Abstract
Post-traumatic stress disorder (PTSD) is difficult to treat but one promising strategy is to block memory reconsolidation of the traumatic event. This study aimed to evaluate the efficacy of traumatic memory reactivation under the influence of propranolol, a noradrenergic beta-receptor blocker, in reducing PTSD symptoms as well as comorbid major depression (MD) symptoms. We conducted a double blind, placebo-controlled, randomised clinical trial in 66 adults diagnosed with longstanding PTSD. Propranolol or a placebo was administered 90 min before a brief memory reactivation session, once a week for 6 consecutive weeks. Measures included the SCID PTSD module, the PTSD Check List (PCL-S) and the Beck Depression Inventory-II (BDI-II). PTSD symptoms decreased both in the pre-reactivation propranolol group (39.28%) and the pre-reactivation placebo group (34.48 %). During the 6 treatment sessions, PCL-S and BDI-II scores decreased to similar extent in both groups and there were no treatment differences. During the 3-month follow-up period, there were no treatment effects for the mean PCL-S and BDI-II scores. However, in patients with severe PTSD symptoms (PCL-S ≥ 65) before treatment, PCL-S and BDI-II scores continued to decline 3 months after the end of treatment in the propranolol group while they increased in the placebo group. Repeated traumatic memory reactivation seemed to be effective for PTSD and comorbid MD symptoms. However, the efficacy of propranolol was not greater than that of placebo 1 week post treatment. Furthermore, in this traumatic memory reactivation, PTSD symptom severity at baseline might have influenced the post-treatment effect of propranolol.
Similar content being viewed by others
Introduction
It is a well-known fact that stressful events such as man-made traumas, natural disasters or worldwide pandemics such as Covid-19 [1, 2] can have a significant mental health impact and lead to conditions such as depression and PTSD. Remission of post-traumatic stress disorder (PTSD) can be obtained by trauma-focused psychotherapies, but treatment duration and the limited availability of therapists are major limitations. The benefits of such treatments decline over time [3] and a recent study of community-based treatment for anxiety highlighted that fewer than 5% of patients received exposure-based treatment [4]. These psychotherapies may or may not be associated with pharmacotherapy such as selective serotonin reuptake inhibitors (SSRIs). However, 43% of patients receiving SSRI treatment do not respond adequately and SSRIs are only superior to placebo by a small effect size (d = −0.23) [5, 6].
One possible novel strategy is pharmacological impairment of memory reconsolidation [7]. Reactivated memory becomes temporarily labile and requires stabilisation referred to as reconsolidation [8,9,10]. The beta-blocking compound, propranolol, has been shown to interfere with memory reconsolidation [8], suggesting its potential use as a pharmacological treatment in conditions involving increased memory intrusions such as PTSD [11].
The noradrenergic system is critical in modulating memory processes, and β-noradrenergic receptor stimulation has been found to facilitate emotional memory reconsolidation [12]. Moreover, a hyper-noradrenergic state is implicated in the pathophysiology of PTSD [13, 14].
In healthy humans, the β-noradrenergic receptor antagonist propranolol was found to block memory reconsolidation in a fear conditioning test [15] and studies have reported successful propranolol-induced disruption of reconsolidation that persisted for at least one month and resisted fear reinstatement [16].
From a therapeutic perspective, propranolol has been tested for use in substance abuse [17] and phobia [18]. Brunet et al. [19] conducted the first clinical trial in PTSD patients. They administered a single dose of propranolol following traumatic memory reactivation. One week later, this treatment reduced physiological responses to mental imagery of the traumatic event. In three other independent open-label studies, six brief trauma reactivation sessions under the influence of propranolol resulted in significant PTSD symptom improvement [11]. Propranolol may well have blocked traumatic memory reconsolidation, leading in turn to symptom reduction [20]. However, Wood et al. [21] failed to find significant group differences in PTSD symptoms with propranolol, with or without memory reactivation, in veterans, all of whom had been diagnosed with combat-related PTSD.
Recently, in a randomised clinical trial [22] with “pre-reactivation propranolol therapy”, PTSD participants who actively recalled their traumatic event under the influence of propranolol once a week for up to 6 weeks showed a substantial decrease in symptom scores (PTSD symptom improvement = 36%) compared to those who underwent pre-reactivation with the placebo (PTSD symptom improvement = 13%). Although the results of the randomised trial were promising, there was no long-term patient follow-up. Furthermore, 50% of the patients either did not complete the six treatment sessions or were excluded. We decided to replicate this study to confirm the efficacy of propranolol in reducing PTSD symptoms and to observe the 3-month post-treatment effects. Traumatic exposure is also associated with alcohol use disorder, attempted suicide and major depression (MD) [23]. Considering that approximately 30–50% of the patients with a lifetime diagnosis of PTSD also meet the diagnostic criteria for MD [24], we also examined the efficacy of propranolol in reducing comorbid symptoms of depression, which has never been done before. Therefore, the main study objective was to assess the efficacy of propranolol in the context of traumatic memory reactivation in reducing the severity of PTSD and MD symptoms 1 week after the end of the treatment. The secondary objective was to assess efficacy 3 months after treatment had ended. Lastly, for exploratory purposes, we assessed the efficacy of propranolol according to the level of severity of PTSD symptoms at baseline.
Methods
Study design and participants
Participants were enroled from January 2013 to October 2017 from three French regional outpatient services specialising in psychotraumatology in Toulouse, Lille and Tours University Hospitals [25]. Participants had to have a primary diagnosis of PTSD according to the DSM-IV criteria [26], with a PTSD Checklist–Specific (PCL-S) score ≥ 45. Participants gave their written informed consent to take part. All procedures were reviewed and approved by institutional review boards (ClinicalTrials.gov identifier: NCT01713556). The inclusion criteria were as follows: treatment-seeking adults aged 18–65 years; suffering from PTSD for at least 3 consecutive months and having a PCL-S score ≥ 45 at the first session (W1). The exclusion criteria were as follows: contraindication to propranolol (hypotension, higher than a first-degree heart block, bronchial asthma etc.); medication recommended or suggested for PTSD treatment; psychotherapy; basal systolic blood pressure <100 mm Hg; basal heart rate <50 bpm; psychotic or bipolar disorders; traumatic brain injury; current substance or alcohol dependence; acute suicidal ideation; pregnancy and breast feeding.
Randomisation and masking
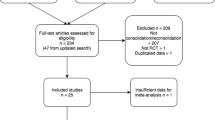
Participants were waiting for PTSD treatment and were randomised by the Toulouse University Hospital pharmacy according to a double-blind design. Following inclusion, participants were assigned by randomised complete block, with a 1:1 ratio to either the “traumatic memory reactivation+propranolol” group, hereafter referred to as the propranolol group (n = 33, 20 females) or the “traumatic memory reactivation+placebo” group, referred to as the placebo group (n = 33, 21 females) (Fig. 1).
The randomisation list was drawn up by the Methodology and Data Management Centre statistician before the start of the study and randomisation was stratified by centre.
Patients were allocated to groups according to the chronological order of enrolment in the protocol. Participants, care providers and those assessing outcomes were blinded. The placebo capsule contained only pharmaceutical grade lactose. The placebo was the same size and colour as the active drug capsules in order to maintain blind status
Procedures
Measures
The PTSD Check List (PCL-S) [27] was used to assess PTSD symptoms. Scores ranged from 17 to 85, with higher scores reflecting increased levels of PTSD symptoms. Scores ≥45 reflect a condition that warrants clinical attention. For additional analyses, we split our general population into two groups of patients based on symptom severity. For this purpose, we used the median and the mean PCL-S in the general population at session 1. The median for the PCL-S score was 66 and the mean 64.075. We therefore chose the value of 65, which is also the median value between the two terminals values of the PCL-S scale for patients with PTSD (45 and 85), to split our population into two groups, namely non-severe PTSD (45 ≤ scores < 65) and severe PTSD (scores ≥ 65).
The PTSD module’s trauma screen of the Structured Clinical Interview for DSM-IV (SCID) [28] is a well-established semi-structured interview to establish diagnoses consistent with the DSM-IV. The PTSD module was applied and required participants to refer to their traumatic experience when answering the questions.
The Beck Depression Inventory-II (BDI-II) [29] is a 21-item self-report inventory to assess the severity of depressive symptomatology. Each symptom is rated from 0 to 3. The total score can range from 0 to 63 with scores ≥ 29 indicating symptoms of severe depression. Percentage change in the BD-II score was used as a response metric, with treatment response defined as a greater than 50% reduction in BDI scores over the course of treatment [30].
Study medication
Propranolol hydrochloride is a lipophilic, centrally acting β1- and β2-noradrenergic receptor blocker [31, 32], commonly prescribed for hypertension or migraine [33]. For the first treatment session, each subject received 0.67 mg/kg of short-acting (SA) propranolol or a placebo. If the SA dose was well tolerated, subjects received 1.0 mg/kg of long-acting propranolol or a placebo 90 min later. Both doses were given simultaneously for the remaining treatment sessions. Unfortunately, it was not possible to administer the drugs just after reactivation to ensure that only memory reconsolidation was affected since propranolol reaches its peak systemic concentration 75 min after ingestion [34]. Propranolol or the placebo was administered as soon as the patient arrived at the centre, 90 min prior to reactivation of the traumatic memory, mainly to ensure that an optimal blood level was achieved at the time of memory reactivation. A nurse monitored the participants’ vital signs every 30 min throughout this 90-min period. Two (3.03%) participants in the propranolol group reported side effects. One had a pulse rate below 50 bpm after the first treatment session and the other developed insomnia after the fourth treatment session. Both subjects were excluded from the study (Fig. 1).
Trauma reactivation
This procedure is described in detail elsewhere [19, 22]. In the clinical centres, 90 min after ingesting the medication, participants wrote a one-page trauma narrative in the present tense, first person singular, focusing on the event’s most disturbing moments. This task took up to 30 min. The therapist asked the patient to add some emotional details, if possible. Prior to the second session (W2), a “traumatic script” was prepared. In subsequent treatment sessions, 90 min after receiving the medication, participants read their trauma script to the study therapist. Participants were asked whether they wished to amend or supplement their narrative.
Statistical analyses
We conducted an Intention-To-Treat (ITT) analysis and a Per Protocol analysis. Due to the fact that only four patients (12.12%) per group dropped out between the first and seventh weeks, results were very similar in both analyses and we decided to present only the ITT analysis. The sample size (n > 28 subjects per group) was calculated to estimate an effect size (d) comparable to that observed in the meta-analysis by Bradley et al. [35] concerning psychotherapies for PTSD, and taking into account 30% of patients lost to follow-up.
All statistical analyses were conducted using SPSS for Windows version 22.0 (IBM, Armonk, N.Y.). For the demographic data, group comparisons were calculated with Chi-square, Fisher’s exact test or the independent t-test. PCL-S and BDI-II scores were analysed using a Student t test or ANOVA analysis with repeated measures (between factor: treatment; within factor: weeks). The effect size for pre- to post-treatment according to certain conditions was also provided for both these scores and the SCID. Cohen’s d is generally interpreted as follows: ≥1.0 very large, 1 > d ≥ 0.8 large, 0.8 > d ≥ 0.5 moderate, 0.2–0.4 small. For the SCID and for severe symptoms of depression (BDI ≥ 29), group comparisons were calculated with Chi-square or the independent t-test.
Moreover, we added an analysis of the PCL-S score according to the level of PTSD before the beginning of treatment (W1). To this end, we analysed data for the PCL-S scores in patients with severe PTSD symptoms (PCL-S score ≥ 65; propranolol n = 18, placebo n = 15) or with moderate PTSD symptoms (PCL-S 45 ≤ score < 65; propranolol n = 11, placebo n = 14).
Result
Demographic data
Patient characteristics are summarised in Table 1.
Thirty-three subjects were randomly assigned to the propranolol group and 33 to the placebo group. The remaining patient characteristics are summarised in Table 1. Only the ages of the patients differed in the two groups (propranolol 35.6 ± 12.8 years; placebo 42.2 ± 12.7 years; t(1.64)=2.114 p = 0.038).
For our primary outcomes measures, we found a highly significant difference in PCL-S scores between W1 and W7 for both the propranolol group [t(28) = 5.683 p < 0.001] and the placebo group [t(28) = 7.410 p < 0.001] (Fig. 2A). A complementary analysis showed that, for the PCL-S score during the treatment assessment, there were no general treatment effects [F(1,55) = 0.267 p = 0.607] and no interaction between the treatment and the session [F(6,330) = 1.682 p = 0.125] but a general significant session effect [F(6,330) = 47.719 p < 0.001]. Thus, the PCL scores decreased to similar extent in both groups. The pre- to post-treatment effect sizes (Cohen’s d) were 1.249 in the propranolol group and 1.411 in the placebo group. For each session (W2–W7), no significant differences were found between the propranolol and placebo groups.
A Mean PCL-S score. 33 patients attended the first treatment session and 29 during week 7 for both groups. B Change in MD symptoms during the treatment period. PCL-S = PTSD Checklist; BDI-II = the Beck Depression Inventory-II, propranolol = “traumatic memory reactivation + propranolol” group; placebo = “traumatic memory reactivation + placebo” group.
The SCID Post-treatment PTSD diagnosis decreased in both groups with no significant intergroup differences (p = 0.707) at W7. Almost two-thirds of the patients were in remission 1 week post treatment: 60.72% in the propranolol group and 65.52% in the placebo group.
The BDI-II scores for the two groups are presented in Fig. 2B. The treatment assessment did not highlight any general treatment effects [F(1,55) = 0.199 p = 0.658] or any interaction between the treatment and the session [F(6,330)=0.486 p = 0.819] but a significant session effect was apparent [F(6,330) = 38.321 p < 0.001]. Therefore, BDI-II scores decreased to similar extent in both groups. The pre- to post-treatment effect sizes (Cohen’s d) were 0.941 in the propranolol group and 0.974 in the placebo group.
Before the first treatment session, BDI-II scores ≥ 29 (severe) were recorded in 54.5% (18/33) and 57.6% (19/33) of the propranolol and placebo patients, respectively. BDI-II scores ≥ 29 decreased significantly in both groups between W1 and W7 (p < 0.001). After six treatment sessions, the level of severe depression dropped dramatically to 10.7% in the propranolol group and to 10.3% in the placebo group. Treatment response rates of 7/18 (38.88%) and 6/15 (40%) were documented in the propranolol and placebo groups, respectively. No significant differences were noted in these two measurements.
3-month follow-up
The rates of SCID PTSD diagnoses at 3 months post treatment were low (14.3% in the propranolol group and 24% in the placebo group) but there was no significant difference between the two groups (p = 0.408). However, these low rates are partly biased because some patients were withdrawn from the study after session 7 for another treatment.
At the 3-month follow-up consultation, there were no treatment effects for the mean PCL-S scores during the 18th week (propranolol 45.16 ± 19.55; placebo 41.48 ± 19.04; t(1,49) = 0.680 p = 0.500) or for the change in this score between weeks 7 and 18 (propranolol −3.30 ± 8.10; placebo −1.28 ± 11.0; t(1,49)=0.739 p = 0.464).
Three months post treatment, low BDI-II scores were recorded in both groups (propranolol: 15.64 ± 13.01; placebo: 17.76 ± 15.12). These BDI scores continued to decrease between W7 and W18 for the propranolol group (−2.64) but increased slightly for the placebo group (+1). However, this change was not deemed significant (t(1,47)=1.738 p = 0.089).
Analysis by PTSD level
We checked whether propranolol treatment had the same effects in patients with severe PTSD symptoms (PCL-S score ≥ 65) and those with non-severe PTSD symptoms (PCL-S 45 ≤ score < 65) prior to treatment. During treatment sessions (W1–W7), we noted no difference in the PCL-S and BDI-II scores between the propranolol and placebo groups for patients with severe or non-severe PTSD symptoms (Fig. 3).
A Change in PTSD symptoms during the treatment period depending on the initial level of PTSD (mean PCL-S score), B change in MD symptoms during the treatment period depending on the initial level of PTSD (mean PCL-S score). Propranolol = “traumatic memory reactivation + propranolol” group; Placebo = “traumatic memory reactivation + placebo” group. *p < 0.05, **p < 0.01.
Three months post treatment, for non-severe PTSD symptoms groups, PCL-S and BDI scores continued to decrease slightly in both groups of patients and the relationship between change and treatment was not significant [PCL-S: F(1,18)=0.094 p = 0.76; BDI: F(1,18) = 0.154 p = 0.700]. At W18, the PCL-S scores seemed higher in propranolol patients than in placebo patients but this difference was not significant [PCL-S: t (19) = 1.415 p = 0.173; BDI: t (18) = 0.810 p = 0.428]. The between group effect size (Cohen’s d) was 0.168 for the BDI score and 0.404 for the PCL-S score but in favour of the placebo group. In contrast, for the severe PTSD symptoms groups, both the PCL-S score and the BDI-II score for propranolol patients continued to decrease, which was not the case for the placebo patients. The relationship between change in the PCL-S score and treatment was significant for both scores [PCL-S: F(1,26) = 4.315 p = 0.048; BDI: F(1,27) = 8.457 p = 0.007] and the between group effect size was moderate for the PCL-S score (d = 0.520) and very large for the BDI score (d = 1.06). However, at W18, we found no significant treatment effect for either score [PCL-S: t (26) = 0.825 p = 0.417; BDI; t (19) = 1.700 p = 0.101].
Discussion
In this randomised, double-blind, placebo-controlled study of propranolol vs. placebo, we were unable to replicate the results of Brunet et al. [22]. In fact, at the end of treatment, we found no difference in the PTSD scores between the propranolol and placebo groups. However, as was expected [11, 22], participants who actively recalled their traumatic event under the influence of propranolol once a week for up to 6 weeks showed a substantial decrease in PTSD symptoms (W7–W1 PCLs score: −17.1). The non-replication of Brunet’s study can be particularly attributed to the placebo group. Even a pre-reactivation placebo effect was expected but, surprisingly, the PCL-S scores (W7-W1 PCL-S score: −18.9) showed a comparable improvement to that of the propranolol group. This significant improvement in the placebo group could be attributed to the exposure procedure which was repeated six times and to the fact that patients completed 7 PCL-S which could also have served as a form of exposure. However, the duration of the reactivation sessions in our study was very short, from 3 to 10 min, without psychotherapeutic intervention during memory reactivation. This period is extremely short compared to trauma-focused psychotherapy sessions and should only activate the reconsolidation process and not the extinction process [36]. It should be noted that this brief reactivation of traumatic memory was much more direct and certainly more destabilising than in other therapies.
However, Brunet et al. [22] recorded only a minor improvement (W7-W1 PCL-S score: −5) in the placebo group. This difference in PCL-S scores cannot be explained by a difference in protocol because we used exactly the same reactivation procedure as in the Brunet’s study. However, the setting for this reactivation procedure may differ in the two studies. In our investigation, we asked patients to reactivate their traumatic memory in a safe environment, i.e., at clinical trial sites in university hospitals. In addition, nurses and all medical staff were empathetic towards patients. When reactivation was linked to major emotional symptoms (crying, tremor, malaise, agitation, etc.), the psychologist had words of comfort to the participants but was instructed not to adopt a psychotherapeutic approach. This succession of human and empathic interactions certainly partly explains the protocol effect for participants who received the placebo. An important factor that could have influenced the evolution of PCL-S scores was the female/male ratio. However, this ratio was very similar in both studies (58.3% in Brunet’s study and 62.1% in our study). Sample size is also an important factor in this type of study. Nevertheless, at the beginning of the protocol (W1), the sample sizes were very similar in the two studies; 30 patients per group for the Brunet’s study and 33 per group for our study. However, the only important difference between the two studies was the number of patients remaining at the end of the treatment protocol (W7); 15 patients per group for the Brunet’s study and 29 per group for our study. Therefore, in our study, 12.12% (8/66) of the patients dropped out compared to 50% in the Brunet’s study, hence 30 of the 60 patients did not complete the 6 treatment sessions. This was the first instance of follow-up 3 months after treatment and 77.3% (51/66) of the patients were still participating in the study. Improvement was extremely stable in the propranolol group. However, in the placebo group, the PCL-S score increased again in 21% of the patients. This difference in stabilisation depended mainly on our patients’ PCL-S baseline levels. For non-severe PCL scores at baseline, stabilisation was evident in both patient groups. In contrast, for subjects with severe PCL scores at baseline, we noted a slight increase in these scores in the placebo group while the propranolol group scores continued to improve.
In addition, we showed that comorbid symptoms of depression were also sensitive to this treatment. We noted a significant decrease in BDI-II scores during the various treatment sessions and the number of patients with severe symptoms of depression decreased from 56% before starting treatment to 10% at the end of treatment. Once again, no significant difference between propranolol and placebo was observed in any patient during the six treatment sessions. In contrast, during the 3-month follow-up period, propranolol treatment was very effective in the management of depression in patients with a severe PCL score before treatment. In this group, the BDI-II score continued to decline 3 months after treatment had ended, whereas it increased in placebo patients. This significant decrease in BDI-II scores also could be linked to an improvement in negative alterations in cognition and mood associated with the traumatic event, and mainly concerning rumination (shared by PTSD and MD) [37]. Moreover, in the cognitive model of depression, cognitive functions (biased attention, biased processing, biased thoughts and rumination, biased memory, and dysfunctional attitudes and schemas) have been consistently associated with the onset, continuation and recurrence of the disorder [38, 39]. Focusing on autobiographical memory dysfunction, which is frequently reported with this disorder (mainly over general memory recollection), Kohler et al. suggested that manipulation of the reconsolidation of autobiographical memories might represent a novel therapeutic approach for depression treatment [39]. This could partially explain the significant decrease in BDI-II scores highlighted in our study.
Propranolol appears to be more effective in decreasing PTSD and comorbid depression symptoms in patients with severe PTSD scores prior to treatment. Given the high and positive correlation between CSF norepinephrine levels and the severity of PTSD symptoms [40], one possible explanation is that the reactivation of traumatic memory in a patient with severe PTSD may result in the release of a higher level of norepinephrine than in traumatic memory reactivation in a patient with non-severe PTSD.
This study has several limitations. Our hypothesis concerning the results is that blocked traumatic memory reconsolidation could in turn lead to symptom reduction. However, as propranolol reaches its peak systemic concentration 75 min after ingestion, this medication was given before reactivation and we did not have the necessary controls (such as preservation of post-reactivation short-term memory) to prove that memory reconsolidation blockade was the active mechanism. The long-term efficacy of such interventions and continued gains following termination have not yet been assessed. Further studies should include long-term follow-up of at least 6-month duration. It seems very difficult to strictly analyse the effect of propranolol and the effect of human interactions associated with this type of protocol separately. Artificially and theoretically, we could design a protocol without any human intervention. However, such total dehumanisation in the management of patients suffering from a mental disorder would be incongruent and unethical.
Conclusion
This study showed that the traumatic script reactivation procedure is effective, since in all patients, we obtained effect sizes comparable to those observed in trauma-focused therapies and greater than those associated with pharmacological treatments. However, at the end of the treatment we did not find propranolol to be more effective than placebo, which was the main purpose of this study. Our secondary objective was to examine changes in the PCL-S and BDI scores 3 months after treatment had ended. These scores stabilised well but this seemed to depend on the level of severity of PTSD at baseline. Although this was only an exploratory objective, stabilised clinical improvement in the propranolol group did not differ from that observed in the placebo group in patients with non-severe PTSD, whereas propranolol seemed effective in stabilising improvement in patients with severe PTSD.
In addition, some placebo patients showed marked improvement in their symptomatology, which was comparable to the improvement observed in the propranolol group. These results not only show that the procedure with reactivation and propranolol seems to be effective, but that the same reactivation procedure with placebo can also be effective. It is important to note that these new results are not entirely “negative findings” as both procedures tested demonstrate a strong decrease in the PCL-S score during treatment sessions and allow remission in many patients. These innovative results suggest interesting perspectives in terms of understanding the mechanisms underlying these procedures as well as open clinical perspectives. In fact, these results could indicate that effective reactivation of traumatic memory can lead to total destabilisation of memory traces of the traumatic event as has already been demonstrated in animal research [14, 41]. It seems essential and perhaps even sufficient to achieve remission in case of non-severe PTSD. This assumption is purely exploratory and requires further investigation in order to conclude whether or not the level of intensity of PTSD and depressive symptoms at baseline could affect the therapeutic response. However, if this hypothesis is verified, the results of this study open up very interesting clinical perspectives. In fact, two therapeutic procedures could be proposed: for severe PTSD, therapy with propranolol and brief intense reactivation of traumatic memory, and for less severe PTSD, the same procedure without propranolol and without the potential of frequent adverse effects (bradycardia, insomnia, nightmares, etc.). In the latter case, this would allow rapid and inexpensive non-medical management and could benefit patients with contraindications to propranolol (asthma, etc.) or those who refuse any pharmacological treatment.
Funding and disclosure
None of the authors reported any financial interests or potential conflicts of interest. This work was supported by a grant from the French Ministry of Health’s Hospital Program for Clinical Research. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or report compilation. The corresponding author had full access to all study data and was responsible for deciding whether or not to submit this paper for publication.
References
Yin Q, Sun Z, Liu T, Ni X, Deng X, Jia Y, et al. Posttraumatic stress symptoms of health care workers during the corona virus disease 2019. Clin. Psychol. Psychother. https://doi.org/10.1002/cpp.2477(2020).
Guo Q, Zheng Y, Shi J, Wang J, Li G, Li C, et al., Immediate psychological distress in quarantined patients with COVID-19 and its association with peripheral inflammation: a mixed-method study. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2020.05.038(2020).
Tran K, Moulton K, Santesso N, Rabb D, CADTH Health Technology Assessments, Cognitive processing therapy for post-traumatic stress disorder: a systematic review and meta-analysis. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2016.
Kline AC, Cooper AA, Rytwinksi NK, Feeny NC. Long-term efficacy of psychotherapy for posttraumatic stress disorder: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2018;59:30–40.
Ipser JC, Stein DJ. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD). Int J Neuropsychopharmacol. 2012;15:825–40.
Asnis GM, Kohn SR, Henderson M, Brown NL. SSRIs versus non-SSRIs in post-traumatic stress disorder: an update with recommendations. Drugs. 2004;64:383–404.
Krystal JH, Davis LL, Neylan TC, M AR, Schnurr PP, Stein MB, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82:e51–e59.
Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19:6623–8.
Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7:73–84.
Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–6.
Brunet A, Poundja J, Tremblay J, Bui E, Thomas E, Orr SP, et al. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J Clin Psychopharmacol. 2011;31:547–50.
Debiec J, Bush DE, LeDoux JE. Noradrenergic enhancement of reconsolidation in the amygdala impairs extinction of conditioned fear in rats-a possible mechanism for the persistence of traumatic memories in PTSD. Depression Anxiety. 2011;28:186–93.
Strawn JR, Geracioti TD Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depression Anxiety. 2008;25:260–71.
Villain H, Benkahoul A, Drougard A, Lafragette M, Muzotte E, Pech S et al. Effects of propranolol, a beta-noradrenergic antagonist, on memory consolidation and reconsolidation in mice. Front Behav Neurosci. 2016;10:49.
Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–8.
Lonergan MH, Olivera-Figueroa LA, Pitman RK, Brunet A. Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: a meta-analysis. J psychiatry Neurosci. 2013;38:222–31.
Lonergan M, Saumier D, Tremblay J, Kieffer B, Brown TG, Brunet A. Reactivating addiction-related memories under propranolol to reduce craving: a pilot randomized controlled trial. J Behav Ther Exp Psychiatry. 2016;50:245–9.
Soeter M, Kindt M. An abrupt transformation of phobic behavior after a post-retrieval amnesic agent. Biol Psychiatry. 2015;78:880–6.
Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–6.
Brunet A, Ashbaugh AR, Saumier D, Nelson M, Pitman RK, Tremblay J, et al. Does reconsolidation occur in humans: a reply. Front Behav Neurosci. 2011;5:74
Wood NE, Rosasco ML, Suris AM, Spring JD, Marin MF, Lasko NB, et al. Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: three negative psychophysiological studies. Psychiatry Res. 2015;225:31–39.
Brunet A, Saumier D, Liu A, Streiner DL, Tremblay J, Pitman RK. Reduction of PTSD symptoms with pre-reactivation propranolol therapy: a randomized controlled trial. Am J Psychiatry. 2018;175:427–33.
Estévez-Lamorte N, Pitzurra R, Foster S, Gmel G, Mohler-Kuo M, Schnyder U. Exposure to potentially traumatic events in young Swiss men: associations with socio-demographics and mental health outcomes (alcohol use disorder, major depression and suicide attempts). Eur J Psychotraumatol. 2019;10:1611093.
Elhai JD, Grubaugh AL, Kashdan TB, Frueh BC. Empirical examination of a proposed refinement to DSM-IV posttraumatic stress disorder symptom criteria using the National Comorbidity Survey Replication data. J Clin Psychiatry. 2008;69:597–602.
El-Hage W, Birmes P, Jehel L, Ferreri F, Benoit M, Vidailhet P et al. Improving the mental health system for trauma victims in France. Eur J Psychotraumatol. 2019;10:1617610.
First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders, research version, non-patient edition (SCID-I/NP). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 2002.
Ventureyra VA, Yao SN, Cottraux J, Note I, De Mey-Guillard C. The validation of the Posttraumatic Stress Disorder Checklist Scale in posttraumatic stress disorder and nonclinical subjects. Psychother Psychosom. 2002;71:47–53.
First M, Spitzer R, Gibbon M. Structured clinical interview for DSMIV (Axis I Disorders): Nonpatient edition. New York, NY: Biometrics Research Department, NY State Psychiatric Institute; 1995.
Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Personal Assess. 1996;67:588–97.
Hirschfeld RM, Montgomery SA, Aguglia E, Amore M, Delgado PL, Gastpar M et al. Partial response and nonresponse to antidepressant therapy: current approaches and treatment options, The. J Clin Psychiatry. 2002;63:826–37.
Mueller HS, Ayres SM. Propranolol decreases sympathetic nervous activity reflected by plasma catecholamines during evolution of myocardial infarction in man. J Clin Investig. 1980;65:338–46.
Mueller HS, Ayres SM. Propranolol decreases sympathetic nervous activity reflected by plasma catecholamines during evolution of myocardial infarction in man. J Clin Investig. 1980;65:338–46.
Hoffman BB. Cathecolamines, sympathomimetic drugs and adrenergic receptor antagonists. In: Brunton L, editor. Goodman & Gilman’s: the pharmacological basis of therapeutics. New York: McGraw-Hill; 2001, p. 215–68.
Dey M, Brisson J, Davis G, Enever R, Pray K, Zaim B, et al. Relationship between plasma propranolol concentration and dose in young, healthy volunteers. Biopharm Drug Dispos. 1986;7:103–11.
Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005;162:214–27.
Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–95.
Roley ME, Claycomb MA, Contractor AA, Dranger P, Armour C, Elhai JD. The relationship between rumination, PTSD, and depression symptoms. J Affect Disord. 2015;180:116–21.
Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–77.
Köhler CA, Carvalho AF, Alves GS, McIntyre RS, Hyphantis TN, Cammarota M. Autobiographical memory disturbances in depression: a novel therapeutic target? Neural Plast. 2015;2015:759139.
Geracioti TD,Jr., Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–30.
De Jaeger X, Courtey J, Brus M, Artinian J, Villain H, Bacquie E, et al. Characterization of spatial memory reconsolidation. Learn Mem. 2014;21:316–24.
Acknowledgements
The authors would like to thank the CIC of Toulouse University Hospital and all the patients who participated in this trial.
Author information
Authors and Affiliations
Contributions
PR and PB had full access to all study data and assume responsibility for the integrity and accuracy of the data analysis. Study concept and design: PR, PL, CT, PB, statistical analysis: PR, CT, LJ, PB, article compilation: PR, PB, AY, critical revision of the article for key intellectual content: All authors. Final approval of the version to be published: all authors.
Corresponding author
Ethics declarations
Ethical approval
Participants received detailed information about the study and gave their written informed consent to participate. All procedures were reviewed and approved by institutional review boards (ClinicalTrials.gov identifier: NCT01713556).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roullet, P., Vaiva, G., Véry, E. et al. Traumatic memory reactivation with or without propranolol for PTSD and comorbid MD symptoms: a randomised clinical trial. Neuropsychopharmacol. 46, 1643–1649 (2021). https://doi.org/10.1038/s41386-021-00984-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-00984-w