions, e.g., dorsolateral and ventrolateral prefrontal cortex (dlPFC, vlPFC) and medial regions, i.e., rostral and dorsal anterior cingulate cortex (rACC, dACC) implicated, respectively, in effortful and implicit emotional regulation sub-processes [26]. In BIOS, we previously reported that OBP showed greater rACC activity and greater amygdala-dACC functional connectivity (FC) vs. OCP during emotional regulation, and that increase in amygdala-dACC FC during emotional regulation over two scans approximately three years apart in all groups was associated with concurrent increases in affective lability [27], suggesting ineffectual emotion regulation strategies in OBP in particular. These findings parallel reports in healthy OBP, and unaffected relatives, of elevated amygdala and altered dlPFC activity during emotion processing [28,29,30] and altered amygdala-vlPFC FC during emotion regulation [31,32,33]. Results also parallel reports in youth and adults with BD of altered amygdala activity to emotional stimuli [34,35,36], altered activity in prefrontal cortical regions supporting emotion regulation [26, 37,38,39,40,41,42,43], and altered vlPFC-amygdala FC [44,45,46,47,48,49,50,51,52,53]. Thus, abnormalities in emotion regulation neural circuitry in OBP might confer risk for the development or worsening of affective disorders, but confirmation is needed in larger, longitudinal studies.
The above studies examined how activity in key prefrontal cortical-amygdala emotion regulation circuitry predicts affective symptoms. Yet, an increasing literature has established consistent patterns of deactivation in regions which subserve self-monitoring processes [54, 55] during performance of executive function tasks, including tasks that are integral to emotion regulation attentional processes [56], e.g., working memory. Such deactivation is thought to reduce interference from self-monitoring processes on cognitive task performance [57,58,59,60]. Whereas not all studies indicate the necessity of deactivation in these regions for this kind of task performance [61], several studies indicate that, alongside increased activity in regions associated with executive functioning, deactivation in self-monitoring regions and inverse FC (anticorrelation) between regions associated with self-monitoring and regions supporting executive function during performance of such tasks are normative and critical for task performance in adults [56, 60] and youth [62, 63]. In parallel, studies in adults with AD indicate abnormally reduced deactivation in self-monitoring regions during executive function tasks [41, 64,65,66,67,68,69,70,71,72,73]. This pattern is also present in relatives of BD probands [74]. More normative deactivation in these regions and more normative anticorrelations between these regions and those supporting executive function were associated with better antidepressant treatment response in adults with MDD [75]. Additional studies reported abnormal increases in prefrontal cortical activity during executive function task performance in adults with affective disorders [41, 64,65,66,67,68,69,70,71,72,73], which might represent a compensatory response. The extent to which deactivation in self-monitoring regions during executive function tasks is related to future worsening of affective symptoms in OBP and OCP is, however, unknown.
Our goal was to examine activation and FC in neural regions supporting executive function, and self-monitoring, in OBP and OCP during performance of an emotional n-back task and the extent to which these neural measures predicted future affective symptom severity. Our specific hypotheses, based on the literature, were:
-
(1)
At baseline (Time-1), less deactivation in self-monitoring regions, greater activity in regions associated with executive function during emotion regulation, and greater positive FC between these two sets of regions during performance on an emotional n-back task, would predict greater future (Time-2) depression and hypo/mania severity in OBP and OCP.
-
(2)
Longitudinal (Time-1 to Time-2) decreases in deactivation in self-monitoring regions, increases in activation in regions associated with executive function during emotion regulation, and increases in positive FC between these two sets of regions during task performance, would be associated with greater future (Time-2) depression and hypo/mania symptom severity in OBP and OCP.
Patients and methods
Participants
BIOS participants had two levels of familial risk for AD: Offspring of a parent with BD, OBP, and offspring of a parent with a non-BD Axis-1 disorder, OCP. Participants with frank BD at study entry were excluded. A sample of n = 52 at Time-1 (OBP n = 27, OCP n = 25), was used to identify neural regions supporting executive function and self-monitoring. No sibling pairs were included. Primary analysis of change in affective symptoms was tested with the subsample of individuals (n = 34) who were scanned and assessed twice, (~4 years apart; OBP n = 15 and OCP n = 19). The University of Pittsburgh Human Research Protection Office approved this study. Parent/guardian consent and child assent were obtained.
Clinical assessments
Assessments included the Depression Rating Scale [KDRS; 76] and Mania Rating Scale [KMRS; 77], both derived from the K-SADS-PL [76, 77], to ascertain depressive or hypo/manic symptom severity over the prior two months; Mood and Feelings Questionnaire [MFQ; 78] to assess depressive symptoms; Child Affective Lability Scale [CALS; 79] to assess affective lability; Screen for Child Anxiety Related Emotional Disorders [SCARED; 80] to assess anxiety symptoms. The mean lag between assessment and scan-day for Time-1 = 10.26 weeks, Time-2 = 19.65 weeks, prior to the scan). Medication use at scan was assessed by parent report if the participant was under 18 years or self-report otherwise. See the supplement for additional details.
Neuroimaging parameters
Paradigm
The emotional face n-back (EFNBACK) task is a modified version of the n-back working memory task that has been used previously [81]. To examine neural circuitry underlying the ability to direct attention away from emotional faces during a 2-back working memory task, our analysis focused on the 2-back with face distracters (fearful, happy, neutral) condition (executive function during emotion regulation; EF+/ER+) in which greater activation is more likely to occur in prefrontal cortical-striatal regions versus the 0-back with no face distracters (low executive function, EF−) condition (see the supplement for additional details).
Neuroimaging data acquisition and processing
fMRI data for Time-1 were collected on a 3T Siemens Trio and for Time-2 on a Siemens Prisma scanner (see the supplement for details).
Activation/Deactivation
Models were constructed to examine EF+/ER+ > EF− and EF− > EF+/ER+ contrasts at Time-1 using a single anatomical mask previously used with this task [27, 82], created from the WFU PickAtlas [Wake Forest University, Winston-Salem; 83]. The mask included regions associated with executive function during emotional regulation, i.e., bilateral amygdala, caudate and putamen, dlPFC; Brodmann Areas (BA) 9 and 46, ventrolateral prefrontal cortex [vlPFC; BA 47; 26], and regions implicated in self-monitoring, i.e., anterior and mid-cingulate cortex (ACC; BA 24 and 32). A key advance of the current analyses over previous reports was the examination of neural activation and FC during both contrasts within this mask (cluster forming threshold = p < 0.001, uncorrected; extent threshold p < 0.05, FWE small-volume corrected). Time-1 and Time-2 data were extracted from the identified clusters and used to test our hypotheses. Whole brain analyses at Time-1 were performed in the larger group (n = 52) to determine the extent of activation during both contrasts.
Functional connectivity (FC)
Generalized psychophysiological interaction [gPPI; 84] examined FC between regions identified above. To constrain the number of inferential tests, we used a combined seed from clusters deactivated less to the EF− compared to the EF+/ER+ conditions, with targets defined by the individual clusters activated more during the EF+/ER+ than the EF− conditions.
Change in neuroimaging measures
To calculate change in neuroimaging measures from Time-1 to Time-2, given that data from each timepoint were collected on different scanners, extracted BOLD signal and gPPI parameter estimates at each timepoint were normalized separately for each scanner against values from samples of healthy individuals scanned during the same task in the respective scanners (Trio: n = 12; Prisma: n = 8, see Table S1b for characteristics of the healthy sample. No differences between scanners were observed in the identified regions, Table S11).
Statistical analyses
An a priori procedure was used to identify other potential clinical and demographic covariates. Each potential covariate (age, gender, IQ, MFQ, CALS, SCARED) was added separately to a model containing Time-1 symptoms, group membership, and time-interval between scans. Any variable associated with Time-2 KDRS or KMRS scores at p < 0.10 was retained.
Primary analyses focused on the prediction of KDRS and KMRS scores at Time-2, controlling for Time-1 symptoms, group membership, the time-interval between scans, and additional covariates identified above. For each symptom type (KMRS and KDRS), four negative binomial regression models were examined containing the following independent variables: (A) mean activation in each significant cluster in the anatomic mask at Time-1; (B) change in mean activation in each of these clusters from Time-1 to Time-2; (C) FC at Time-1 from all EF− > EF+/ER+ clusters to each EF+/ER+ > EF− cluster; and (D) change in FC from Time-1 to Time-2 from the former clusters to each of the latter clusters. Group membership was controlled in all models to ensure that identified neural markers would have incremental predictive validity over and above known, family-history based predictors. Project data are available upon request and were submitted to the National Data Archive (Collection ID:2336).
We used a positive False Discovery Rate (pFDR) correction for multiple comparisons to calculate FDR corrected q values (see the supplement) [85, 86].
Results
Demographic and clinical data for the primary sample (n = 34) are presented in Table 1 (Table S1a for the larger n = 52 sample). OBP and OCP only differed on Time-1 hypo/mania severity. Although mean symptoms were low, OBP had greater hypo/mania severity than OCP (χ2(1) = 6.01, p = 0.01). A small proportion (<16%) of children were receiving psychiatric medication at either scan visit, and task accuracy was high (>90%).
Task Effects
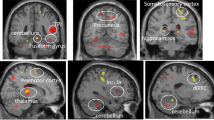
We observed three clusters with significantly greater activation to EF+/ER+ > EF− (executive function during emotion regulation regions): Left dlPFC (BA 9,46; k = 289, FWE p < 0.001, peak: t = 7.11, −42,5,31); Right dlPFC (BA 9, 46; k = 111, FWE p = 0.01, peak: t = 4.01, 42,35,19); and right caudate (k = 75, FWE p = 0.03, peak: t = 4.33, 12,11,−2). Furthermore, we observed three clusters associated with less deactivation to EF− than to EF+/ER+. A large region in medial prefrontal cortex extending posteriorly to the pregenual anterior cingulate cortex (BA 24, 32) and dorsally to medial BA 9 (k = 639, FWE p < 0.001, peak: t = 7.38, −9,50,−2); mid-cingulate cortex (BA 24, k = 204, FWE p = .001, peak: t = 6.27, 9,−19,43); and right putamen tail (k = 87, FWE p = 0.02, peak: t = 5.01, 36,−13,−2 (Fig. 1). The whole brain map of task effects revealed that the clusters above were generally part of larger clusters (Table S2).
Panel A displays regions with significant BOLD signal (cluster forming: p < 0.001 uncorrected, cluster extent: p < 0.05 FWE small-volume corrected to the anatomical mask) to EF+/ER+ vs EF− (2-back emotional-face minus 0-back no-face, in red) and EF− vs EF+/ER+ (0-back no-face minus 2-back emotional-face, in blue) contrasts. Analyses were conducted in an anatomical mask that contained: bilateral amygdala, caudate and putamen, dorsolateral prefrontal cortical regions (Brodmann Areas (BA) 9 and 46), anterior and mid cingulate cortex (BA 24 and 32), and ventrolateral prefrontal cortex (BA 47). Panel B displays extracted BOLD signal from each element of the contrasts. Blue bars represent signal to the EF− vs EF+/ER+ contrast and red bars represent signal to the EF+/ER+ vs EF− contrast. Darker bars represent the 2-back emotional face (EF+/ER+) condition and lighter bars represents the 0-back no face (EF−) condition. Region A = medial prefrontal cortex; B = mid-cingulate; C = right putamen tail; D = left dorsolateral prefrontal cortex; E = right caudate; F = right dorsolateral prefrontal cortex.
Covariate selection
No additional covariates were identified at p < 0.10 for the prediction of Time-2 KDRS or KMRS scores (Tables S3a and S3b). All models below control for relevant Time-1 symptoms (KDRS or KMRS), OBP/OCP group membership, and the time lag between scans.
Depressive symptoms
Hypothesis 1
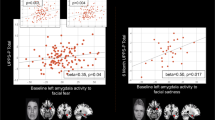
In the model examining whether baseline activation in the six clusters identified above predicted Time-2 KDRS scores, we observed a significant effect1 for deactivation in the mid-cingulate, such that less deactivation in this region to EF+/ER+ relative to EF− was associated with higher KDRS scores at Time-2 (Table 2; Fig. 2 displays the relationship in a reduced model in which the regions not showing a significant effect were removed, rate ratio (RR) = 3.07, 95% CI:1.09–8.66 χ2(1) = 4.48, p = 0.03). Follow-up analyses revealed that both components of the contrast contributed to this effect. Less deactivation in this region during EF+/ER+ condition (RR = 2.31, 95%CI:1.09–4.94, χ2(1) = 4.85, p = 0.03) and greater deactivation to EF− condition (RR = 0.34, 95% CI = 0.13–0.91, χ2(1) = 4.88, p = 0.03) were independently associated with greater Time-2 KDRS scores.
Panels A and B display the prediction of KDRS scores at Time-2 from BOLD response in the mid-cingulate at Time-1 (Panel A = model estimates; Panel B = raw scores). Panel C and D display the prediction of Time-2 KDRS scores from change in generalized psychophysiological interaction (gPPI) measured functional connectivity between the three EF− regions and the right caudate (Panel C = model estimates; Panel D = raw scores). Bands represent 95% confidence intervals.
We observed no significant relationships for Time-1 gPPI measures (all χ2(1) < 2.80; Table S4).
Hypothesis 2
In the model examining changes between Time-1 and -2 in FC among the clusters, we observed a significant effectFootnote 1 of changing FC between the combined EF− > EF+/ER+ cluster mask and the right caudate (an EF+/ER+ > EF− region) in predicting Time-2 KDRS scores (Table 3; Fig. 2 displays the relationship in a reduced model in which the regions not showing a significant effect have been removed, RR = 1.48, 95% CI:1.15–1.91, χ2(1) = 8.32, p = 0.004). Here, increases in FC to EF+/ER+ vs. EF− conditions between Time-1 and Time-2 were associated with greater KDRS scores at Time-2. Follow-up analyses revealed that the overall effect was largely driven by reductions between Time-1 and Time-2 in FC to the EF− condition (RR = 0.69 95% CI 0.53–0.90, χ2(1) = 7.08, p = 0.008).
We observed no significant relationships for Time-1-Time-2 change in BOLD response (all χ2(1) < 1.01); Table S5).
Sensitivity analyses
Controlling for diagnoses, both effects reported above remained significant (mid-cingulate: RR = 2.30, 95% CI: 1.18–4.48, χ2(1) = 5.98, p = 0.01; FC change: RR = 1.57, 95% CI: 1.21–2.04, χ2(1) = 9.92, p = 0.002); likewise when psychiatric medications were controlled (mid-cingulate: RR = 2.07, 95% CI: 1.20–3.57, χ2(1) = 6.70, p = 0.01; FC change: RR = 1.46, 95% CI: 1.13–1.88, χ2(1) = 7.44, p = 0.006).
Moderation by group
Group moderated the effect of mid-cingulate deactivation on KDRS scores (χ2(1) = 4.72, p = 0.03), such that the effect was stronger for OCP (RR = 4.79, 95% CI: 1.63–13.64) than for OBP (RR = 1.28, 95% CI: 0.69–2.37; Fig. S1). Group did not moderate the effect of FC change (χ2(1) = 0.06, p = 0.81).
Secondary analyses
OBP showed a trend for less Time-1 mid-cingulate cortical deactivation to EF+/ER+ versus EF− conditions than OCP (t(32) = 1.83, p = 0.08), but there was no group difference in Time-1-Time-2 FC change (t(32) = 0.15, p = 0.88).
Combining effects
When both Time-1 BOLD response in the mid-cingulate and change in FC from EF− regions to the right caudate were added to the same model, change in FC remained significant (RR = 1.41, 95% CI: 1.11–1.79, χ2(1) = 7.58, p = 0.006). BOLD response in the mid-cingulate cortex at Time-1 did not cross the conventional threshold for statistical significance (RR:2.36, 95% CI: 0.997–5.59, χ2(1) = 3.73, p = 0.054), although the direction of the relationship remained unchanged (Table S6).
Hypo/manic symptoms
Hypothesis 1
There were no Time-1 BOLD response or Time-1 FC predictors of KMRS scores at Time-2 (Tables S7 and 8).
Hypothesis 2
Change in deactivation from Time-1 to Time-2 in one EF− > EF+/ER+ region (right putamen tail) initially appeared to predict Time-2 KMRS scores (Table S9), however this effect was not robust when regions not showing a significant effect were removed from the model (RR:1.68, 95% CI:0.85–3.32, χ2(1) = 2.48, p = 0.12. Likewise, when controlling for diagnoses or medication, the effect was not significant (RR = 1.81 95% CI 0.68–4.84; χ2(1) = 1.60, p = .21, and RR = 2.35 95% CI 0.86–6.42; χ2(1) = 3.26, p = 0.07, respectively).
There were no FC change predictors of Time-2 KMRS scores (Table S10).
Discussion
We aimed to determine for the first time the extent to which future affective symptom severity in OBP and OCP was predicted by activation and deactivation within and FC between neural regions supporting executive function during emotion regulation (EF+/ER+ regions) and neural regions subserving self-monitoring during a lower executive load task condition (EF− regions) at baseline, and by changes in these neural measures over follow-up. The most robust finding was that increases in FC between EF− and an EF+/ER+ region during emotional regulation over follow-up predicted greater future depression severity. We previously reported that changes in FC in EF+/ER+ regions were associated with concurrent changes in affective lability in a smaller group of BIOS youth [27]. The present study is the first, however, to examine activation, deactivation and FC within and between EF− and EF+/ER+ regions during emotional regulation, and the first to determine the extent to which these neural measures predict future affective symptom severity.
Our findings specifically show that relative increases in FC from Time-1 to Time-2 between EF− and an EF+/ER+ region to the executive function during emotion regulation condition predicted greater Time-2 depression severity in OBP and OCP, which resulted primarily from decreased FC during the EF− condition over follow-up. Previous studies highlight the importance of activation within and inverse FC between EF− and EF+/ER+ regions during attentionally demanding executive function tasks [56,57,58]. Findings suggest that a greater influence of EF− regions on EF+/ER+ regions during executive function tasks interferes with cognitive function. Our findings are the first to show, however, that relative increases in FC between EF− and EF+/ER+ regions over time, likely reflecting greater influence of EF− regions during emotion regulation, predicts worsening future depression severity in youth across a range of familial risk for AD.
This pattern of increased FC between regions was specific to the caudate. The caudate is a key striatal component of the task positive, fronto-striatal network implicated in stimulus-response learning [87, 88]. Furthermore, lower FC between the caudate and prefrontal cortex following traumatic brain injury is associated with executive dysfunction [89, 90]. Our findings suggest that relative increases in FC over follow-up specifically between EF− regions and the caudate during emotional regulation may be a potential neural mechanism predisposing to cognitive dysfunction and, in turn, higher levels of future depression in OBP and OCP.
Less normative deactivation [57] in one of the EF− regions, the mid-cingulate cortex, at Time-1 also predicted future (Time-2) depression severity. This effect resulted from less deactivation in this cluster to the EF+/ER+ condition and greater deactivation to the EF− condition. The effect just missed significance when accounting for the above FC predictive relationship. The mid-cingulate finding parallels previous reports of lower levels of EF− region deactivation during executive task performance in individuals with MDD and BD [41, 64,65,66,67,68,69,70,71,72,73, 75]. The mid-cingulate cortex is implicated in the prediction and monitoring of the outcomes of one’s own and others’ decisions [91], as well as the integration of pain and negative affect [92, 93]. Lower mid-cingulate deactivation at baseline during the EF+/ER+ condition might reflect greater baseline outcome monitoring and negative affect. This process may compromise the ability to redirect attention away from the emotional faces and toward more recent, relevant task stimuli. Additionally, decreases in deactivation from Time-1 to Time-2 in the putamen tail were initially identified as predicting greater hypo/mania severity at Time-2, but this effect was not robust to changes in the model’s covariates. We advise caution in interpreting this effect, and suggest that it could be used to guide hypotheses for future work.
The most robust predictive relationship was shown for increases in FC between EF− and EF+/ER+ regions over the four-year scan interval. It is notable that no EF+/ER+ region activity at Time-1, nor activity in the amygdala, predicted Time-2 symptoms. Together, this indicates a potentially greater role for increases in FC over time between EF− and EF+/ER+ regions than baseline measures of activation and FC for future symptom severity. These findings further highlight increasing influence of EF− over EF+/ER+ regions over time as a potential mechanism predisposing to higher levels of future depression in OBP and OCP.
The relationships between neural measures and future affective symptoms were similar in OBP and OCP, indicating that these relationships might reflect neural mechanisms of risk for worsening of affective symptoms in youth across a range of familial risk. The mid-cingulate cortical deactivation relationship with future depression severity was stronger in OCP than OBP, potentially reflecting the trend-level lower deactivation in this region at Time-1 in OBP than OCP and should be examined further in future studies.
Limitations
Some participants had psychiatric diagnoses at study entry or were medicated, however sensitivity analyses revealed no impact of these predictors on future depression severity for the majority of findings. There was a greater range of Time-2 depression than hypo/mania severity, which might have impacted these findings. All included participants showed high accuracy, curtailing our ability to examine relationships with task-performance. Future work could evaluate cognitive functioning during emotion regulation assessed outside of the scanner. Such measures could serve as additional markers of AD risk and could further illuminate relationships among neural function, emotion regulation deficits, and future symptoms. We used the EF− condition in our task as a proxy for processes in which we expect greater activation in self-monitoring regions, given that it was the task condition requiring the least cognitive and emotion regulatory effort. Future work could examine more specifically the processes that take place in these regions during low and high effort conditions. While the average lag between the assessment of Time-2 affective symptoms and scan was notable (~20 weeks), the central predictors of Time-2 affective symptoms included neural measures at Time-1 and change in neural measures between Time-1 to Time-2, which encompassed an approximately four-year period preceding the second symptom assessment. Thus, while it is possible that some neural changes from Time-1 to Time-2 might have occurred after the second symptom assessment, Time-1 neural measures and the majority of the Time-1 to Time-2 neural measure changes had already occurred in the time period prior to the second symptom assessment. The scanner changed between Time-1 and Time-2. Although we cannot rule out the possibility that this change affected the reported results, we believe it unlikely that it contributed to the patterns of significant associations we identified. Data from offspring of healthy parents were used to normalize neuroimaging measures for each scanner. Secondary analyses did not reveal significant differences among these offspring as a function of scanner in any of the regions we examined. Furthermore, it is not clear how the change in scanner, which affected all participants, could account for the patterns we observed which reflected individual differences in brain function and symptoms. Future work can identify predictors of diagnostic conversion and compare neurodevelopmental trajectories with lower AD risk offspring. Finally, sample size was small, reflecting the challenges in recruiting and following familial risk samples. While future studies can examine the above predictive relationships in larger sample sizes, participants in the present study were recruited across a range of future AD risk and had a long interscan interval.
Conclusions
We show that patterns of activation and deactivation during a task requiring both executive function and emotion regulation, and that changes over time in the functional connectivity between deactivated and activated neural regions during this task, predict future depression severity in youth across a range of familial risk for AD. As objective measures, these neural markers can provide objective, “early-warning” signs that might predate the increases in affective symptoms associated with the risk for the development or worsening of AD in at-risk youth. Additionally, they could be used to monitor the effect of treatments in future trials, and to evaluate whether interventions designed to address emotion regulation dysfunction can affect the functioning of these systems. If not, novel interventions may be needed to reduce the detrimental influence of neural networks supporting self-monitoring and negative affect upon neural networks supporting cognitive functions that are critical for everyday activities and, ultimately, mental health.
Funding and disclosures
This work was supported by MH060952 (PIs: BB & MLP) from the National Institute of Mental Health and the Pittsburgh Foundation (PI: MLP). The sources of funding exerted no influence over the present work.
BB has or will receive royalties from for publications from Random House, Inc (New Hope for Children and Teens with BD) and Lippincott Williams & Wilkins (Treating Child and Adolescent Depression). JCF has or will receive royalties from Guilford Press (Cognitive Therapy for Personality Disorders, 3rd edition) and has received consulting fees from Happify.com. All other authors report no competing interests.
Notes
Two individuals had higher KDRS scores than the rest of the cohort. When removed from the larger models, the associations remained significant: BOLD response in the mid-cingulate continued to predict Time-2 KDRS (RR = 9.15, χ2 (1) = 6.34, p = 0.01), as did change in connectivity between the task-negative seeds and the right caudate between Time-1 and Time-2 (RR = 1.51, χ2 (1) = 6.47, p = 0.01).
References
Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. A J Psychiatry. 2000;157:1925–32.
Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the Global Burden of Disease study. J Psychiatr Res. 2020;126:134–40.
Ferrari AJ, Stockings E, Khoo J-P, Erskine HE, Degenhardt L, Vos T, et al. The prevalence and burden of bipolar disorder: Findings from the Global Burden of Disease Study 2013. Bipolar Disord. 2016;18:440–50.
Burke KC, Burke JD, Regier DA, Rae DS. Age at onset of selected mental disorders in five community populations. Arch Gen Psychiatry. 1990;47:511–18.
Leibenluft E, Rich B. Pediatric bipolar disorder. Ann Rev Clin Psychol. 2008:4;163–87.
Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18:1023–35.
DelBello MP, Hanseman D, Adler CM, Fleck DE, Strakowski SM. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. A J Psychiatry. 2007;164:582–90.
Chang K, Howe M, Gallelli K, Miklowitz D. Prevention of pediatric bipolar disorder: Integration of neurobiological and psychosocial processes. Ann NY Acad Sci. 2006;1094:235–47.
Birmaher B Bipolar Disorders In: Martin A, Volkmar F, editors. Lewis’s Child and Adolescent Psychiatry: A Comprehensive Textbook. Philadelphia: Wolters Kluwer; 2018.
Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: The Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66:287–96.
Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44:846–71.
Goodwin F, Jamison K, SNG Manic-depressive illness: Bipolar disorders and recurrent depression. Oxford University Press: New York, N.Y; 2007.
Merikangas KR, Jin R, He J-P, Kessler RC, Lee S, Sampson NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–51.
Axelson D, Goldstein B, Goldstein T, Monk K, Yu H, Hickey MB, et al. Diagnostic precursors to bipolar disorder in offspring of parents with bipolar disorder: a longitudinal study. A J Psychiatry. 2015;172:638–46.
Birmaher B. Longitudinal course of pediatric bipolar disorder. Am J Psychiatry. 2007;16:537–39.
Chang KD, Steiner H, Ketter TA. Psychiatric phenomenology of child and adolescent bipolar offspring. J Am Acad Child Adolesc Psychiatry. 2000;39:453–60.
Chang K, Steiner H, Ketter T. Studies of offspring of parents with bipolar disorder. Am J Med Genet Part C: Semin Med Genet. 2003;123:26–35.
Parker G. Parental representations and affective symptoms: examination for an hereditary link. Br J Med Psychol. 1982;55:57–61.
Warner V, Weissman M, Fendrich M, Wickramaratne P, Moreau D. The course of major depression in the offspring of depressed parents: Incidence, recurrence, and recovery. Arch Gen Psychiatry. 1992;49:795–801.
Weissman MM, Wickramaratne P, Gameroff MJ, Warner V, Pilowsky D, Kohad RG, et al. Offspring of depressed parents: 30 years later. A J Psychiatry. 2016;173:1024–32.
Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. A J Psychiatry. 2006;163:1001–08.
Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet. 2003;123C:48–58.
Hillegers M, Reichart C, Wals M, Verhulst F, Ormel J, Nolen W. Five‐year prospective outcome of psychopathology in the adolescent offspring of bipolar parents. Bipolar Disord. 2005;7:344–50.
Duffy A. The early natural history of bipolar disorder: what we have learned from longitudinal high-risk research. Can J Psychiatry. 2010;55:477–85.
Joormann J, Gotlib IH. Emotion regulation in depression: relation to cognitive inhibition. Cognition Emot 2010;24:281–98.
Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–57.
Acuff H, Versace A, Bertocci M, Ladouceur C, Hanford L, Manelis A, et al. Association of neuroimaging measures of emotion processing and regulation neural circuitries with symptoms of bipolar disorder in offspring at risk for bipolar disorder. JAMA Psychiatry. 2018;75:1241–51.
Cattarinussi G, Di Giorgio A, Wolf RC, Balestrieri M, Sambataro F. Neural signatures of the risk for bipolar disorder: a meta‐analysis of structural and functional neuroimaging studies. Bipolar Disord. 2019;21:215–27.
Olsavsky AK, Brotman MA, Rutenberg JG, Muhrer EJ, Deveney CM, Fromm SJ, et al. Amygdala hyperactivation during face emotion processing in unaffected youth at risk for bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:294–303.
Tseng W-L, Bones B, Kayser R, Olsavsky A, Fromm S, Pine D, et al. An fMRI study of emotional face encoding in youth at risk for bipolar disorder. Eur Psychiatry. 2015;30:94–98.
Ladouceur CD, Farchione T, Diwadkar V, Pruitt P, Radwan J, Axelson DA, et al. Differential patterns of abnormal activity and connectivity in the amygdala-prefrontal circuitry in bipolar-I and bipolar-NOS youth. J Am Acad Child Adolesc Psychiatry. 2011;50:1275–89.e2.
Ladouceur C, Diwadkar V, White R, Bass J, Birmaher B, Axelson D, et al. Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm. Developmental Cogn Neurosci. 2013;5:185–96.
Manelis A, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, et al. Altered amygdala-prefrontal response to facial emotion in offspring of parents with bipolar disorder. Brain. 2015;138:2777–90.
Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–87.
Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology. 2005;183:308–13.
Korgaonkar MS, Erlinger M, Breukelaar IA, Boyce P, Hazell P, Antees C, et al. Amygdala activation and connectivity to emotional processing distinguishes asymptomatic patients with bipolar disorders and unipolar depression. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2019;4:361–70.
Drapier D, Surguladze S, Marshall N, Schulze K, Fern A, Hall M-H, et al. Genetic liability for bipolar disorder is characterized by excess frontal activation in response to a working memory task. Biol Psychiatry. 2008;64:513–20.
Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–28.
Hafeman DM, Bebko G, Bertocci MA, Fournier JC, Bonar L, Perlman SB, et al. Abnormal deactivation of the inferior frontal gyrus during implicit emotion processing in youth with bipolar disorder: attenuated by medication. J Psychiatr Res. 2014;58:129–36. (C)
Lee M-S, Anumagalla P, Talluri P, Pavuluri MN. Attentional engagement increases inferior frontal gyrus activity and mutes limbic activity in pediatric bipolar disorder: Meta-analyses of fMRI studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;91:14–19.
Alonso‐Lana S, Moro N, McKenna PJ, Sarró S, Romaguera A, Monté GC, et al. Longitudinal brain functional changes between mania and euthymia in bipolar disorder. Bipolar Disord. 2019;21:449–57.
Smucny J, Lesh TA, Newton K, Niendam TA, Ragland JD, Carter CS. Levels of cognitive control: a functional magnetic resonance imaging-based test of an RDoC domain across bipolar disorder and schizophrenia. Neuropsychopharmacology. 2018;43:598–606.
Borgelt L, Strakowski SM, DelBello MP, Weber W, Eliassen JC, Komoroski RA, et al. Neurophysiological effects of multiple mood episodes in bipolar disorder. Bipolar Disord. 2019;21:503–13.
Foland-Ross LC, Bookheimer SY, Lieberman MD, Sugar CA, Townsend JD, Fischer J, et al. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. Neuroimage. 2012;59:738–44.
Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry. 2013;73:127–35.
Altshuler L, Bookheimer S, Townsend J, Proenza MA, Sabb F, Mintz J, et al. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–17.
Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–88.
Townsend JD, Bookheimer SY, Foland‐Ross LC, Moody TD, Eisenberger NI, Fischer JS, et al. Deficits in inferior frontal cortex activation in euthymic bipolar disorder patients during a response inhibition task. Bipolar Disord. 2012;14:442–50.
Delvecchio G, Fossati P, Boyer P, Brambilla P, Falkai P, Gruber O, et al. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol. 2012;22:100–13.
Passarotti AM, Ellis J, Wegbreit E, Stevens MC, Pavuluri MN. Reduced functional connectivity of prefrontal regions and amygdala within affect and working memory networks in pediatric bipolar disorder. Brain Connectivity. 2012;2:320–34.
Kim P, Thomas LA, Rosen BH, Moscicki AM, Brotman MA, Zarate J, et al. Differing amygdala responses to facial expressions in children and adults with bipolar disorder. A J Psychiatry. 2012;169:642–49.
Garrett AS, Reiss AL, Howe ME, Kelley RG, Singh MK, Adleman NE, et al. Abnormal amygdala and prefrontal cortex activation to facial expressions in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:821–31.
Ladouceur CD, Farchione T, Diwadkar V, Pruitt P, Radwan J, Axelson DA, et al. Differential patterns of abnormal activity and connectivity in the amygdala–prefrontal circuitry in bipolar-I and bipolar-NOS youth. J Am Acad Child Adolesc Psychiatry. 2011;50:1275–89. e2.
Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–47.
Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–72.
Piccoli T, Valente G, Linden DE, Re M, Esposito F, Sack AT, et al. The default mode network and the working memory network are not anti-correlated during all phases of a working memory task. PloS One. 2015;10:e0123354.
Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra‐subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27:694–705.
Zuo N, Salami A, Yang Y, Yang Z, Sui J, Jiang T. Activation‐based association profiles differentiate network roles across cognitive loads. Hum Brain Mapp. 2019;40:2800–12.
Fuentes-Claramonte P, Martín-Subero M, Salgado-Pineda P, Alonso-Lana S, Moreno-Alcázar A, Argila-Plaza I, et al. Shared and differential default-mode related patterns of activity in an autobiographical, a self-referential and an attentional task. Plos One. 2019;14:e0209376.
Gu H, Hu Y, Chen X, He Y, Yang Y. Regional excitation-inhibition balance predicts default-mode network deactivation via functional connectivity. Neuroimage. 2019;185:388–97.
Čeko M, Gracely JL, Fitzcharles M-A, Seminowicz DA, Schweinhardt P, Bushnell MC. Is a responsive default mode network required for successful working memory task performance? J Neurosci. 2015;35:11595–605.
Huang AS, Klein DN, Leung H-C. Load-related brain activation predicts spatial working memory performance in youth aged 9–12 and is associated with executive function at earlier ages. Developmental Cogn Neurosci. 2016;17:1–9.
Satterthwaite TD, Wolf DH, Erus G, Ruparel K, Elliott MA, Gennatas ED, et al. Functional maturation of the executive system during adolescence. J Neurosci. 2013;33:16249–61.
Breukelaar IA, Erlinger M, Harris A, Boyce P, Hazell P, Grieve SM, et al. Investigating the neural basis of cognitive control dysfunction in mood disorders. Bipolar Disord. 2020;22:286–95.
Gärtner M, Ghisu ME, Scheidegger M, Bönke L, Fan Y, Stippl A, et al. Aberrant working memory processing in major depression: evidence from multivoxel pattern classification. Neuropsychopharmacology. 2018;43:1972–79.
Rodríguez-Cano E, Sarró S, Monté G, Maristany T, Salvador R, McKenna P, et al. Evidence for structural and functional abnormality in the subgenual anterior cingulate cortex in major depressive disorder. Psychol Med. 2014;44:3263.
Alonso-Lana S, Goikolea JM, Bonnin CM, Sarró S, Segura B, Amann BL, et al. Structural and functional brain correlates of cognitive impairment in euthymic patients with bipolar disorder. PloS One. 2016;11:e0158867.
Rose EJ, Simonotto E, Ebmeier KP. Limbic over-activity in depression during preserved performance on the n-back task. Neuroimage. 2006;29:203–15.
Fernández-Corcuera P, Salvador R, Monté GC, Sarró SS, Goikolea JM, Amann B, et al. Bipolar depressed patients show both failure to activate and failure to de-activate during performance of a working memory task. J Affect Disord. 2013;148:170–78.
Rodríguez‐Cano E, Alonso‐Lana S, Sarró S, Fernández‐Corcuera P, Goikolea JM, Vieta E, et al. Differential failure to deactivate the default mode network in unipolar and bipolar depression. Bipolar Disord. 2017;19:386–95.
Pomarol-Clotet E, Alonso-Lana S, Moro N, Sarro S, Bonnin MC, Goikolea JM, et al. Brain functional changes across the different phases of bipolar disorder. Br J Psychiatry. 2015;206:136–44.
Pomarol-Clotet E, Moro N, Sarró S, Goikolea JM, Vieta E, Amann B, et al. Failure of de-activation in the medial frontal cortex in mania: Evidence for default mode network dysfunction in the disorder. World J Biol Psychiatry. 2012;13:616–26.
Bartova L, Meyer BM, Diers K, Rabl U, Scharinger C, Popovic A, et al. Reduced default mode network suppression during a working memory task in remitted major depression. J Psychiatr Res. 2015;64:9–18.
Alonso-Lana S, Valentí M, Romaguera A, Sarri C, Sarró S, Rodríguez-Martínez A, et al. Brain functional changes in first-degree relatives of patients with bipolar disorder: evidence for default mode network dysfunction. Psychol Med. 2016;46:2513.
Meyer BM, Rabl U, Huemer J, Bartova L, Kalcher K, Provenzano J, et al. Prefrontal networks dynamically related to recovery from major depressive disorder: a longitudinal pharmacological fMRI study. Transl Psychiatry. 2019;9:1–10.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–88.
Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, et al. A preliminary study of the Kiddie schedule for affective disorders and schizophrenia for school-age children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13:463–70.
Angold A, Costello EJ, Messer SC, Pickles A. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int J Methods Psychiatr Res. 1995;5:237–49.
Gerson AC, Gerring JP, Freund L, Joshi PT, Capozzoli J, Brady K, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. 1996;65:189–98.
Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38:1230–6.
Ladouceur CD, Silk JS, Dahl RE, Ostapenko L, Kronhaus DM, Phillips ML. Fearful faces influence attentional control processes in anxious youth and adults. Emotion. 2009;9:855–64.
Bertocci M, Bebko G, Dwojak A, Iyengar S, Ladouceur C, Fournier J, et al. Longitudinal relationships among activity in attention redirection neural circuitry and symptom severity in youth. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2017;2:336–45.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–39.
McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–86.
Storey JD. A direct approach to false discovery rates. J R Stat Soc Ser B-Stat Methodol. 2002;64:479–98.
Storey JD, Taylor JE, Siegmund D. Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach. J R Stat Soc Ser B-Stat Methodol. 2004;66:187–205.
Rogers RD, Andrews T, Grasby P, Brooks D, Robbins T. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12:142–62.
Packard MG. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc Natl Acad Sci USA. 1999;96:12881–86.
De Simoni S, Jenkins PO, Bourke NJ, Fleminger JJ, Hellyer PJ, Jolly AE, et al. Altered caudate connectivity is associated with executive dysfunction after traumatic brain injury. Brain. 2018;141:148–64.
Stoffers D, Altena E, van der Werf YD, Sanz-Arigita EJ, Voorn TA, Astill RG, et al. The caudate: a key node in the neuronal network imbalance of insomnia? Brain. 2014;137:610–20.
Apps MA, Lockwood PL, Balsters JH. The role of the midcingulate cortex in monitoring others’ decisions. Front Neurosci. 2013;7:251.
Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67.
Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero‐Gallagher N, Laird AR, et al. The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp. 2014;35:2741–53.
Author information
Authors and Affiliations
Contributions
JCF, MB, and MLP conceived the analytic plan, conducted or interpreted the statistical analyses, and drafted the manuscript. MLP and BB were the principal investigators and oversaw the project. Additionally, CDL, AV, JPLS, SI aided substantially in elements of the design or interpretation of the results. LB, KM, and HA-W made substantial contributions to the acquisition of the data. All authors made significant contributions to the manuscript, and all approved the final version.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fournier, J.C., Bertocci, M., Ladouceur, C.D. et al. Neural function during emotion regulation and future depressive symptoms in youth at risk for affective disorders. Neuropsychopharmacol. 46, 1340–1347 (2021). https://doi.org/10.1038/s41386-021-01001-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01001-w