Abstract
New treatment development for psychiatric disorders depends critically upon the development of physiological measures that can accurately translate between preclinical animal models and clinical human studies. Such measures can be used both as stratification biomarkers to define pathophysiologically homogeneous patient populations and as target engagement biomarkers to verify similarity of effects across preclinical and clinical intervention. Traditional “time-domain” event-related potentials (ERP) have been used translationally to date but are limited by the significant differences in timing and distribution across rodent, monkey and human studies. By contrast, neuro-oscillatory responses, analyzed within the “time-frequency” domain, are relatively preserved across species permitting more precise translational comparisons. Moreover, neuro-oscillatory responses are increasingly being mapped to local circuit mechanisms and may be useful for investigating effects of both pharmacological and neuromodulatory interventions on excitatory/inhibitory balance. The present paper provides a roadmap for development of neuro-oscillatory responses as translational biomarkers in neuropsychiatric treatment development.
Similar content being viewed by others
Introduction
New treatment development in psychiatry depends on the availability of biomarkers that permit translation between preclinical and clinical research. Event related potential (ERP)-based measures, such as auditory N1, mismatch negativity (MMN) or P300, have proven among the strongest biomarkers of brain dysfunction in schizophrenia [1, 2], but translational utility of these measures has been hampered by the significant differences in timing, polarity and scalp distribution across species. Here, we review the degree to which recent advances in the use of neuro-oscillatory approaches to analyze both human and rodent ERP data permits greater utility and rigor within translational research.
For ERPs, continuous electroencephalographic activity (EEG) is recorded along with the timing of “events,” such as auditory/visual stimuli or motor responses. The continuous EEG is then segmented into discrete epochs relative to the timing tags. In traditional ERP approaches, the epochs are then averaged within the “time domain,” giving rise to a series of positive and negative peaks that vary across time and scalp distribution. Peaks with consistent polarity, timing, scalp distribution and response to parametric manipulation (e.g. loudness, probability) are termed “components” (e.g. refs. [3, 4]). While effective, ERPs capture only a small portion of the information inherent in the EEG signal [5].
Oscillatory analyses of EEG/ERP data depend on the use of spectral decomposition or “time-frequency” (TF) analysis. In spectral analyses, data are analyzed as a function of amplitude (or power) over time within specific frequency-bands. While computationally more demanding than traditional ERP analyses, such approaches potentially provide greater insights into underlying physiological mechanisms, as well as greater ability to translate between human and animal studies in relationship to the oscillatory “connectome” [6]. Here we provide an overview of current translational usage of both time- and TF (“spectral”) approaches for the analysis of ERP data, as well as a roadmap for studies needed to further validate translational neuro-oscillatory approaches to support novel treatment development.
Computational aspects of neuro-oscillatory activity
Neuro-oscillatory activity is typically analyzed in discrete frequency-bands that were originally described based on empirical observation and are named in order of discovery. Thus, alpha (8–12 Hz) rhythms, which predominate over occipital brain regions during wakefulness, were first described in the 1920s based upon changes in amplitude during eyes-closed vs. eyes-open conditions [7]. Beta (12–24 Hz) rhythms were described shortly thereafter based upon pre-movement activity over motor cortex [8]; gamma (>24 Hz) rhythms based on “psychical activation” [9], delta (1–4 Hz) rhythms based on sleep [10]; and theta (4–7 Hz) rhythms based on psychomotor seizures [11] (Table 1).
Despite the empirical origins of these rhythms, ongoing research has reified these definitions and has begun to characterize the underlying neural mechanisms [12, 13]. Given the limited number of frequency-bands relative to the number of potential cellular/network generation mechanisms, it is clear that multiple mechanisms may contribute to activity within each band. Specific generator mechanisms may also cross traditional frequency-bands [14]. Nevertheless, current nomenclature permits efficient communication of results [13].
Evoked vs. single-trial spectral analysis
Within each frequency-band, several measures are obtained that offer complementary information regarding underlying generator mechanisms (Fig. 1). Specific measures obtained from TF analyses include (1) evoked-power, (2) single-trial power, (3) intertrial coherence (ITC) [15]—also termed phase-locking factor (PLF) [16, 17], and (4) event-related desynchronization (Fig. 1a, left). These measures all provide mechanistic information that is lost in traditional time-domain ERP approaches.
a Schematic diagram of processing scheme. Unshaded boxes represent time-domain measures. Shaded boxes represent time-frequency (TF) domain or “spectral” measures. For time-domain measures, random EEG activity and waves that are not time or phase-locked to event onsets do not survive signal averaging across epochs and are not captured in the ERP (see also Fig. 2). TF decomposition can be applied either to averaged time-domain files, yielding evoked-power analyses, or to the single-trials prior to averaging, yielding separate assessments of intertrial coherence (ITC) and total power (evoked + induced). Estimation of power from the single trial epochs preserves and quantifies all of the neuro-oscillatory activity present, irrespective of whether the oscillations elicited by the event are “evoked” or “induced”. Total ongoing power in humans tends to be dominated by ongoing alpha activity. Therefore, total power is typically baseline corrected to permit better visualization of stimulus-induced changes. b Cartoon of the recently developed interleaved visual presentation paradigm (“JH-Flkr”) showing initial stimulus onset at 0 msec, followed by motion onset at 600 msec and steady-state stimulation onset at 1400 msec. c Time-frequency plots for Evoked-Power, Intertrial coherence (ITC, “phase locking”), Ongoing (i.e. non-baseline corrected) single-trial Total Power and Baseline corrected total power. Note that the response evoked by stimulus onset occurs primarily in the theta frequency range (solid box) and is accompanied by alterations in both ITC and total power. By contrast, the response evoked by motion onset occurs primarily in the delta frequency range (dashed box) and is associated with alterations only in ITC but not total power, suggesting differential underlying local circuit mechanisms. Finally, the stimulus-induced alpha event-related desynchronization (ERD) (asterisk) is not associated with alterations in either evoked-power or ITC but appears only in single-trial power analyses. Adapted from ref. [61] .
Evoked-power (“power of the average”) represents the spectral decomposition of the traditional ERP into the TF-domain and permits differentiation of spectral components that are superimposed in the traditional ERP. Any of a number of TF decomposition approaches may be used, including fast Fourier transform (FFT) or Wavelet decomposition (e.g. “Morlets” [18]). For wavelets, the number of cycles (typically 3–6) is tailored to specific frequency-bands [19, 20].
Single-trial power (“average of the power”) is calculated by first decomposing each individual response into the TF-domain and then averaging the single trials (Fig. 1a, right). An additional measure, “induced power”, may be obtained by subtracting or regressing evoked-power from total power [21, 22], isolating components that are not phase-locked. As with evoked-power, total single-trial power is typically represented in µV² (or single-trial amplitude as µV),
Intertrial coherence (ITC, PLF) represents the consistency of responses across trials, and is represented as percentage (0–1), or percent (0–100%) coherence across trials. Traditional evoked responses may reflect either pure phase-resetting of ongoing oscillatory activity, pure added power, or a combination, which can only be determined by single-trial analyses [23, 24]. Moreover, these processes may be differentially mediated by driver vs. modulatory inputs from thalamus to cortex (e.g. [25]).
Event-related desynchronizations (ERD) represent a reduction in ongoing power following stimulus presentation. Conceptually, surface-recorded oscillatory activity is largest when individual local-circuit ensembles oscillate in-phase with each other, leading to summation at a distance [26]. By contrast, when ensembles desynchronize as occurs when individual ensembles are brought “on-line” for cognitive operations, the surface-recorded activity decreases. ERD are particularly relevant for alpha activity, which increases with eye-closure and is thought to represent “idling” of the visual system. Alpha amplitude is reduced tonically during visual attention (vs. rest) and phasically by individual stimuli, leading to a stimulus-driven alpha ERD [27, 28].
Examples of these processes are shown in Figs. 1 and 2. Figure 1b shows a paradigm in which visual responses are obtained both to the onset of a stimulus and its subsequent motion. In the traditional time-domain ERP both responses are represented simply as positive and negative peaks in the waveform (Fig. 1a). By contrast, in TF analyses, the two separate response types are resolved into different underlying frequency bands (Fig. 1c), suggesting that different local circuit ensemble types are involved. Similar representations are observed in evoked-power and ITC measures.
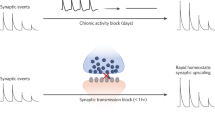
a Stimulus evoked phase-resetting of ongoing gamma oscillations. Pure phase-resetting occurs when a stimulus evokes a change in the phase of the ongoing oscillations without evoking a change in the magnitude of the oscillations. Because the phase of the stimulus evoked-oscillation is reset in a consistent manner across trials, the single trial oscillations survive averaging and are evident in the ERP. Note that these oscillations are not evident in the pre-stimulus ERP baseline because their random phase leads to their being cancelled out when epochs are averaged to generate the ERP. In contrast, the oscillations show strong phase synchrony across trials for the first 200 msec post-stimulus and are therefore evident after averaging trials to derive the ERP. b In pure phase-resetting by a stimulus, the phase of ongoing oscillations is reset by the stimulus in a consistent manner across trials, resulting in high intertrial coherence (ITC). In this scenario, the TF analysis shows prominent gamma band evoked-power and ITC. However, because the stimulus does not evoke a change in the magnitude of the ongoing oscillations, there is no appreciable total gamma power response to the stimulus once baseline correction is performed. c Stimulus induced increase in the power of ongoing gamma oscillations with no phase resetting. As with a, oscillations during the prestimulus baseline period do not survive averaging during the generation of the ERP. In contrast to A, the stimulus induces an increase in the magnitude of oscillations, but because the post-stimulus oscillations are not phase synchronized across trials, very little of this activity survives averaging across epochs when generating the ERP. d With a stimulus-induced increase in power, but oscillations with random phase across trials, there is a clear increase in total power, but no appreciable evoked power or intertrial coherence. Adapted from ref. [17].
Total power measures provide complementary information. For example, in addition to the evoked activity, stimulus presentation also desynchronizes ongoing alpha activity (Fig. 1c, asterisk), which is thought to reflect bringing the region “on-line”. This effect is further highlighted by correcting for baseline activity (Fig. 1d). Similarly, in gamma steady-state paradigms, presentation of auditory stimuli aligns ongoing gamma activity, leading to an increase in evoked-power and ITC. By contrast, stimulus-induced changes in gamma power that are not phase-locked will not appear in time-domain or evoked-power analyses and will only be detectable using single-trial measures (Fig. 2).
Significant coupling also occurs between frequencies. Typically, frequencies are hierarchically organized such that peaks of high-frequency activity occur at specific phases of the low-frequency cycle (“phase-amplitude coupling”) [29,30,31,32]. In general, oscillatory activity represents an interplay between excitatory glutamatergic pyramidal neurons in cortex and local circuit GABAergic interneurons, modulated by additional neurotransmitter systems, and thus may be particularly useful as indices of excitation/inhibition (E/I) balance across disorders [26, 33,34,35].
Clinical findings
The majority of clinical studies using spectral decomposition approaches have focused on gamma activity, which may also be derived from narrow-band filtering of the traditional ERP. More recent studies have explored the spectral content of validated ERP measures such as auditory MMN, visual P1 or task-related P300. Single-trial analysis studies have also investigated alpha and beta ERD as measures of task engagement during sensory and cognitive processing.
Gamma
Gamma deficits have been studied extensively in schizophrenia using both auditory steady-state response (ASSR) and evoked-activity paradigms. For ASSR, stimuli are presented at 40-Hz permitting assessment of integrity of gamma-generating circuit elements. Deficits in ASSR have been widely replicated across the psychosis spectrum [33,37,38,39,40,41,42,43] and in relevant genetic risk syndromes (e.g. 22q11) [44], although literature in autism spectrum disorder (ASD) is conflicting [45]. Progressive reduction in auditory evoked gamma is seen over time in first-episode schizophrenia patients, but not in clinical high risk (CHR), suggesting that evoked gamma may index progressive neurodegenerative processes that occur over the early course of the illness [46].
Theta
Deficits in theta frequency responses are observed prominently during both auditory and visual processing in schizophrenia. Deficits in early auditory processing, including impaired N1 [47] and MMN [48,49,50] generation have been demonstrated consistently in schizophrenia, including prodromal phases of the illness [51,52,53]. Both N1 and MMN were subsequently mapped into the theta-frequency-band using narrow spectral filtering [54] as well as more comprehensive TF approaches [33, 55,56,57,58,59] (Fig. 3).
a Mismatch negativity (MMN) in schizophrenia vs. control patients, showing deficits in evoked-power within the theta frequency range. From ref. [200]. b MMN prior to and following treatment with the NMDAR glycine-site agonist D-serine in schizophrenia (Sz) vs. placebo. From ref. [138], *p < 0.05. c MMN in rodents pre/post treatment with placebo (Ctl), PCP alone, or PCP + glycine in rodents. Note decrease in MMN-related theta activity pre/post PCP alone treatment, and prevention of the difference by simultaneous glycine treatment. From ref. [55].
In the visual system, sensory-driven P1 potentials also show prominent activity in the theta frequency range and are reduced in schizophrenia, particularly in response to low spatial frequency, magnocellular-biased stimuli [60,61,62]. Visual fixation-related potentials (FRP) also reflect primarily phase-reset of ongoing theta frequency rhythms and are also significantly reduced in schizophrenia [63]. Finally, theta activation over frontal regions may index adaptive cognitive controls [64, 65], including response inhibition [64, 66], and thus may index impairments of these processes in schizophrenia.
As opposed to the reductions observed in schizophrenia, increases in visual theta response have recently been observed in ASD, related to impaired face emotion processing [62]. Acute alcohol may disrupt theta generation across a range of processes including auditory N1 and MMN [67], especially in the context of bipolar disorder [68], visual sensory potentials, and cognitive activities such as visual P300 [69]. In individuals with chronic alcohol use disorder, reduced theta activation during reward processing has been found to correlate with increased impulsivity [69], consistent with the role of prefrontal theta in response inhibition.
Delta
Delta rhythms were initially defined in the context of slow-wave (non-rapid eye movement) sleep, in which thalamic neurons impose T channel-mediated slow coherent activity throughout cortex [70, 71]. During adolescence, there is a marked decline in delta-sleep activity [72, 73] that correlates with parallel reductions in cortical thickness and gray matter volume, all of which are thought to reflect age-related synaptic pruning [72, 74]. Reductions in delta amplitude during slow-wave sleep are well established in schizophrenia [75, 76] as are reductions in cortical thickness and gray matter volume, and thus support the potential role of erroneous- or hyper-pruning in schizophrenia [77].
During wakefulness, delta amplitudes are low, reflecting desynchronization of delta generators across cortex. However, during attention-dependent processing, regional delta rhythms become entrained to the rhythm of presented stimuli and increase in the selectivity and efficiency of local processing [78, 79]. Violations of these entrained rhythms may underlie the generation of the widely-studied P300 potential, which also shows spectral power within the delta range [80,81,82].
In schizophrenia, deficits in auditory delta entrainment [79] and steady-state delta response [83] correlate with impaired P300 generation, auditory perceptual abnormalities and working memory deficits. P300 deficits are observed consistently not only across the illness course in schizophrenia [84] but also in the prodromal period preceding psychosis onset [85,86,87] other neuropsychiatric disorders including bipolar disorder [88], alcohol use disorder [89, 90] and neurodegenerative disorders [91].
Finally, in the visual system, the onset of motion elicits a characteristic negative potential with peak at ~200 ms termed the motion-N2 (N2m) [92]. As opposed to other stimulus-driven activity, N2m has recently been shown to have a spectral signature within the delta frequency range (Fig. 1c) and thus may be particularly useful for investigating underlying circuits. N2m/delta deficits have recently been reported in both schizophrenia and CHR [61] subjects.
Alpha/Beta
Alpha rhythms play a predominant role in coordination within the visual system whereas beta rhythms are particularly important with regard to sensory-motor processing and coordination of information across cortical regions [6, 93]. For both sets of measures, amplitudes are high during brain “idling,” but are suppressed when brain systems are engaged [27]. Thus, posterior alpha is reduced in a “top down” fashion during sustained visual attention. Alpha “blocking” also occurs in response to stimulus presentation, leading to a stimulus driven ERD [28]. Both task and stimulus-induced modulation of alpha activity reflect in part reciprocal interactions between the pulvinar nucleus of the thalamus and visual regions of cortex [61, 94,95,96,97].
Deficits in alpha blocking in schizophrenia were initially discovered shortly after the discovery of the EEG itself [98], and were extensively replicated in the pre-ERP era (e.g. refs. [99, 100]). More recently, deficits in alpha/beta ERD over visual areas [101, 102] and beta ERD over motor areas [102, 103] have been observed during visuo-motor tasks, and shown to correlate with impaired task performance. By contrast to stimulus-driven activity, sustained alpha suppression during visual attention appears to be preserved [28, 79]. Alpha synchrony deficits in schizophrenia may also be studied using steady-state visual evoked potential (ssVEP) approaches [61, 104]. In children with ASD, reductions in ongoing alpha activity correlate with cognitive impairment [105], whereas in high-functioning ASD increases are observed in both resting alpha and ssVEP activity [62], suggesting involvement of alpha-generating circuits across pathophysiological disorders.
Translational neuro-oscillation studies
The characterization of oscillatory disturbances in neuropsychiatric disorders provides increased opportunities for cross-species translation. For many measures, particularly those utilizing passive sensory stimulation, paradigms are extremely similar across species, permitting direct cross-species comparison. Animal models can then be used to both dissect local circuit mechanisms and evaluate the effect of interventions, such as N-methyl-D-aspartate receptor (NMDAR) antagonists, that are known to produce schizophrenia-like deficits in humans.
Gamma
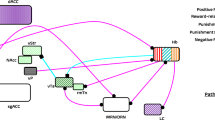
Gamma activity, in general, is thought to reflect increased excitatory drive to specific cortical regions [106], which then can trigger rhythmic feedback inhibition from local fast-spiking parvalbumin (PV)-type basket interneurons [107] (Fig. 4). The characteristic frequency is imposed by the kinetics of AMPA-type glutamate receptors on the PV interneurons, as well as KV3.1/3.2K+ channels that maintain narrow action potentials; and GABAA receptors, which drive the feedback inhibition of pyramidal neurons [108].
Frequency-bands and example circuit motifs whose activation is associated with frequency specific rhythmic activity in the neocortex. The frequency axis illustrates that oscillatory bands are partly overlapping (varying e.g. with excitatory and inhibitory drive). The circuit diagrams illustrate that activation of specific cells (e.g. fast spiking parvalbumim (PV+) expressing interneurons in superficial layers; somatostain (SOM+) interneurons) and their cortical connectivity are associated with rhythmic activity at bandlimited frequency-bands.
The cross-species translatability of the ASSR at this time requires further investigation. Whereas the ASSR in humans shows a consistent 40 Hz resonance, more variable results have been observed in rodents (e.g., refs. [109,110,111]. In some reports, NMDAR antagonism [112, 113] and genetic knockout of NMDAR on PV-expressing interneurons [114, 115] have been shown to decrease the 40 Hz ASSR in rodents, but in other studies the ASSR is increased by these manipulations [110, 111, 116].
In computational modeling, reductions in ASSR are most attributable to reductions of PV and GAD65 in PV interneurons [117], which may be a downstream consequence of NMDAR blockade [118], rather than to NMDAR dysfunction itself [117]. In rodents, effects of developmental NR1 knock-down may be reversed by GABAB agonists [119], although the applicability of this finding to humans has not been tested.
Task-related gamma modulation may also be observed in rodents. For example, cortico-hippocampal gamma synchronization transiently increases during working memory encoding [120] and retrieval [121]. In animal models, as in humans, gamma activity may also be extensively coupled to slower theta and delta rhythms [29, 30, 122,123,124]. Furthermore, in rodents, modulatory neurotransmitters such as dopamine may shift patterns of coupling [125]. Animal models thus provide an opportunity to determine the degree to which gamma deficits reflect dysfunction within specific gamma generating mechanism vs. the degree to which they are an indirect reflection of impaired generation of slower rhythms, or impaired cross-frequency coupling.
Theta
The MMN paradigm has also been extensively validated in both monkeys [126,127,128,129] and rodents [55, 113, 130,131,132], and as in humans depends strongly on NMDAR function. As in humans, rodent [55, 133] and monkey [134], MMN activity maps predominantly to the theta frequency range. Moreover, schizophrenia-like MMN deficits are induced by acute [135,136,137] and chronic [55] NMDAR blockade and prevented by the simultaneous administration of an NMDAR agonist [55], paralleling human studies [138] (Fig. 3c).
At the local circuit level, cortical theta rhythms depend primarily on the functioning of local circuit somatostatin (SOM)-type GABAergic interneurons [13] (Fig. 4). Moreover, in rodents both theta rhythms and MMN generation are selectively impaired by optogenetic silencing of somatostatin interneurons within cortex [139]. In rodents, increased theta synchrony, especially within the septo-hippocampal-entorhinal system [6], is also associated with working memory encoding [140] and retrieval [141, 142], and may thus serve as a model system for theta-related frontal cognitive deficits in schizophrenia.
Delta
In animals, mechanisms underlying generation of delta rhythms have been studied most extensively in the context of sleep, in which thalamic neurons impose T-channel mediated slow coherent activity throughout cortex [70, 71]. Type 1 nitrergic cortical interneurons, which are known to co-express both somatostatin, neurokinin 1 (NK1) receptors, and NMDAR may also play a critical role in both NREM sleep generation and sleep-related consolidation [143], although the role of these cells in attention-dependent processing remains relatively unknown.
In monkeys, delta entrainment to stimulus regularities are observed that are similar to those in humans and are linked to synchrony between cortex and subcortical structures including the pulvinar [144,145,146]. P300-like activity is also elicited during active discrimination tasks in rodents. Furthermore, as in humans evidence suggests that cholinergic signaling in the medial septum (MS) and the nucleus basalis magnocellularis (NBM) may be important modulators of task-related delta activity of cortical ERP in the rat [147, 148] and can be modulated by pharmacological perturbation of cholinergic tone [149]. Additionally, selective lesions in the MS or NBM produce profound changes in both and synchrony in both cortical and limbic sites [150, 151].
Alpha/beta
In monkeys, as in humans, alpha rhythms within visual cortex appear to be driven by synchrony between pulvinar nucleus and cortex [96, 152], as well as feedback propagation from higher to lower tiers of the visual system [153]. Furthermore, NMDAR antagonists inhibit alpha while increasing ongoing gamma activity [153, 154], similar to the pattern observed in schizophrenia. Glutamatergic contributions to alpha generation may also be mediated, in part, by mGluR1 receptors [93] Other receptor systems involved in alpha generation, including muscarinic cholinergic [93, 155] and 5-HT2A [156] receptors, may be relevant for changes observed in neurodegenerative disorders and the effects of hallucinogenic psychostimulants, respectively.
In rodents, pulvinar nucleus is much less developed than in primates [157], and pulvinar contributions to neuro-oscillatory activity remains relatively unstudied [158]. Integrative beta function is also relatively unstudied in rodent schizophrenia models. Motor-related beta activity in rodents is critically dependent upon gap junction and M- currents mediated through Kc7-type potassium channels and may be modulated by both GABAA and NMDAR modulation [159, 160]. In rodents, NMDAR antagonists such as ketamine or PCP induce reductions in ongoing beta activity that may reflect impaired corticothalamic connectivity. Supporting this possibility, chemogenetic inhibition of the mediodorsal thalamus in mice decreases synchronization between mediodorsal thalamus and medial prefrontal cortex [161].
Cross-species validation issues
Despite the promise of neuro-oscillations as translational biomarkers, several methodological issues still need to be clarified before their widespread use for treatment development. The main “disconnect” between spectral activity obtained from human scalp-recorded activity and intracranially recorded oscillations in animals is the need for greater signal averaging in human recordings to differentiate task-related activity from background EEG.
A critical question, therefore, is the degree to which the oscillatory signature obtained from spectral decomposition of human ERP data reflects added energy “created” by the stimulus that coincidentally falls within specific frequency-bands vs. stimulus-induced activation/modulation of the same circuits that generate ongoing oscillatory brain activity.
This is especially the case with regard to conditions such as steady-state potentials, where the oscillatory structure is imposed by the eliciting stimulus, and by paradigms in which the exogenous stimulation evokes an increase in power that may therefore indicate a response that is superimposed on ongoing brain rhythms. In such cases, artifactual increases in ITC may be observed as well [32, 78]. Nevertheless, several translational measures, including ASSR, MMN [55, 162] and delta entrainment [79] involve primarily phase-reset of ongoing rhythms and thus appear to translatable across species [24, 163].
Further support for homology comes from the possibility that even continuously recorded endogenous potentials may, in fact, be synchronized to unmeasured events. For example, in humans and monkeys, saccadic eye movements lead to reset of ongoing theta rhythms not only within visual regions but also hippocampus [164, 165], a process termed “active sensing” [166]. The relationship can only be assessed, however, if eye movements are explicitly measured. In rodents, a similar relationship is observed related to whisker movement (“active whisking”) [167], suggesting that even endogenous rhythms may, in fact, be synchronized to discrete events [168].
Treatment development
Current medication classes such as antipsychotics, antidepressants and anti-anxiety agents were discovered fortuitously and then “reverse engineered” primarily using radioreceptor approaches to translate across human and rodent models and demonstrate clinical target engagement. Many current treatment targets (e.g. ionotropic/metabotropic glutamate receptors) have low agonist affinity and thus do not support receptor-based approaches for determination of target engagement. Moreover, treatment development is increasingly focused on modification of circuit-level disturbances, such as impaired E/I balance, using multi-targeted treatment approaches. Neuro-oscillatory approaches specifically index local circuit activity and are translatable across rodents, monkeys and humans, supporting their use in development of next-generation treatment approaches.
For example, ASSR may be particularly related to chronic oxidative stress, which leads to downregulation of PV-type interneurons [169]. In rodents, both increases [109,110,111, 116] and decreases [113, 170] in ASSR are also reported, potentially explainable based on varying degrees of NMDAR occupancy across studies [112]. To date, the effects of manipulation of other receptor types such as Ca2+ permeable AMPA channels on local ASSR generation has not been assessed.
Even in humans, many aspects of the ASSR remain relatively unexplored, such as differential impairment of early (0–100 ms) vs. late (300–500 ms) response in CHR vs. first-episode schizophrenia [45], and differential effects of increasing interstimulus interval length/variability [37, 171], and stimulus duration [36] in controls vs. patients. Systematic exploration of the same parameter space in both humans and rodents, combined with rodent pharmacological and genetic studies are needed to refine the biomarker and increase its applicability across disorders.
By contrast, theta-band deficits such as indexed by MMN have shown significant sensitivity to NMDAR antagonists such as PCP, ketamine or MK-801 across rodent [133, 135], primate [126, 134] and human [172,173,174] models, suggesting NMDAR involvement within the SOM-containing E/I circuit [133]. Similar effects are also observed on theta activity within the paired click paradigm [175]. In rodents, as in humans, MMN generation may be modulated by NMDAR agonists such as glycine, D-serine or N-acetylcysteine [55, 138, 176, 177], suggesting utility for translational treatment development.
Nevertheless, in current animal models, MMN deficits are induced primarily by pharmacological challenge. Development of animals that more closely capture pathophysiological features of the disorder may lead to improved understanding of the basis for MMN deficits in schizophrenia and other disorders, and thus to improved treatments. Additionally, sub-chronic exposure to ketamine leads to lasting reductions in evoked theta activity, as well as cognitive performance that may rely on the networks that generate this rhythm [178,179,180,181].
The basis for these persistent changes needs to be understood, and may provide improved treatment targets for schizophrenia, alcohol use disorders, or others. Finally, development of translational biomarkers, in general, requires pre-competitive collaboration between industry, academia and government to support an FDA biomarkers approval [3]. Such a consortium has recently been established for MMN (www.erpbiomarkers.org), potentially paving the way for its use in patient stratification and outcome assessment.
Disturbances of alpha modulation are documented across a number of disorders including schizophrenia [62, 100, 101, 105] and ASD [62, 105]. Even though the alpha rhythm was first described almost 100 years ago, basic mechanisms underlying alpha generation remain unresolved. More etiological work is needed to understand and manipulate these circuits. Increasingly, subcortical structures such as pulvinar nucleus [61, 96] are implicated in alpha regulation and should be increased targets for treatment-related research.
Alterations in P300 generation, reflecting delta-band dysfunction, are caused not only by NMDAR antagonists [182] but also by other pharmacological agents including GABA agonists [182], 5-HT2A antagonists [183, 184], cannabinoids [185, 186] and muscarinic antagonists [187, 188]. The GABAB agonist gamma-hydroxyoxybutyrate may improve both delta-sleep time and cognition in schizophrenia [189], suggesting interactive contributions of multiple neurochemical pathways to delta generation. Although attention-dependent paradigms are difficult to implement in rodents, entrainment phenomena may be possible
Finally, oscillatory approaches may also be critical for demonstration of target engagement with non-invasive brain stimulation approaches, such as transcranial magnetic (TMS) or direct-current stimulation (tDCS), which target disturbances in intrinsic oscillatory activity [190, 191] in disorders such as schizophrenia [192,193,194] or depression [190, 191, 195,196,197]. For example, one interventional strategy involves entrainment of oscillatory activity at frequencies and phase relationships that are natural to the underlying neuronal circuits [13] and evident in the timing of cognitive processes [198, 199]. However, further exploration of these processes in both rodents and humans is required.
In summary, neuro-oscillatory approaches are ideally suited for integration across pre-clinical and clinical stages of new treatment development, especially for treatments targeting network level dysfunction and impaired E/I balance. Unanswered questions remain concerning the homology between specific oscillatory measures across human and animal models. Nevertheless, the strong conservation of the oscillatory frequencies across species provides novel opportunities for bidirectional cross-species translation and assessment of target engagement by novel pharmacological and brain-stimulation-based approaches.
Funding and disclosure
This study was funded by the following: DCJ: MH49334, MH109289. SJS: 5KL2TR001854, R01 MH075916-05. KMS: Department of Veterans Affairs I01 CX001443, NIH R01 MH093450. DHM: U01 MH076989. LEH: R01MH112180, R01MH116948. AM: MH49334. CLE: AA006059, 019969,026248,027316AIA: Janssen Fellowship in Translational Neuroscience, OHSU Physician Scientist Award; TT: MH120117, MH114223, MH113041PL: R01DC012947, P50MH109429. TW: NIH NIBIB R01EB028161, NIH NINDS UF1-NS17666-01. This study has the following conflict of interest: DCJ: Intellectual property for NMDAR agonists in schizophrenia, NMDAR antagonist in depression, fMRI for prediction of ECT response and apparatus for diagnosis of mental disorders. Equity in Glytech, AASI and NeuroRx. Scientific advisory board NeuroRx, Promentis. Consultant payments Concert, Lundbeck, Phytec, Autifony, SK Life Sciences, Biogen, Cadence, and Pfizer, Boehringer Ingelheim. Research support Cerevance. SJS: Grants from Astellas Research Institute of America (ARIA) and consultant to Zynerba. KMS: Consultant payments from Biogen. DHM: Consultant payments from Boehringer-Ingelheim, Cadence, Aptinyx, and Greenwich Bioscience LEH: Consulting, scientific advisory board or research project payments from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho, Heptares, Pfizer, Luye Pharma, Sound Pharma, Takeda, and Regeneron AM: no conflicts CLE: no conflicts AIA: no conflicts TT: no conflicts PL: no conflicts TW: no conflicts.
References
Javitt DC, Freedman R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry. 2015;172:17–31.
Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate C, Merchant K, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3:102mr2.
Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev. 2008;7:68–83.
Luck SJ, Mathalon DH, O’Donnell BF, Hamalainen MS, Spencer KM, Javitt DC, et al. A roadmap for the development and validation of event-related potential biomarkers in schizophrenia research. Biol Psychiatry. 2011;70:28–34.
Cohen MX. Analyzing neural time series data: theory and practice. Cambridge: MIT press; 2014 .
Buzsaki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. 2013;80:751–64.
Berger H. Über das Elektrenkephalogramm des Menschen (On the EEG in humans). Arch für Psychiatre und Nervenkrankheiten. 1929;87:527.
Jasper HH, Anderews HL. Brain potentials and voluntary muscle activity in man. J Neurophysiol. 1938;1:87–100.
Rohracher H. Die gehirnelektrischen Erscheinungen bei geistiger Arbeit [Brain waves in mental work]. Z für Angew Psychologie und Charakterkd. 1935;136:308–24.
Davis H, Davis PA, Loomis AL, Harvey EN, Hobart G. Changes in Human Brain Potentials during the Onset of Sleep. Science. 1937;86:448–50.
Lennox M, Brody BS. Paroxysmal slow waves in the electroencephalograms of patients with epilepsy and with sub-cortical lesions. J Nerv Ment Dis. 1946;104:237–48.
Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiological Rev. 2010;90:1195–268.
Womelsdorf T, Valiante TA, Sahin NT, Miller KJ, Tiesinga P. Dynamic circuit motifs underlying rhythmic gain control, gating and integration. Nat Neurosci. 2014;17:1031–9.
Kopell N, Kramer MA, Malerba P, Whittington MA. Are different rhythms good for different functions? Front Hum Neurosci. 2010;4:187.
Tallon-Baudry C. Oscillatory synchrony and human visual cognition. J Physiol Paris. 2003;97:355–63.
Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci methods. 2004;134:9–21.
Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–26.
Bartnik EA, Blinowska KJ, Durka PJ. Single evoked potential reconstruction by means of wavelet transform. Biol Cyber. 1992;67:175–81.
Pesaran B, Vinck M, Einevoll GT, Sirota A, Fries P, Siegel M, et al. Investigating large-scale brain dynamics using field potential recordings: analysis and interpretation. Nat Neurosci. 2018;21:903–19.
Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–40.
Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–62.
Herrmann CS, Knight RT. Mechanisms of human attention: event-related potentials and oscillations. Neurosci Biobehav Rev. 2001;25:465–76.
Canavier CC. Phase-resetting as a tool of information transmission. Curr Opin Neurobiol. 2015;31:206–13.
Voloh B, Womelsdorf T. A role of phase-resetting in coordinating large scale neural networks during attention and goal-directed behavior. Front Syst Neurosci. 2016;10:18.
Mo C, Petrof I, Viaene AN, Sherman SM. Synaptic properties of the lemniscal and paralemniscal pathways to the mouse somatosensory thalamus. Proc Natl Acad Sci USA. 2017;114:E6212–E21.
Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci. 2015;16:535–50.
Klimesch W. alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012;16:606–17.
Martinez A, Hillyard SA, Bickel S, Dias EC, Butler PD, Javitt DC. Consequences of magnocellular dysfunction on processing attended information in schizophrenia. Cereb Cortex. 2012;22:1282–93.
Chrobak JJ, Buzsaki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci. 1998;18:388–98.
Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–11.
Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–8.
Aru J, Aru J, Priesemann V, Wibral M, Lana L, Pipa G, et al. Untangling cross-frequency coupling in neuroscience. Curr Opin Neurobiol. 2015;31:51–61.
Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–42.
Gao R, Peterson EJ, Voytek B. Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage. 2017;158:70–78.
Sohal VS, Rubenstein JLR. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol Psychiatry. 2019;24:1248–57.
Hamm JP, Gilmore CS, Clementz BA. Augmented gamma band auditory steady-state responses: support for NMDA hypofunction in schizophrenia. Schizophr Res. 2012;138:1–7.
Kim S, Jang SK, Kim DW, Shim M, Kim YW, Im CH, et al. Cortical volume and 40-Hz auditory-steady-state responses in patients with schizophrenia and healthy controls. NeuroImage Clin. 2019;22:101732.
Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–5.
Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85.
Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–40.
Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70:293–302.
Reilly TJ, Nottage JF, Studerus E, Rutigliano G, Micheli AI, Fusar-Poli P, et al. Gamma band oscillations in the early phase of psychosis: A systematic review. Neurosci Biobehav Rev. 2018;90:381–99.
Thune H, Recasens M, Uhlhaas PJ. The 40-Hz auditory steady-state response in patients with schizophrenia: a meta-analysis. JAMA Psychiatry. 2016;73:1145–53.
Parker DA, Hamm JP, McDowell JE, Keedy SK, Gershon ES, Ivleva EI, et al. Auditory steady-state EEG response across the schizo-bipolar spectrum. Schizophr Res. 2019;209:218–26.
Tada M, Kirihara K, Koshiyama D, Fujioka M, Usui K, Uka T, et al. Gamma-band auditory steady-state response as a neurophysiological marker for excitation and inhibition balance: a review for understanding schizophrenia and other neuropsychiatric disorders. Clin EEG Neurosci. 2019. https://doi.org/10.1177/1550059419868872.
Oribe N, Hirano Y, Del Re E, Seidman LJ, Mesholam-Gately RI, Woodberry KA, et al. Progressive reduction of auditory evoked gamma in first episode schizophrenia but not clinical high risk individuals. Schizophr Res. 2019;208:145–52.
Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia-a critical review. Psychiatry Res 2008;161:259–74.
Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000;57:1131–7.
Thomas ML, Green MF, Hellemann G, Sugar CA, Tarasenko M, Calkins ME, et al. Modeling deficits from early auditory information processing to psychosocial functioning in schizophrenia. JAMA Psychiatry. 2017;74:37–46.
Avissar M, Xie S, Vail B, Lopez-Calderon J, Wang Y, Javitt DC. Meta-analysis of mismatch negativity to simple versus complex deviants in schizophrenia. Schizophr Res. 2018;191:25–34.
Friedman T, Sehatpour P, Dias E, Perrin M, Javitt DC. Differential relationships of mismatch negativity and visual p1 deficits to premorbid characteristics and functional outcome in schizophrenia. Biol Psychiatry. 2012;71:521–9.
Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH, et al. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry. 2014;75:459–69.
Bodatsch M, Brockhaus-Dumke A, Klosterkotter J, Ruhrmann S. Forecasting psychosis by event-related potentials-systematic review and specific meta-analysis. Biol Psychiatry. 2015;77:951–8.
Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111:1733–7.
Lee M, Balla A, Sershen H, Sehatpour P, Lakatos P, Javitt DC. Rodent mismatch negativity/theta neuro-oscillatory response as a translational neurophysiological biomarker for N-methyl-d-aspartate receptor-based new treatment development in schizophrenia. Neuropsychopharmacology. 2018;43:571–82.
Kaser M, Soltesz F, Lawrence P, Miller S, Dodds C, Croft R, et al. Oscillatory underpinnings of mismatch negativity and their relationship with cognitive function in patients with schizophrenia. PLoS ONE 2013;8:e83255.
Hsiao FJ, Wu ZA, Ho LT, Lin YY. Theta oscillation during auditory change detection: An MEG study. Biol Psychol. 2009;81:58–66.
Fuentemilla L, Marco-Pallares J, Munte TF, Grau C. Theta EEG oscillatory activity and auditory change detection. Brain Res. 2008;1220:93–101.
Hochberger WC, Joshi YB, Zhang W, Thomas ML, Consortium of Genomics in Schizophrenia invetigators, Braff DL, et al. Decomposing the constituent oscillatory dynamics underlying mismatch negativity generation in schizophrenia: distinct relationships to clinical and cognitive functioning. Int J Psychophysiol. 2018;145:23–29.
Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, Jalbrzikowski M, et al. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116:2204–15.
Martinez A, Gaspar PA, Hillyard SA, Andersen SK, Lopez-Calderon J, Corcoran CM, et al. Impaired motion processing in schizophrenia and the attenuated psychosis syndrome: etiological and clinical implications. Am J Psychiatry. 2018;175:1243–54.
Martinez A, Tobe R, Dias EC, Ardekani BA, Veenstra-VanderWeele J, Patel G, et al. Differential patterns of visual sensory alteration underlying face emotion recognition impairment and motion perception deficits in schizophrenia and autism spectrum disorder. Biol Psychiatry. 2019;86:557–67.
Dias EC, Van Voorhis AC, Braga F, Todd J, Lopez-Calderon J, Martinez A, et al. Impaired fixation-related theta modulation predicts reduced visual span and guided search deficits in schizophrenia. Cereb Cortex. 2019;30:2823–33.
Reinhart RM, Zhu J, Park S, Woodman GF. Synchronizing theta oscillations with direct-current stimulation strengthens adaptive control in the human brain. Proc Natl Acad Sci USA. 2015;112:9448–53.
Best MW, Milanovic M, Shamblaw AL, Muere A, Lambe LJ, Hong IK, et al. An examination of the moderating effects of neurophysiology on treatment outcomes from cognitive training in schizophrenia-spectrum disorders. Int J Psychophysiol. 2019;176:297–306.
Hoptman MJ, Parker EM, Nair-Collins S, Dias EC, Ross ME, DiCostanzo JN, et al. Sensory and cross-network contributions to response inhibition in patients with schizophrenia. NeuroImage Clin. 2018;18:31–39.
Ramlakhan JU, Zomorrodi R, Downar J, Blumberger DM, Daskalakis ZJ, George TP, et al. Using mismatch negativity to investigate the pathophysiology of substance use disorders and comorbid psychosis. Clin EEG Neurosci. 2018;49:226–37.
Chitty KM, Lagopoulos J, Kaur M, Hickie IB, Hermens DF. The N-methyl-D-aspartate receptor as a neurobiological intersection between bipolar disorder and alcohol use: a longitudinal mismatch negativity study. Int J Neuropsychopharmacol. 2015;18:pyu113.
Rangaswamy M, Porjesz B. Understanding alcohol use disorders with neuroelectrophysiology. Handb Clin Neurol. 2014;125:383–414.
McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215.
Crunelli V, Cope DW, Hughes SW. Thalamic T-type Ca2+ channels and NREM sleep. Cell Calcium. 2006;40:175–90.
Feinberg I, Campbell IG. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. Am J Physiol Regul Integr Comp Physiol. 2013;304:R296–303.
Feinberg I, Campbell IG. Shorter sleep durations in adolescents reduce power density in a wide range of waking electroencephalogram frequencies. PLoS ONE. 2019;14:e0210649.
Goldstone A, Willoughby AR, de Zambotti M, Franzen PL, Kwon D, Pohl KM, et al. The mediating role of cortical thickness and gray matter volume on sleep slow-wave activity during adolescence. Brain Struct Funct. 2018;223:669–85.
Hiatt JF, Floyd TC, Katz PH, Feinberg I. Further evidence of abnormal non-rapid-eye-movement sleep in schizophrenia. Arch Gen Psychiatry. 1985;42:797–802.
Keshavan MS, Reynolds CF 3rd, Miewald MJ, Montrose DM, Sweeney JA, Vasko RC Jr, et al. Delta sleep deficits in schizophrenia: evidence from automated analyses of sleep data. Arch Gen Psychiatry. 1998;55:443–8.
Feinberg I. Cortical pruning and the development of schizophrenia. Schizophr Bull. 1990;16:567–70.
Lakatos P, Musacchia G, O’Connel MN, Falchier AY, Javitt DC, Schroeder CE. The spectrotemporal filter mechanism of auditory selective attention. Neuron. 2013;77:750–61.
Lakatos P, Schroeder CE, Leitman DI, Javitt DC. Predictive suppression of cortical excitability and its deficit in schizophrenia. J Neurosci. 2013;33:11692–702.
Ford JM, Roach BJ, Hoffman RS, Mathalon DH. The dependence of P300 amplitude on gamma synchrony breaks down in schizophrenia. Brain Res 2008;1235:133–42.
Doege K, Bates AT, White TP, Das D, Boks MP, Liddle PF. Reduced event-related low frequency EEG activity in schizophrenia during an auditory oddball task. Psychophysiology. 2009;46:566–77.
Almeida PR, Vieira JB, Silveira C, Ferreira-Santos F, Chaves PL, Barbosa F, et al. Exploring the dynamics of P300 amplitude in patients with schizophrenia. Int J Psychophysiol. 2011;81:159–68.
Puvvada KC, Summerfelt A, Du X, Krishna N, Kochunov P, Rowland LM, et al. Delta vs gamma auditory steady state synchrony in schizophrenia. Schizophr Bull. 2018;44:378–87.
Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701.
Hamilton HK, Roach BJ, Bachman PM, Belger A, Carrion RE, Duncan E, et al. Association between P300 responses to auditory oddball stimuli and clinical outcomes in the psychosis risk syndrome. JAMA Psychiatry. 2019;76:1187–97.
Hamilton HK, Woods SW, Roach BJ, Llerena K, McGlashan TH, Srihari VH, et al. Auditory and visual oddball stimulus processing deficits in schizophrenia and the psychosis risk syndrome: forecasting psychosis risk with P300. Schizophr Bull. 2019;45:1068–80.
van Tricht MJ, Nieman DH, Koelman JH, van der Meer JN, Bour LJ, de Haan L, et al. Reduced parietal P300 amplitude is associated with an increased risk for a first psychotic episode. Biol Psychiatry. 2010;68:642–8.
Wada M, Kurose S, Miyazaki T, Nakajima S, Masuda F, Mimura Y, et al. The P300 event-related potential in bipolar disorder: a systematic review and meta-analysis. J Affect Disord. 2019;256:234–49.
Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, Padmanabhapillai A, et al. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin Neurophysiol. 2006;117:2128–43.
Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, et al. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int J Psychophysiol. 2007;63:3–15.
Morrison C, Rabipour S, Knoefel F, Sheppard C, Taler V. Auditory event-related potentials in mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res. 2018;15:702–15.
Kuba M, Kubová Z, Kremláček J, Langrová J. Motion-onset VEPs: characteristics, methods, and diagnostic use. Vis Res. 2007;47:189–202.
Vijayan S, Kopell NJ. Thalamic model of awake alpha oscillations and implications for stimulus processing. Proc Natl Acad Sci USA. 2012;109:18553–8.
Liu Z, de Zwart JA, Yao B, van Gelderen P, Kuo LW, Duyn JH. Finding thalamic BOLD correlates to posterior alpha EEG. Neuroimage 2012;63:1060–9.
Green JJ, Boehler CN, Roberts KC, Chen LC, Krebs RM, Song AW, et al. Cortical and subcortical coordination of visual spatial attention revealed by simultaneous EEG-fMRI recording. J Neurosci. 2017;37:7803–10.
Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–6.
Lakatos P, O’Connell MN, Barczak A. Pondering the Pulvinar. Neuron. 2016;89:5–7.
MacMahon JF, Walter WG. The electroencephalogram in schizophrenia. J Ment Sci. 1938;84:781–7.
Salamon I, Post J. Alpha blocking and schizophrenia. I. Methodology and initial studies. Arch Gen Psychiatry. 1965;13:367–74.
Blum RH. Alpha-rhythm responsiveness in normal, schizophrenic, and brain-damaged persons. Science. 1957;126:749–50.
Martinez A, Gaspar PA, Hillyard SA, Bickel S, Lakatos P, Dias EC, et al. Neural oscillatory deficits in schizophrenia predict behavioral and neurocognitive impairments. Front Hum Neurosci. 2015;9:371.
Dias EC, Bickel S, Epstein ML, Sehatpour P, Javitt DC. Abnormal task modulation of oscillatory neural activity in schizophrenia. Front Psychol. 2013;4:540.
Kantrowitz JT, Epstein ML, Beggel O, Rohrig S, Lehrfeld JM, Revheim N, et al. Neurophysiological mechanisms of cortical plasticity impairments in schizophrenia and modulation by the NMDA receptor agonist D-serine. Brain 2016;139(Pt 12):3281–95.
Murphy M, Ongur D. Decreased peak alpha frequency and impaired visual evoked potentials in first episode psychosis. NeuroImage Clin. 2019;22:101693.
Dickinson A, DiStefano C, Senturk D, Jeste SS. Peak alpha frequency is a neural marker of cognitive function across the autism spectrum. Eur J Neurosci. 2018;47:643–51.
Whittington MA, Cunningham MO, LeBeau FE, Racca C, Traub RD. Multiple origins of the cortical gamma rhythm. Dev Neurobiol. 2011;71:92–106.
Cardin JA. Inhibitory interneurons regulate temporal precision and correlations in cortical circuits. Trends Neurosci. 2018;41:689–700.
Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–25.
Vohs JL, Chambers RA, O’Donnell BF, Krishnan GP, Morzorati SL. Auditory steady state responses in a schizophrenia rat model probed by excitatory/inhibitory receptor manipulation. Int J Psychophysiol. 2012;86:136–42.
Leishman E, O’Donnell BF, Millward JB, Vohs JL, Rass O, Krishnan GP, et al. Phencyclidine disrupts the auditory steady state response in rats. PLoS ONE. 2015;10:e0134979.
Sullivan EM, Timi P, Hong LE, O’Donnell P. Effects of NMDA and GABA-A receptor antagonism on auditory steady-state synchronization in awake behaving rats. Int J Neuropsychopharmacol. 2015;18:pyu118.
Sivarao DV, Chen P, Senapati A, Yang Y, Fernandes A, Benitex Y, et al. 40 Hz auditory steady-state response is a pharmacodynamic biomarker for cortical NMDA receptors. Neuropsychopharmacology. 2016;41:2232–40.
Schuelert N, Dorner-Ciossek C, Brendel M, Rosenbrock H. A comprehensive analysis of auditory event-related potentials and network oscillations in an NMDA receptor antagonist mouse model using a novel wireless recording technology. Physiol Rep. 2018;6:e13782.
Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17:537–48.
Nakao K, Nakazawa K. Brain state-dependent abnormal LFP activity in the auditory cortex of a schizophrenia mouse model. Front Neurosci. 2014;8:168.
Balla A, Ginsberg SD, Abbas AI, Sershen H, Javitt DC. Translational neurophysiological biomarkers of N-methyl-D-aspartate receptor dysfunction in serine racemase knockout mice. Biomark Neuropsychiatry.
Metzner C, Zurowski B, Steuber V. The role of parvalbumin-positive interneurons in auditory steady-state response deficits in schizophrenia. Sci Rep. 2019;9:18525.
Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–7.
Gandal MJ, Sisti J, Klook K, Ortinski PI, Leitman V, Liang Y, et al. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl psychiatry. 2012;2:e142.
Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–14.
Yamamoto J, Suh J, Takeuchi D, Tonegawa S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell. 2014;157:845–57.
Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–15.
Voloh B, Valiante TA, Everling S, Womelsdorf T. Theta-gamma coordination between anterior cingulate and prefrontal cortex indexes correct attention shifts. Proc Natl Acad Sci USA. 2015;112:8457–62.
Lopes-Dos-Santos V, van de Ven GM, Morley A, Trouche S, Campo-Urriza N, Dupret D. Parsing hippocampal theta oscillations by nested spectral components during spatial exploration and memory-guided behavior. Neuron. 2018;100:940–52 e7.
Andino-Pavlovsky V, Souza AC, Scheffer-Teixeira R, Tort ABL, Etchenique R, Ribeiro S. Dopamine modulates delta-gamma phase-amplitude coupling in the prefrontal cortex of behaving rats. Front Neural Circuits. 2017;11:29.
Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci USA. 1996;93:11962–7.
Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG Jr, Arezzo JC. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res 1994;667:192–200.
Javitt DC, Schroeder CE, Steinschneider M, Arezzo JC, Vaughan HG Jr. Demonstration of mismatch negativity in the monkey. Electroencephalogr Clin Neurophysiol. 1992;83:87–90.
Gil-da-Costa R, Stoner GR, Fung R, Albright TD. Nonhuman primate model of schizophrenia using a noninvasive EEG method. Proc Natl Acad Sci USA. 2013;110:15425–30.
Featherstone RE, Shin R, Kogan JH, Liang Y, Matsumoto M, Siegel SJ. Mice with subtle reduction of NMDA NR1 receptor subunit expression have a selective decrease in mismatch negativity: Implications for schizophrenia prodromal population. Neurobiol Dis. 2014;73C:289–95.
Ahnaou A, Huysmans H, Biermans R, Manyakov NV, Drinkenburg W. Ketamine: differential neurophysiological dynamics in functional networks in the rat brain. Transl psychiatry. 2017;7:e1237.
Featherstone RE, Melnychenko O, Siegel SJ. Mismatch negativity in preclinical models of schizophrenia. Schizophr Res. 2018;191:35–42.
Javitt DC, Lee M, Kantrowitz JT, Martinez A. Mismatch negativity as a biomarker of theta band oscillatory dysfunction in schizophrenia. Schizophr Res. 2018;191:51–60.
Lakatos P, O’Connell MN, Barczak A, McGinnis T, Neymotin S, Schroeder CE, et al. The thalamocortical circuit of auditory mismatch negativity. Biol. Psychiatry. 2019. https://doi.org/10.1016/j.biopsych.2019.10.029.
Ehrlichman RS, Maxwell CR, Majumdar S, Siegel SJ. Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. J Cogn Neurosci. 2008;20:1403–14.
Siegel SJ, Connolly P, Liang Y, Lenox RH, Gur RE, Bilker WB, et al. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology. 2003;28:675–82.
Ehrlichman RS, Gandal MJ, Maxwell CR, Lazarewicz MT, Finkel LH, Contreras D, et al. N-methyl-d-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience 2009;158:705–12.
Kantrowitz JT, Epstein ML, Lee M, Lehrfeld N, Nolan KA, Shope C, et al. Improvement in mismatch negativity generation during d-serine treatment in schizophrenia: correlation with symptoms. Schizophr Res. 2018;191:70–79.
Hamm JP, Yuste R. Somatostatin interneurons control a key component of mismatch negativity in mouse visual cortex. Cell Rep. 2016;16:597–604.
Abbas AI, Sundiang MJM, Henoch B, Morton MP, Bolkan SS, Park AJ, et al. Somatostatin interneurons facilitate hippocampal-prefrontal synchrony and prefrontal spatial encoding. Neuron. 2018;100:926–39 e3.
Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402.
O’Neill PK, Gordon JA, Sigurdsson T. Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J Neurosci. 2013;33:14211–24.
Zielinski MR, Atochin DN, McNally JM, McKenna JT, Huang PL, Strecker RE, et al. Somatostatin+/nNOS+ neurons are involved in delta electroencephalogram activity and cortical-dependent recognition memory. Sleep 2019;42:zsz143.
Barczak A, O’Connell MN, McGinnis T, Ross D, Mowery T, Falchier A, et al. Top-down, contextual entrainment of neuronal oscillations in the auditory thalamocortical circuit. Proc Natl Acad Sci USA. 2018;115:E7605–E14.
Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–3.
Besle J, Schevon CA, Mehta AD, Lakatos P, Goodman RR, McKhann GM, et al. Tuning of the human neocortex to the temporal dynamics of attended events. J Neurosci. 2011;31:3176–85.
Pirch JH, Corbus MJ, Rigdon GC, Lyness WH. Generation of cortical event-related slow potentials in the rat involves nucleus basalis cholinergic innervation. Electroencephalogr Clin Neurophysiol. 1986;63:464–75.
Robledo P, Weissenborn R, Robbins TW, Everitt BJ. Effects of lesions of the nucleus basalis magnocellularis on the acquisition of cocaine self-administration in rats. Eur J Neurosci. 1998;10:1946–55.
Ahnaou A, Biermans R, Drinkenburg W. Cholinergic mechanisms of target oddball stimuli detection: the late “P300-Like” event-related potential in rats. Neural Plast. 2018;2018:4270263.
Sanchez-Alavez M, Robledo P, Wills DN, Havstad J, Ehlers CL. Cholinergic modulation of event-related oscillations (ERO). Brain Res 2014;1559:11–25.
Sanchez-Alavez M, Ehlers CL. Event-related oscillations (ERO) during an active discrimination task: Effects of lesions of the nucleus basalis magnocellularis. Int J Psychophysiol. 2016;103:53–61.
Fiebelkorn IC, Kastner S. The puzzling pulvinar. Neuron. 2019;101:201–03.
van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, Poort J, van der Togt C, et al. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc Natl Acad Sci USA. 2014;111:14332–41.
Skoblenick KJ, Womelsdorf T, Everling S. Ketamine alters outcome-related local field potentials in monkey prefrontal cortex. Cereb Cortex. 2016;26:2743–52.
Wan L, Huang H, Schwab N, Tanner J, Rajan A, Lam NB, et al. From eyes-closed to eyes-open: role of cholinergic projections in EC-to-EO alpha reactivity revealed by combining EEG and MRI. Hum Brain Mapp. 2019;40:566–77.
Kometer M, Schmidt A, Jancke L, Vollenweider FX. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on alpha oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci. 2013;33:10544–51.
Chalfin BP, Cheung DT, Muniz JA, de Lima Silveira LC, Finlay BL. Scaling of neuron number and volume of the pulvinar complex in New World primates: comparisons with humans, other primates, and mammals. J Comp Neurol. 2007;504:265–74.
Zhou NA, Maire PS, Masterson SP, Bickford ME. The mouse pulvinar nucleus: Organization of the tectorecipient zones. Vis Neurosci 2017;34:E011.
Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–73.
Sherman MA, Lee S, Law R, Haegens S, Thorn CA, Hamalainen MS, et al. Neural mechanisms of transient neocortical beta rhythms: converging evidence from humans, computational modeling, monkeys, and mice. Proc Natl Acad Sci USA. 2016;113:E4885–94.
Parnaudeau S, O’Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL, et al. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77:1151–62.
Lee M, Sehatpour P, Hoptman MJ, Lakatos P, Dias EC, Kantrowitz JT, et al. Neural mechanisms of mismatch negativity dysfunction in schizophrenia. Mol Psychiatry. 2017;22:1585–93.
Voloh B, Womelsdorf T. Cell-type specific burst firing interacts with theta and beta activity in prefrontal cortex during attention states. Cereb Cortex. 2018;28:4348–64.
Rajkai C, Lakatos P, Chen CM, Pincze Z, Karmos G, Schroeder CE. Transient cortical excitation at the onset of visual fixation. Cereb Cortex 2008;18:200–9.
Hoffman KL, Dragan MC, Leonard TK, Micheli C, Montefusco-Siegmund R, Valiante TA. Saccades during visual exploration align hippocampal 3-8 Hz rhythms in human and non-human primates. Front Syst Neurosci. 2013;7:43.
Gordon G, Ahissar E. Hierarchical curiosity loops and active sensing. Neural Netw. 2012;32:119–29.
Ranade S, Hangya B, Kepecs A. Multiple modes of phase locking between sniffing and whisking during active exploration. J Neurosci. 2013;33:8250–6.
Jensen O, Spaak E, Park H. Discriminating valid from spurious indices of phase-amplitude coupling. eNeuro 2016;3:ENEURO.0334–16.2016.
Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–30.
Sivarao DV, Frenkel M, Chen P, Healy FL, Lodge NJ, Zaczek R. MK-801 disrupts and nicotine augments 40 Hz auditory steady state responses in the auditory cortex of the urethane-anesthetized rat. Neuropharmacology. 2013;73:1–9.
Hamm JP, Bobilev AM, Hayrynen LK, Hudgens-Haney ME, Oliver WT, Parker DA, et al. Stimulus train duration but not attention moderates gamma-band entrainment abnormalities in schizophrenia. Schizophr Res. 2015;165:97–102.
Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–47.
Rosburg T, Kreitschmann-Andermahr I. The effects of ketamine on the mismatch negativity (MMN) in humans - a meta-analysis. Clin Neurophysiol. 2016;127:1387–94.
Avissar M, Javitt D. Mismatch negativity: a simple and useful biomarker of N-methyl-d-aspartate receptor (NMDAR)-type glutamate dysfunction in schizophrenia. Schizophr Res. 2018;191:1–4.
Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22:1452–64.
Greenwood LM, Leung S, Michie PT, Green A, Nathan PJ, Fitzgerald P, et al. The effects of glycine on auditory mismatch negativity in schizophrenia. Schizophr Res. 2018;191:61–69.
Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology 2008;33:2187–99.
Featherstone RE, Liang Y, Saunders JA, Tatard-Leitman VM, Ehrlichman RS, Siegel SJ. Subchronic ketamine treatment leads to permanent changes in EEG, cognition and the astrocytic glutamate transporter EAAT2 in mice. Neurobiol Dis. 2012;47:338–46.
Nagy LR, Featherstone RE, Hahn CG, Siegel SJ. Delayed emergence of behavioral and electrophysiological effects following juvenile ketamine exposure in mice. Transl psychiatry. 2015;5:e635.
Featherstone RE, Nagy LR, Hahn CG, Siegel SJ. Juvenile exposure to ketamine causes delayed emergence of EEG abnormalities during adulthood in mice. Drug Alcohol Depend. 2014;134:123–7.
Dodman K, Featherstone RE, Bang J, Liang Y, Siegel SJ. Ceftriaxone reverses ketamine-induced lasting EEG and astrocyte alterations in juvenile mice. Drug Alcohol Depend. 2015;156:14–20.
Watson TD, Petrakis IL, Edgecombe J, Perrino A, Krystal JH, Mathalon DH. Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. Int J Neuropsychopharmacol. 2009;12:357–70.
Umbricht D, Vollenweider FX, Schmid L, Grubel C, Skrabo A, Huber T, et al. Effects of the 5-HT2A agonist psilocybin on mismatch negativity generation and AX-continuous performance task: implications for the neuropharmacology of cognitive deficits in schizophrenia. Neuropsychopharmacology. 2003;28:170–81.
Heekeren K, Daumann J, Neukirch A, Stock C, Kawohl W, Norra C, et al. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacol (Berl). 2008;199:77–88.
Roser P, Juckel G, Rentzsch J, Nadulski T, Gallinat J, Stadelmann AM. Effects of acute oral Delta(9)-tetrahydrocannabinol and standardized cannabis extract on the auditory P300 event-related potential in healthy volunteers. Eur Neuropsychopharmacol 2008;18:569–77.
D’Souza DC, Fridberg DJ, Skosnik PD, Williams A, Roach B, Singh N, et al. Dose-related modulation of event-related potentials to novel and target stimuli by intravenous Delta(9)-THC in humans. Neuropsychopharmacology. 2012;37:1632–46.
Brown SB, van der Wee NJ, van Noorden MS, Giltay EJ, Nieuwenhuis S. Noradrenergic and cholinergic modulation of late ERP responses to deviant stimuli. Psychophysiology. 2015;52:1620–31.
Meador KJ, Loring DW, Hendrix N, Nichols ME, Oberzan R, Moore EE. Synergistic anticholinergic and antiserotonergic effects in humans. J Clin Exp Neuropsychol. 1995;17:611–21.
Kantrowitz JT, Oakman E, Bickel S, Citrome L, Spielman A, Silipo G, et al. The importance of a good night’s sleep: an open-label trial of the sodium salt of gamma-hydroxybutyric acid in insomnia associated with schizophrenia. Schizophr Res. 2010;120:225–6.
Polania R, Nitsche MA, Ruff CC. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci 2018;21:174–87.
Khademi F, Royter V, Gharabaghi A. State-dependent brain stimulation: power or phase? Brain Stimul. 2019;12:296–99.
Brunoni AR, Shiozawa P, Truong D, Javitt DC, Elkis H, Fregni F, et al. Understanding tDCS effects in schizophrenia: a systematic review of clinical data and an integrated computation modeling analysis. Expert Rev Med devices. 2014;11:383–94.
Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny MF, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169:719–24.
Kantrowitz JT, Sehatpour P, Avissar M, Horga G, Gwak A, Hoptman MJ, et al. Significant improvement in treatment resistant auditory verbal hallucinations after 5 days of double-blind, randomized, sham controlled, fronto-temporal, transcranial direct current stimulation (tDCS): A replication/extension study. Brain Stimul. 2019;12:981–91.
Meron D, Hedger N, Garner M, Baldwin DS. Transcranial direct current stimulation (tDCS) in the treatment of depression: systematic review and meta-analysis of efficacy and tolerability. Neurosci Biobehav Rev. 2015;57:46–62.
Mutz J, Edgcumbe DR, Brunoni AR, Fu CHY. Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: A systematic review and meta-analysis of randomised sham-controlled trials. Neurosci Biobehav Rev. 2018;92:291–303.
Shiozawa P, Fregni F, Bensenor IM, Lotufo PA, Berlim MT, Daskalakis JZ, et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;17:1443–52.
VanRullen R. Perceptual cycles. Trends Cogn Sci. 2016;20:723–35.
Fiebelkorn IC, Kastner S. A rhythmic theory of attention. Trends Cog Sci. 2018;23:87–101.
Lee M, Sehatpour P, Dias EC, Silipo GS, Kantrowitz JT, Martinez AM, et al. A tale of two sites: differential impairment of frequency and duration mismatch negativity across a primarily inpatient versus a primarily outpatient site in schizophrenia. Schizophr Res 2018;191:10–17.
Acknowledgements
This paper includes concepts and ideas developed in a study group for the 2018 Annual Meeting of the American College of Neuropsychopharmacology. We acknowledge the helpful comments of Dr. Irwin Feinberg both at the study group and in response to the paper.
Author information
Authors and Affiliations
Contributions
DCJ participated in drafting the work or revising it critically for important intellectual content; final approval of the submitted version; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SJS participated in drafting the work or revising it critically for important intellectual content; final approval of the submitted version; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. KMS participated in drafting the work or revising it critically for important intellectual content; final approval of the submitted version; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. DHM participated in drafting the work or revising it critically for important intellectual content; final approval of the submitted version; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. LEH participated in drafting the work or revising it critically for important intellectual content; final approval of the submitted version; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AM participated in drafting the work or revising it critically for important intellectual content; final approval of the submitted version; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CLE participated in drafting the work or revising it critically for important intellectual content; final approval of the submitted version; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AIA participated in drafting the work or revising it critically for important intellectual content; final approval of the submitted version; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. TT participated in drafting the work or revising it critically for important intellectual content; final approval of the submitted version; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. PL participated in drafting the work or revising it critically for important intellectual content; final approval of the submitted version; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. TW participated in drafting the work or revising it critically for important intellectual content; final approval of the submitted version; and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Javitt, D.C., Siegel, S.J., Spencer, K.M. et al. A roadmap for development of neuro-oscillations as translational biomarkers for treatment development in neuropsychopharmacology. Neuropsychopharmacol. 45, 1411–1422 (2020). https://doi.org/10.1038/s41386-020-0697-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-020-0697-9