Abstract
Tourette’s Disorder (TD) is characterized by tics that cause distress and impairment. While treatment guidelines recommend behavior therapy as a first-line intervention, patients with TD may exhibit limited therapeutic response. Given the need to improve treatment outcomes, this study examined the efficacy of augmenting behavior therapy with d-cycloserine (DCS) to reduce tic severity in a placebo-controlled quick-win/fast-fail trial. Twenty youth with TD completed a baseline assessment to characterize tic severity, premonitory urges, medical history, and psychiatric comorbidity. Youth were randomly assigned to receive a single session of habit reversal training (HRT) augmented by either 50 mg of DCS or placebo. Two bothersome tics on the Hopkins Motor/Vocal Tic Scale (HM/VTS) were targeted for treatment during HRT. One week after the HRT session, youth completed a posttreatment assessment to evaluate change in the severity of bothersome tics. All assessments were completed by independent evaluators masked to treatment group. There was a Treatment Group by Time Interaction in favor of DCS-augmented HRT (p < 0.01), controlling for baseline tic severity, tic medication, and attention deficit hyperactivity disorder. Follow-up comparisons revealed small group differences at the treatment visit (d = 0.27), with the DCS group exhibiting slightly greater severity for targeted tics. There was a large group difference at posttreatment, in which the DCS group exhibited lower severity for targeted tics (d = 1.30, p < 0.001) relative to the placebo group. Findings demonstrate the preliminary enhancement of tic severity reductions by augmenting HRT with DCS compared with placebo augmentation.
Similar content being viewed by others
Introduction
Tourette’s Disorder and persistent tic disorders (collectively referred to as TD) are neuropsychiatric conditions characterized by recurrent motor and/or vocal tics. For many patients with TD, the severity of tics causes impairment and contributes to a poor quality of life [1]. Behavior therapy has shown considerable efficacy for reducing tic severity [2], which has led to its recommendation as a first-line treatment across professional organizations [3, 4]. However, evidence suggests that only 50% of youth with TD exhibit a positive treatment response and many treatment responders continue to experience bothersome tics [5]. Thus, it is critical to investigate strategies that improve therapeutic outcomes from behavior therapy for patients with TD.
Behavioral therapies like habit reversal training (HRT) and comprehensive behavioral intervention for tics recognize the neurological origin of tics, but highlight that tic expression is influenced by internal and external factors [6]. Prominently, many patients with TD experience aversive premonitory urges that are temporarily relieved by the expression of tics. Consequently, this urge-tic association becomes negatively reinforced and strengthened due to the reduction of premonitory urge distress. This model receives empirical support from experimental studies [7], tic suppression studies [8], and provides evidence that tic expression is influenced via associative learning and reward learning processes.
In behavior therapy, treatment emphasizes building awareness to early tic warning signs like premonitory urges (awareness training) and implementing competing responses contingent upon identified early tic warning signs (competing response training), which prevents tic expression and its associated urge relief. These therapeutic exercises facilitate the formation of a new learned association (urge-competing response) to both inhibit the initial tic association (urge-tic response) and discontinue the negative reinforcement cycle between tic expression and urge reduction. Therefore, treatment strategies that strengthen the formation of new learned associations (i.e., urge-competing response) may make behavioral therapies more effective at inhibiting the urge-tic association and reduce the severity of tics.
One approach to strengthen associative learning during treatment involves pairing therapeutic skills with cognitive enhancers. Translational research suggests that cognitive enhancers like d-cycloserine (DCS) strengthen newly learned associations in animal studies [9, 10] and show promise to improve treatment outcomes when combined with cognitive behavioral therapy (CBT) [10,11,12]. While prior studies have focused on enhancing therapeutic outcomes in anxiety disorders, post-traumatic stress disorder, and obsessive-compulsive disorder (OCD) [10,11,12], the combination of DCS and behavior therapy may efficiently strengthen the formation of therapeutic associations (urge-competing response) to inhibit initial associations (urge-tic associations) to reduce the severity of tics.
We examined the efficacy of augmenting behavior therapy with DCS in a randomized controlled quick-win/fast-fail trial for youth with TD. Given the focus on individual tic associations, our a priori outcome was the reduction in tic severity for bothersome tics targeted in behavior therapy. We also explore reductions in tic severity for all bothersome tics, and reductions in premonitory urge severity.
Methods
Participants
Twenty youth were enrolled in the parallel group randomized controlled trial. To participate youth had to be: 8–17 years old; have TD or a Persistent Motor Tic Disorder; a total tic severity score ≥14 on the Yale Global Tic Severity Scale (YGTSS; or ≥10 for youth with only motor or vocal tics); and medication free or taking a stable dose of psychiatric medication for at least 6 weeks. Youth were excluded from participation if they had any of the following: a medical condition contraindicated for DCS; a lifetime diagnosis of schizophrenia, bipolar disorder, psychosis, or autism spectrum disorder; greater than four sessions of behavior therapy; or were taking an antipsychotic medication. Participants were predominantly male, White, and exhibited moderate tic severity on the YGTSS (see Table 1). Most youth had a diagnosis of TD, and 35% were taking an alpha-agonist. Co-occurring psychiatric conditions included ADHD, anxiety disorders, and oppositional defiant disorder (see Table 1).
Measures
Yale Global Tic Severity Scale (YGTSS) [13]
The YGTSS is a clinician-administered scale that measures global tic severity. It has excellent psychometric properties of reliability and validity [13,14,15].
Hopkins Motor Vocal Tic Scale (HM/VTS) [16]
Participants nominated up to five motor and five vocal bothersome tics at baseline and these tics were reassessed at the treatment and posttreatment visits. Clinicians rated the severity of bothersome tics on a five-point scale: (0) none, (1) mild, (2) moderate, (3) moderate–severe, and (4) severe. Ratings incorporated the frequency, forcefulness, interference, and distress of each tic. The HM/VTS has demonstrated good reliability, validity, and treatment sensitivity to behavior therapy [16, 17].
Individualized Premonitory Urge for Tics Scale (I-PUTS) [18]
The I-PUTS is a clinician-administered measure that assesses the frequency and intensity of premonitory urges for individual tics. After identifying the presence of an urge for each tic, raters inquire about the frequency (1 = “Urge occurs 0–25% of the time” to 4 = “Urge occurs 75–100% of the time”) and intensity (1 = “Minimal Intensity” to 4 = “Strong intensity”) of each endorsed urge on a four-point scale. Items are summed to create a total urge frequency (I-PUTS Frequency) and intensity (I-PUTS Intensity) score [18].
Session summary sheet
Therapists completed a session summary sheet that included information about the fidelity of HRT delivered for the two tics that were targeted for treatment on the HM/VTS. Following the instruction of HRT skills, therapists were asked to rate participants’ ability to implement the identified competing response contingent upon early tic detection (e.g., premonitory urge, early tic movements) for each tic on a Likert-type scale that ranged from poor (1) to excellent (7). Therapists also assigned formal practice of HRT skills following the therapy session, and collected parent report of the formal practice time completed over the intervening week between the treatment session and posttreatment visit.
Procedures
Study procedures were approved by the local institutional review board, and the trial was registered on ClinicalTrials.gov prior to initiating enrollment (NCT02582515). The study took place at the University of California Los Angeles from October 2015 to August 2017. Study enrollment was anticipated to be 20 participants during the recruitment period. This would provide adequate power (power = 0.80) to detect medium-sized effects, but would have less power to detect smaller effects. After obtaining consent, participants completed a baseline assessment to characterize medical history, psychiatric diagnoses, global tic severity (YGTSS), bothersome tic severity (HM/VTS), and urge severity (I-PUTS). One week later, participants completed the treatment visit. During this visit, bothersome tics severity (HM/VTS) and premonitory urge severity (I-PUTS) were reassessed. Next, participants were randomly assigned to receive 50 mg of DCS or placebo which was taken immediately. The randomization sequence was created using a random number generator with a 1:1 allocation, and only the research assistant and study pharmacist were aware of participants’ randomization assignment. Afterward, participants received psychoeducation about TD and the neurobehavioral treatment model underlying behavior therapy. Following procedures comparable with other DCS studies [19], HRT procedures were initiated 1 h after ingesting DCS/placebo. Two bothersome tics on the HM/VTS were targeted for treatment during this 1-hour HRT session. Therapists’ rated the participants’ ability to implement HRT skills for the two bothersome tics targeted for treatment on the session summary sheet. One week after the treatment visit, participants returned for the posttreatment assessment to determine the occurrence of any adverse events using a standardized form, inquire about the amount of time spent formally practicing HRT skills, and evaluate reductions in bothersome tic severity (HM/VTS) and premonitory urge severity (I-PUTS). Participants, parents, therapists, and independent evaluators who administered study assessments (i.e., YGTSS, HM/VTS, and I-PUTS) were all masked to treatment assignment.
Analytic plan
T-tests and chi-square tests were performed to evaluate baseline group differences between the DCS and placebo groups. Next, participants’ abilities to implement HRT skills for targeted tics and amount of time spent formally practicing HRT skills were compared between treatment groups. The primary outcome was the change in tic severity for the two bothersome tics nominated for treatment on the HM/VTS (range: 0–8). In addition, we evaluated the change in tic severity for all bothersome tics nominated on the HM/VTS (range: 0–40). Finally, we explored whether group differences emerged for urge frequency and urge intensity on the I-PUTS. The tic severity ratings for bothersome tics (HM/VTS) and premonitory urges (I-PUTS) were examined using a fixed-effects repeated-measures analysis that controlled for baseline tic severity, co-occurring ADHD and alpha-agonist medication, which have been found to influence tic severity outcomes in behavior therapy [2, 20]. The Greenhouse–Geisser correction for sphericity was applied. Our model included one between-group factor (DCS versus placebo), tic severity, co-occurring ADHD and tic medication were entered as covariates, and the repeated measure was time (treatment and posttreatment).
Results
Baseline characteristics
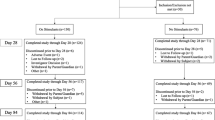
Table 1 presents baseline characteristics for the DCS and placebo groups. There were no differences between groups on demographic or clinical characteristics. One participant in the placebo group dropped out prior to the treatment visit (5% attrition, see Fig. 1), which is comparable with prior behavior therapy studies of youth with TD [5].
Implementation of HRT
Participants demonstrated good abilities to implement competing responses contingent upon early tic detection for tics targeted in the HRT session (M = 5.18, SD = 0.99) and reported some practice of HRT skills between the treatment and posttreatment visit (Mminutes = 117.36, SDminutes = 63.05). There was no significant difference between treatment groups on HRT implementation abilities for tics targeted in treatment (t = 0.16, p = 0.88, d = 0.07) or amount of time dedicated to formal HRT practice between the treatment and posttreatment visits (t = 0.46, p = 0.66, d = 0.25).
Tic severity reductions for tics targeted in behavior therapy
There was a significant Treatment Group by Time Interaction for tics targeted in behavior therapy (see Table 2). Follow-up comparisons revealed small differences between groups at the treatment visit, but large differences at the posttreatment visit in favor of the DCS group (d = 1.28, see Table 3 and Fig. 2).
Tic severity reductions for all bothersome tics
The Treatment Group by Time Interaction for all bothersome tics did not reach statistical significance (p = 0.07, see Table 2), but displayed a similar pattern to the targeted tic analyses. Specifically, small groups differences were identified at the treatment visit, with larger group differences found at the posttreatment visit in favor of the DCS group (d = 0.88, see Table 3).
Premonitory urge frequency and intensity
The Treatment Group by Time interaction was significant for premonitory urge frequency. Follow-up comparisons revealed small group differences at the treatment visit, but large differences at posttreatment in favor of the DCS group (d = 1.07, see Table 3). Meanwhile, the Treatment Group by Time interaction for urge intensity was not statistically significant (p = 0.13, Table 2), but is qualified by a main effect for treatment group that shows group level differences across time points (p = 0.02). While there was a moderate-to-large difference between treatment groups at the treatment visit (d = 0.76), the DCS group exhibited a decline in urge intensity by the posttreatment visit whereas the placebo group did not (see Table 3). This resulted in a large group difference at the posttreatment visit (d = 1.47), which was nearly twice the difference observed at the treatment visit.
Adverse effects
Three participants in the DCS group reported adverse effects in the past week, which appeared unrelated to DCS. These included drowsiness (n = 1), difficulty falling asleep (n = 1), and a sore throat (n = 1). No adverse events reported in the placebo group.
Discussion
This study provides initial evidence that augmenting HRT with DCS produces greater severity reductions for tics targeted in treatment, compared with augmentation with placebo. A similar pattern was observed for all bothersome tics, but did not achieve statistical significance.
While findings highlight the possibility for enhancing tic severity reductions, this trial only used a single session of HRT. Therefore, a larger multi-session clinical trial is critical to determine the optimum number of DCS-augmented behavior therapy sessions and evaluate the durability of its therapeutic effect. While early pilot studies demonstrated similar promising effects for DCS augmentation in related conditions like OCD [21], later definitive trials did not find these effects [22]. However, there are some notable distinctions between these two conditions in regards to the efficacy of evidence-based treatments. Specifically, therapeutic outcomes are fairly large for exposure-based CBT with or without DCS [22, 23]. As a result, there is limited room to enhance current treatment outcomes to achieve clinical and/or statistical significance. In comparison, only 50% of youth with TD exhibit a positive treatment response and many treatment responders continue to experience bothersome tics [5, 17]. Indeed, as a treatment response in TD is associated with a 25–35% reduction in tic severity [24, 25], there is clear room to detect and enhance therapeutic outcomes.
The mechanism by which tic severity was reduced in this study is an important consideration. Evidence suggests that DCS does not broadly enhance motor learning [26] nor do glutamatergic modulators like DCS directly reduce tic severity [27]. In behavior therapy, patients develop new compensatory association (e.g., urge-competing response) to inhibit prior associations that maintain tic expression (e.g., urge-tic). It is likely that the compensatory associations learned in HRT are strenghtened when developed in the presence of DCS compared with placebo. This type of associative learning in which one association inhibits another association—extinction learning—would result in the greater inhibition of tic expression and reductions in tic severity, which was observed in this study. However, the related process of reversal learning may also play a role. Broadly, reversal learning is the ability to actively suppress reward-related responding to disengage an ongoing behavior and adapt to a new reward contingency. In behavior therapy, participants learn to disengage from expressing tics that alleviate premonitory urges (i.e., disengage from an ongoing behavior) and implement a new compensatory behavior to inhibit tic expression that is subsequently positively reinforced (i.e., adapting to a new reward contingency). Indeed, evidence from preclinical studies suggest that DCS may enhance both extinction learning and reversal learning processes [10, 28, 29].
While the specific mechanism requires further research, the learned associations acquired in HRT serve to disrupt the negative reinforcement cycle that maintains tic expression. In doing so, the frequency and intensity of premonitory urges becomes diminished over time and the cycle maintaining tic expression discontinued. This is supported by observations that the DCS group exhibited greater reductions in urge frequency relative to those in the placebo group, with a similar pattern observed for urge intensity that did not achieve statistical significance due to group level differences. A multi-session clinical trial can test the mechanisms (e.g., extinction learning, reversal learning) that are enhanced by DCS during behavior therapy and result in reduced tic severity and premonitory urge severity.
While this quick-win/fast-fail trial has several strengths (e.g., quadruple blinded study), a few limitations are present. First, youth on antipsychotic medications were excluded from participation. Antipsychotic and alpha-agonist medications are common pharmacological interventions to reduce tic severity [3]. However, concurrent antipsychotic medications were excluded to minimize potential confounds, as prior studies have shown that DCS has some interactive effects when administered alongside antipsychotic medications in adults [30, 31]. No such interactive effects were identified for alpha-agonists, which were permitted for inclusion to improve the generalizability of findings to clinical care. Future research should include youth on antipsychotic medications and incorporate medication status into randomization assignment. Second, this trial only utilized a single session of HRT and administered DCS 1 h prior to HRT procedures. Future research should test these promising findings in a multi-session HRT protocol and consider two key aspects of DCS dosing. Specifically, recent evidence shows that the therapeutic effects of DCS level off after about nine dose administrations and participants who received DCS doses more than 60 min before initiating treatment procedures exhibited greater therapeutic effects compared with DCS dose administrations 60 min or earlier [19]. Therefore, when designing and implementing a multi-session clinical trial, it will be important to have treatment be completed in fewer than nine sessions and administer DCS at an optimal time prior to initiating HRT. Third, we utilized the HM/VTS as the primary outcome measure. Although making conceptual sense due to the focus of HRT skills on specific bothersome tics, the scale has received minimal psychometric examination in comparison with gold standard scales like the YGTSS [13]. Furthermore, we did not include direct observation protocols of tic severity. Future research should examine changes in tic severity utilizing a multi-modal assessment approach that includes bothersome tics (HM/VTS), global tic severity (YGTSS), and even tic observation protocols in a multi-session clinical trial. Finally, the sample is predominantly male and White. However, this is comparable with other clinical trials of behavior therapy for youth with TD [5].
In summary, augmentation of behavior therapy with DCS reduced tic severity to a greater degree compared with placebo augmentation and was well-tolerated. The evaluation of DCS in a multi-session behavioral treatment trial is needed to determine the precise mechanism (e.g., associative learning, reward learning) and duration of augmentation over multiple sessions to optimize behavior therapy outcomes for patients with TD.
Funding and disclosure
NG and KR report having nothing to disclose. JFMG reports having received research or grant support from the Tourette Association of America, American Academy of Neurology, the Brain Research Foundation, American Psychological Foundation, and the Hilda and Preston Davis Family Foundation. He has received royalties from Elsevier, and has served as a consultant for Signant Health, Syneos Health, and Luminopia. JKYE reports having received grant support from the Tourette Association of America. EJR reports having received research or grant support from the Tourette Association of America and the National Institute of Health. JTMC has received research or grant support from National Institutes of Health, Seaside Therapeutics, Roche, and Otsuka. He has served as a consultant to BioMarin and PharmaNet. JP has received grant or research support from the National Institute of Mental Health, Pfizer Pharmaceuticals through the Duke University Clinical Research Institute CAPTN Network, Psyadon Pharmaceuticals, and the Tourette Association of America. He has received financial support from the Petit Family Foundation and the Tourette Syndrome Association Center of Excellence Gift Fund. He has received royalties from Guilford Press and Oxford University Press. He has served on the speakers’ bureau of the TAA, the International Obsessive-Compulsive Disorder Foundation (IOCDF), and the Trichotillomania Learning Center (TLC).
References
Conelea CA, Woods DW, Zinner SH, Budman C, Murphy T, Scahill LD, et al. Exploring the impact of chronic tic disorders on youth: results from the Tourette syndrome impact survey. Child Psychiatry Hum Dev. 2011;42:219–42.
McGuire JF, Piacentini J, Brennan EA, Lewin AB, Murphy TK, Small BJ, et al. A meta-analysis of behavior therapy for Tourette Syndrome. J Psychiatr Res. 2014;50:106–12.
Murphy TK, Lewin AB, Storch EA, Stock S. Practice parameter for the assessment and treatment of children and adolescents with tic disorders. J Am Acad Child Adolesc Psychiatry. 2013;52:1341–59.
Pringsheim T, Holler-Managan Y, Okun MS, Jankovic J, Piacentini J, Cavanna AE, et al. Comprehensive systematic review summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology 2019;92:907–15.
Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA. 2010;303:1929–37.
Conelea CA, Woods DW. The influence of contextual factors on tic expression in Tourette’s syndrome: a review. J Psychosom Res. 2008;65:487–96.
Beetsma DJV, van den Hout MA, Engelhard IM, Rijkeboer MM, Cath DC. Does repeated ticking maintain tic behavior? An experimental study of eye blinking in healthy individuals. Behav Neurol. 2014;2014:1–7.
Capriotti MR, Brandt BC, Turkel JE, Lee H-J, Woods DW. Negative reinforcement and premonitory urges in youth with Tourette syndrome: an experimental evaluation. Behav Modif. 2014;38:276–96.
Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with d-cycloserine facilitates learning and retention. Eur J Pharmacol. 1994;257:7–12.
Norberg MM, Krystal JH, Tolin DF. A meta-analysis of d-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–26.
McGuire JF, Wu MS, Piacentini J, McCracken JT, Storch EA. A meta-analysis of d-cycloserine in exposure-based treatment: moderators of treatment efficacy, response, and diagnostic remission. J Clin Psychiatry. 2017;78:196–206.
Mataix-Cols D, Cruz LF, de la, Monzani B, Rosenfield D, Andersson E, Pérez-Vigil A, et al. d-cycloserine augmentation of exposure-based cognitive behavior therapy for anxiety, obsessive-compulsive, and posttraumatic stress disorders: a systematic review and meta-analysis of individual participant data. JAMA Psychiatry. 2017;74:501–10.
Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–73.
Storch EA, Murphy TK, Geffken GR, Sajid M, Allen P, Roberti JW, et al. Reliability and validity of the Yale Global Tic Severity Scale. Psychol Assess. 2005;17:486–91.
McGuire JF, Piacentini J, Storch EA, Murphy TK, Ricketts EJ, Woods DW, et al. A multicenter examination and strategic revisions of the Yale Global Tic Severity Scale. Neurology. 2018;90:e1711–e1719.
Walkup JT, Rosenberg LA, Brown J, Singer HS. The validity of instruments measuring tic severity in Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1992;31:472–7.
McGuire JF, Piacentini J, Scahill L, Woods DW, Villarreal R, Wilhelm S, et al. Bothersome tics in patients with chronic tic disorders: characteristics and individualized treatment response to behavior therapy. Behav Res Ther. 2015;70:56–63.
McGuire JF, McBride N, Piacentini J, Johnco C, Lewin AB, Murphy TK, et al. The premonitory urge revisited: an individualized premonitory urge for tics scale. J Psychiatr Res. 2016;83:176–83.
Rosenfield D, Smits JAJ, Hofmann SG, Mataix-Cols D, de la Cruz LF, Andersson E, et al. Changes in dosing and dose timing of d-cycloserine explain Its apparent declining efficacy for augmenting exposure therapy for anxiety-related disorders: an individual participant-data meta-analysis. J Anxiety Disord. 2019;68:102149.
Sukhodolsky DG, Woods DW, Piacentini J, Wilhelm S, Peterson AL, Katsovich L, et al. Moderators and predictors of response to behavior therapy for tics in Tourette syndrome. Neurology. 2017;88:1029–36.
Storch EA, Murphy TK, Goodman WK, Geffken GR, Lewin AB, Henin A, et al. A preliminary study of d-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry 2010;68:1073–6.
Storch EA, Wilhelm S, Sprich S, Henin A, Micco J, Small BJ, et al. Efficacy of augmentation of cognitive behavior therapy with weight-adjusted d-cycloserine vs placebo in pediatric obsessive-compulsive disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73:779–88.
McGuire JF, Piacentini J, Lewin AB, Brennan EA, Murphy TK, Storch EA. A meta-analysis of cognitive behavior therapy and medication for child obsessive–compulsive disorder: moderators of treatment efficacy, response, and remission. Depress Anxiety. 2015;32:580–93.
Storch EA, De Nadai AS, Lewin AB, McGuire JF, Jones AM, Mutch PJ, et al. Defining treatment response in pediatric tic disorders: a signal detection analysis of the Yale Global Tic Severity Scale. J Child Adolesc Psychopharmacol. 2011;21:621–7.
Jeon S, Walkup JT, Woods DW, Peterson A, Piacentini J, Wilhelm S, et al. Detecting a clinically meaningful change in tic severity in Tourette syndrome: a comparison of three methods. Contemp Clin Trials. 2013;36:414–20.
Günthner J, Scholl J, Favaron E, Harmer CJ, Johansen-Berg H, Reinecke A. The NMDA receptor partial agonist d-cycloserine does not enhance motor learning. J Psychopharmacol 2016;30:994–9.
Lemmon ME, Grados M, Kline T, Thompson CB, Ali SF, Singer HS. Efficacy of glutamate modulators in tic suppression: a double-blind, randomized control trial of D-serine and riluzole in Tourette syndrome. Pediatr Neurol. 2015;52:629–34.
George SA, Rodriguez-Santiago M, Riley J, Abelson JL, Floresco SB, Liberzon I. D-cycloserine facilitates reversal in an animal model of post-traumatic stress disorder. Behav Brain Res. 2018;347:332–8.
Fitzgerald PJ, Seemann JR, Maren S. Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Res Bull. 2014;105:46–60.
Heresco-Levy U, Ermilov M, Shimoni J, Shapira B, Silipo G, Javitt DC. Placebo-controlled trial of d-cycloserine added to conventional neuroleptics, olanzapine, or risperidone in schizophrenia. Am J Psychiatry. 2002;159:480–2.
van Berckel B, Evenblij CN, van Loon B, Maas MF, van der Geld M, Wynne HJ, et al. D-cycloserine increases positive symptoms in chronic schizophrenic patients when administered in addition to antipsychotics: a double-blind, parallel, placebo-controlled study. Neuropsychopharmacology. 1999;21:203–10.
Acknowledgements
This work was supported in part by grants from the Tourette Association of American (JFMG, JKYE), American Academy of Neurology (JFMG), American Psychological Foundation (JFMG), and NIMH T32MH073517 (JTMC and JP). The views expressed within this article represent those of the authors, were not influenced by the funding sources, and are not intended to represent the position of the NIMH.
Author information
Authors and Affiliations
Contributions
JFMG, JTMC, and JP contributed to the design of the study. JFMG, NG, KR, JKYE, EJR, JTMC, and JP contributed to the acquisition, analysis, and/or interpretation of the data for the work. JFMG, JTMC, and JP composed the initial draft of the work, and NG, KR, JKYE, and EJR contributed revisions and important intellectual content to the work. All authors approved the version of the paper, and agree to be accountable for all aspects of the work to ensure that any questions related to the accuracy or integrity are appropriately investigated and resolved.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McGuire, J.F., Ginder, N., Ramsey, K. et al. Optimizing behavior therapy for youth with Tourette’s disorder. Neuropsychopharmacol. 45, 2114–2119 (2020). https://doi.org/10.1038/s41386-020-0762-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-020-0762-4
This article is cited by
-
Tourette Syndrome Treatment Updates: a Review and Discussion of the Current and Upcoming Literature
Current Neurology and Neuroscience Reports (2022)
-
European clinical guidelines for Tourette syndrome and other tic disorders—version 2.0. Part II: psychological interventions
European Child & Adolescent Psychiatry (2022)
-
Mechanisms Underlying Behavior Therapy for Tourette’s Disorder
Current Developmental Disorders Reports (2021)