Abstract
There is a critical need to better understand the neural basis of antidepressant medication (ADM) response with respect to both symptom alleviation and quality of life (QoL) in major depressive disorder (MDD). Reward neurocircuitry has been implicated in QoL, the neural basis of MDD, and the mechanisms of ADM response. Yet, we do not know whether change in reward neurocircuitry as a function of ADM is associated with change in symptoms and QoL. To address this gap in knowledge, we analyzed data from 128 patients with MDD who participated in the iSPOT-D trial and were assessed with functional neuroimaging pre- and post-ADM treatment (randomized to sertraline, venlafaxine-XR, or escitalopram). 58 matched healthy controls were scanned at the same time points. We quantified functional connectivity (FC) of reward neurocircuitry using nucleus accumbens (NAc) seed regions of interest, and then characterized how changes in FC relate to symptom response (primary outcome) and QoL response (secondary outcome). Symptom responders showed an increase in NAc-dorsal anterior cingulate cortex (ACC) FC relative to non-responders (p < 0.001) which was associated with improvement in physical QoL (p < 0.0003), and a decrease in NAc-inferior parietal lobule FC relative to controls (p < 0.001). QoL response was characterized by increases in FC between NAc-ventral ACC for environmental, NAc-thalamus for physical, and NAc-paracingulate gyrus for social domains (p < 0.001). Symptom responders to sertraline were distinguished by a decrease in NAc-insula FC (p < 0.001) and to venlafaxine-XR by an increase in NAc-inferior temporal gyrus FC (p < 0.005). Findings suggest that change in reward neurocircuitry may underlie differential ADM response profiles with respect to symptoms and QoL in depression.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is experienced by over 17 million Americans annually [1], and is a leading cause of disability with devastating effects on quality of life (QoL) [2]. Clinical trials have focused on treatment response based upon clinically assessed symptoms as the outcome measure [3]. Yet, it is increasingly evident that impairments in QoL can persist for years, even after “successful” treatment of depressive symptoms [4]. Thus, the first premise of the current study was that it is important to consider response assessed by change in QoL as well as by change in symptoms. The second premise was that in order to advance our understanding of the neural mechanisms by which some patients do not respond to ADMs, in regard to symptoms and/or QoL, it is important to also undertake trials that incorporate direct measures of neural function such as with functional neuroimaging.
Qualitative analyses indicate that improvement in QoL may be of equal or of greater importance to patients as improvement in symptoms [5]; yet, very few clinical trials to-date have incorporated QoL as an outcome measure of treatment response in depression [6]. Similarly, neuroimaging trials of treatment response have focused on symptom outcomes defined by traditional diagnostic criteria (e.g., the DSM) and assessed clinically by scales such as the Hamilton Depression Rating Scale (HDRS), but not with respect to change in QoL [7, 8]. The use of both clinical symptom and QoL outcomes, together with functional neuroimaging measures to understand the changes in brain circuitry underlying such outcomes, has the potential to contribute to a more nuanced understanding of patients’ lived experience of “response” to treatment [7, 8].
Multiple neuroimaging studies have documented reward circuit dysfunction in MDD (e.g., for meta-analysis [9]; for review [10]), and dysfunction in reward processing has been implicated in ADM response [11, 12]. Yet, we do not know whether change in reward neurocircuitry as a function of ADM is associated with change in symptoms and QoL. To address these gaps in knowledge, in this prospective randomized-controlled ADM treatment trial we focused on intrinsic functional connectivity (FC) of reward circuitry, utilizing repeated functional neuroimaging scans, to characterize change in neural circuitry in relation to improvement with respect to both clinically-defined symptoms (primary outcome) and QoL (secondary outcome). The first objective was to (1a) test the hypothesis that MDD patients who were symptom responders would be characterized by a greater pre-to-post ADM increase in reward circuitry FC, particularly between the NAc and frontal cortical regions, relative to symptom non-responders and healthy controls, and (1b) characterize the relationship between changes in reward circuit FC profiles that distinguished symptom responders from non-responders relative to improvement in QoL. The second objective was to examine the hypothesis that patients with MDD who demonstrate improvement in QoL (referred to as “functional” response) would be characterized by unique profiles of pre-to-post ADM change in reward circuitry. The third objective was to explore differential profiles of change in reward circuit FC according to the type of ADM that each patient was randomized to receive: escitalopram, sertraline, or venlafaxine-XR.
Methods
Study design
Data were collected as part of the International Study to Predict Optimized Treatment for Depression clinical trial (iSPOT-D): a randomized, parallel-model, open-lab repeated measure, longitudinal 8-week trial assessing candidate clinical and behavioral response to three of the most commonly prescribed first-line ADM [7]. For the iSPOT-D neuroimaging substudy, participants’ data were collected at the Brain Dynamics Centre at the University of Sydney and the sample size was selected to provide statistical power of 80% and an effect size of 1 standard deviation for analysis of predictive measures [8].
Participants
As per the iSPOT-D protocol [7, 8] all clinical participants met criteria for MDD as assessed by the Mini-International Neuropsychiatric Interview in accordance with the DSM-IV [13] and had a score of ≥16 (at least moderate severity) on the Hamilton Depression Rating Scale (HDRS17) [14]. All MDD participants were either ADM naive or had undergone a wash-out period of at least five half-lives at the start of the study. Exclusion criteria included current or past diagnosis of psychosis, bipolar disorder, post-traumatic stress disorder, obsessive-compulsive disorder, as well as any contraindication to neuroimaging [7, 8]. The study was conducted in accordance with the principles of the Declaration of Helsinki 2008 and informed consent was obtained from all participants.
The final sample included data for a total of 128 MDD participants and 56 age, gender, and education matched healthy controls with fMRI neuroimaging scans and both symptom and QoL outcome measures acquired prior to treatment and at 8-week follow-up (Supplementary Fig. 1; CONSORT chart). Selection of these participants followed a rigorous artifact identification and removal procedures suited to the goals of intrinsic FC analysis. Participants with excessive artifact on either fMRI scan (i.e., >20% of their volumes identified as exceeding motion criteria) or who had missing clinical assessment data were not included in the present study (for details; Study Supplementary S3).
Baseline age was significantly higher (F1,126 = 8.46, p < 0.001) in symptom non-responders relative to symptom responders and controls. Race significantly differed between groups (\({\upchi}_8^2\) = 19.51, p = 0.01). Duration of illness was longer (t(126)=2.29, p = 0.02) and sertraline dosage was higher (t(40)=3.79, p < 0.001) in symptom non-responders compared to responders. There were no between-group differences in baseline education, gender, or occupation. There were no differences between symptom responders and non-responders with respect to baseline HDRS scores, comorbid anxiety diagnoses, age of MDD onset, escitalopram dosage, or venlafaxine-XR dosage (all p’s>0.05). Participants with a baseline HDRS score of 16–18 (mild-moderate severity) did not significantly differ in symptom response rate relative to participants with moderate-severe MDD (baseline HDRS > 18). Demographic, change in symptom and QoL assessments, and medication information are summarized in Table 1, with additional detail in Supplementary Tables 2 and 3.
Study treatments
MDD participants were randomized to receive one of three first-line ADM: sertraline, escitalopram, or venlafaxine-XR. Randomization was carried out using PhaseForward’s™ validated, Web-based Interactive Response Technology. A blocked randomization procedure (block size of 12) was managed at the level of the Global Coordinating Center in Sydney, Australia. Over the course of study participation, medication doses were adjusted as determined appropriate by clinicians in accordance with routine clinical practice and recommended dose ranges. Due to the practical trial design, clinicians and patients were not blind to treatment assignment.
Criteria for response
The primary outcome—symptom response—was defined as a 50% or greater improvement according to the HDRS [8] (administered by blinded clinician raters) in accordance with the pre-registered iSPOT-D protocol and standard definition of clinical symptom response to ADM [7, 15]. According to this criterion for 57% (n = 73) were classified as symptom responders and 43% (n = 55) as non-responders (Table 1). The predetermined secondary outcome measure of change in QoL was assessed using the World Health Organization QoL Assessment (WHOQOL) with respect to four domains: physical health, psychological health, social relationships, and external environment rated on a scale of 0–100 [16]. Functional response in QoL was defined as a 25% or greater increase on each domain assessed in the WHOQOL, which corresponds with substantial improvement given that the threshold for healthy QoL is considered >70. According to this definition, of patients with MDD 53% (n = 67) were responders in physical and social domains, 76% (n = 95) were responders in the psychological domain, and 30.4% (n = 38) in the environmental domain (Supplementary Table S3). Unsurprisingly, symptom responders had significantly greater improvement in QoL than symptom non-responders pre-to-post treatment (Table 1, Supplementary Table S2). However, there was not a direct overlap between responders and non-responders defined by symptom and QoL criteria (see Supplementary Table S5; Supplementary Fig. S4).
Image acquisition
The neuroimaging data were collected using a 3.0-T GE signa scanner and an eight-channel head coil. MR images were acquired using echo planar imaging (TR = 2500 ms, TE = 27.5 ms, matrix = 64 × 64, FOV = 24 cm, flip angle = 90°). In each volume, 40 slices (each 3.5 mm thick) covered the whole brain. 120 volumes for each task were acquired. Structural T1-weighted images were obtained in the sagittal plane using a 3D spoiled gradient echo sequence (TR = 8.3 ms; TE = 3.2 ms; flip angle = 11°, TI = 500 ms, NEX = 1, ASSET = 1.5, matrix = 256 × 256). 180 contiguous slices (each 1 mm thick) covered the whole brain with an in-plane resolution of 1 × 1 mm2. Please refer to the Study Supplement for a detailed description fMRI data acquisition (S1) and preprocessing (S2) [17].
Intrinsic FC was derived from the residual time series from five tasks with 120 volumes collected from each task for a total scan time of 5 min. The residual time series were concatenated following the removal of task and covariate effects. General linear models (GLMs) were used to model the BOLD responses for each experimental condition for each task. Additional covariates included the mean signal time course extracted from eroded ventricle and white matter masks as well as the temporal masks derived from the volume censoring for each task. The intrinsic FC signal was estimated as the residual images after modeling the BOLD signal for each stimulus of the above tasks as regressors of non-interest after applying a very-low frequency band-pass filter (0.009 < f < 0.08 Hz) [18]. This procedure has been validated to correspond to intrinsic FC extracted from standard resting scans [19,20,21,22].
Analysis of group differences in demographic clinical and QoL data
The symptom responder, non-responder and control groups were compared on demographic, clinical and QoL assessments using one-way ANOVAs with post-hoc Tukey tests and chi-square tests, as appropriate, using SPSS Version 24. Demographic, change in symptom and QoL assessments, and medication information are summarized in Table 1, with additional detail in Supplementary Tables S2 and S3. We used R version 3.6.1 to perform linear regressions.
Analysis of pre-to-post treatment change in reward circuit FC
We anatomically defined a priori bilateral NAc regions of interest (ROIs) using the WFU PickAtlas (Fig. 1a). FC analyses were conducted using a whole-brain seed-to-voxel approach in CONN [17]. Pearson’s r correlation coefficients were computed between the time course of each NAc seed and the time course of all other voxels in the brain. Resulting correlation coefficients were converted to z-scores using Fisher’s transformation and used as inputs in second level GLM analyses to investigate between-group differences. The significance voxel-level threshold was set to p < 0.001 for all primary analyses and to p < 0.005 for exploratory subgroup analysis of ADM. To correct for multiple comparisons, a cluster-level FDR threshold of p < 0.05 based on Gaussian field theory was utilized for all analyses [23].
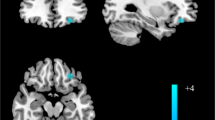
a Right and left NAc seed regions of interest (anatomically defined using the WFU PickAtlas). Significant differences in pre-to-post treatment change in FC between symptom responders versus non-responders. b Symptom responders had a significant increase in FC between the right NAc and the right dorsal anterior cingulate cortex (dACC) (peak coordinates 8, −4, 38) compared to symptom non-responders (p < 0.001 voxel-level). There was a significant association between change in HDRS and right NAc-dACC pre-to-post treatment change in FC (R = −0.31, p < 0.001). c Symptom responders also had a significant pre-to-post treatment decrease in FC between the left NAc and the right inferior parietal lobule (peak coordinates 56, −32, 48) compared to CTL (p < 0.001 voxel-level). d Sertraline responders had a significant pre-to-post treatment decrease in FC between the right NAc and the left insular cortex (peak coordinates −32, −36, 4) compared to sertraline non-responders (p < 0.001 voxel-level). e Venlafaxine responders had a significant pre-to-post treatment increase in FC between the right NAc and the right inferior temporal gyrus (peak coordinates 42, −36, −24) compared to venlafaxine non-responders (p < 0.005 voxel-level). Box-and-Whisker plots depict significant pre-to-post treatment between-group differences in FC. Color bars represents t values from the between-group paired t-tests.
Primary outcome: treatment-related change in reward circuit FC as a function of symptom response status and in relation to QoL
Two-tailed t-tests of change in FC pre-to-post treatment were conducted to directly examine relative differences between change in reward circuitry FC in (1) symptom responders versus symptom non-responders, (2) symptom responders versus controls, and (3) symptom non-responders versus controls (Table 2a). Regression modeling was used to test whether discovered associations between change in HDRS pre-to-post treatment and change in FC in MDD subjects remained significant after correcting for the effect of possible confounding covariates that have been suggested in prior literature to be associated with treatment response including: age [24], gender [25], race [26], duration of illness [27], baseline symptom severity [28], medication type [29], medication dose [30], and comorbid anxiety disorder [31] (Table 2a and d). For interpretation of these results, individual FC values from these significant clusters were extracted post-hoc for follow-up analyses (Section S6 of the Study Supplement; Supplementary Table S4; Supplementary Fig. S3).
Using regression modeling, we then examined whether those changes in FC that were significantly associated with symptom response vs. non-response were also associated with improvement in QoL (Table 3a). Similarly, we assessed whether those changes in FC that were significantly associated with response in QoL were also associated with improvement in clinical symptoms (Table 3b), as well as change in FC associated with improvement in QoL independent of improvement in clinical symptoms (Table 3c). Lastly, we explored whether those changes in FC that were significantly associated with specific ADM symptom response vs. non-response were also associated with improvement in QoL (Table 3d). We used the Benjamini–Hochberg procedure to control the False Discovery Rate at level α = 0.05 for all findings in this analysis.
Secondary outcome: treatment-related change in reward circuit FC as a function of QoL improvement
Two-tailed t-tests of change in FC pre-to-post treatment were conducted to directly examine change in reward circuitry FC in patients with MDD that showed QoL response versus non-response in environmental, physical, psychological, and social domains (Table 2b). For changes in FC that were significantly associated with QoL response, we tested whether these associations were still significant after adjusting for clinical symptoms (baseline HDRS scores and pre-to-post changes in HDRS) and other potentially confounding covariates (see Table 2b; if not significant after adjustment, this would suggest that these associations could be explained by known correlations between QoL and clinical symptoms). Associations were deemed significant if the coefficient corresponding to the change in FC had a p value < 0.05.
We performed a complementary analysis designed to identify changes in FC that correlate with changes in QoL independent of changes in clinical symptoms (Table 2c). We regressed residualized treatment changes in QoL on changes in FC using a two-stage regression approach [32]. First, in patients with MDD, we regressed change in QoL domains on change in HDRS, baseline HDRS, and possible confounding variables (detailed in Table 2c). Next, this regression model was used to estimate the expected change in each QoL domain in the absence of any FC information; these estimates were subtracted from the observed changes in QoL domains in each patient to generate residuals (i.e., residual variation unexplained by treatment, demographics, and changes in clinical symptoms). These residuals were used to conduct a whole-brain search to identify changes in reward circuitry FC that are associated with changes in QoL independent of changes in symptoms.
Exploratory: treatment-related change in FC as a function of specific ADM
Two-tailed t-tests of change in FC pre-to-post treatment were conducted to examine differential change in FC with specific ADM in symptom responders versus non-responders while accounting for the effect of time, with between-group differences as the main outcome of interest and accounting for baseline symptoms and possible confounding covariates (Table 2d).
Results
Primary outcome: treatment-related change in reward circuit FC as a function of symptom response status and in relation to QoL
Symptom responders vs. non-responders
A two-tailed t-test revealed that responders were distinguished from non-responders by a significantly greater increase in right NAc-right dACC FC. These findings remained significant after adjusting for potential confounding covariates (Table 2a, Fig. 1b). There were no significant differences in baseline right NAc-right dACC FC between responders and non-responders. For baseline FC differences between controls and MDD patients, refer to Section S7 of the study supplement (Supplementary Table S6; Supplementary Fig. S5).
Symptom responders vs. controls
Responders showed decreased left NAc-right inferior parietal lobule (IPL) FC relative to healthy controls. These findings remained significant after adjusting for potential confounding covariates (Table 2a, Fig. 1c).
Symptom non-responders vs. controls
Non-responders were distinguished from controls by a comparative increase in FC between the left NAc and the left lateral occipital cortex. These findings remained significant after adjusting for relevant covariates (Table 2a, Section S8 of the Study Supplement, Supplementary Fig. 2).
Pre-to-post treatment increases in NAc-dACC FC were significantly associated with pre-to-post treatment increases (improvement) in the WHOQOL physical health domain (bunstandardized = 32.148, bstandardized = 0.322, p < 0.0003; R2 = 0.104, F1,122 = 14.103, p < 0.0003; Table 3a). This association remained significant after controlling for potential confounding baseline covariates. It did not, however, remain significant after adjusting for pre-to-post treatment changes in HDRS. Change in NAc-dACC FC showed no significant associations with other WHOQOL domains. The item-level questions used to assess change in physical health (and other QoL domains) are presented in Supplementary Table S1.
Secondary outcome: treatment-related change in reward circuit FC as a function of QoL improvement
Between-group comparisons: QoL responders vs. non-responders
Physical QoL
There was a significant increase in right NAc-right thalamus FC in physical QoL responders relative to non-responders at p < 0.001 voxel-level. This difference remained significant after adjusting for potential confounding variables (Table 2b, Fig. 2c). Change in NAc-Thalamus FC was independent of, i.e., not significantly associated with change in HDRS (Table 3b).
Coronal view of the a Right and left NAc seed regions of interest (anatomically defined using the WFU PickAtlas). b Between-group comparison between social QoL responders* and non-responders displayed greater pre-to-post treatment increase in FC between the right NAc seed and the right paracingulate gyrus (peak coordinates +14, +54, −04; this cluster comprises a portion of the frontal medial cortex and ACC) among responders relative to non-responders (WhoSocR > WhoSocNR). c Between-group comparison between physical QoL responders* and non-responders displayed greater pre-to-post treatment increase in FC between the right NAc and the right thalamus (peak coordinates +02, −34, +06) among responders relative to non-responders (WhoPhe-R > WhoPheNR). d Residualized dimensional improvement in environmental QoL (WHO-Env) extracted from patients with MDD showed a significant association with pre-to-post treatment increase in FC between the right NAc and the right ventral anterior cingulate cortex (peak x y z coordinates +04, +46, 00). *QoL responders were defined as exhibiting a 25% or greater improvement in the WHOQOL domain at a voxel-level significant threshold of p < 0.001. Box-and-Whisker plots depict significant pre-to-post treatment between-group FC differences. Color bar represents t values from the between-group paired t-tests.
Social QoL
There was a significant increase in right NAc-right paracingulate gyrus FC in social QoL responders relative to non-responders. This difference was significant after adjusting for potential confounding covariates (Table 2b, Fig. 2b). Change in right NAc-right paracingulate gyrus FC was significantly associated with change in HDRS, though this relationship did not survive FDR-correction (Table 3b).
There were no significant differences in baseline FC between QoL responders and non-responders in any of the domains.
Dimensional improvement in QoL independent of change in HDRS (residuals)
Environmental QoL
Increased right NAc-right ventral ACC (vACC) FC was significantly associated with improvement in environmental QoL pre-to-post treatment independent of change in clinical symptoms (HDRS scores) and potential confounding covariates (Table 2c, Table 3c, Fig. 2d).
Exploratory: treatment-related change in FC as a function of specific ADM
Sertraline responders vs. non-responders
Sertraline responders had a significant pre-to-post treatment decrease in right NAc-left insular cortex FC compared to sertraline non-responders which remained significant at p < 0.001 voxel-level thresholding and after adjusting for potential confounding covariates (Table 2d, Fig. 1d). No significant associations were found between change in FC and QoL pre-to-post treatment with sertraline (Table 3d). In addition, there was a significant association between sertraline dose and change in NAc-insula FC (p < 0.002) (Section S9 of Study Supplement; Supplementary Fig. S6).
Venlafaxine-XR responders vs. non-responders
Venlafaxine-XR responders had a significant pre-to-post treatment increase in right NAc-right inferior temporal gyrus FC compared to venlafaxine-XR non-responders at voxel-level p < 0.005. This association remained significant after controlling for potential confounding covariates (Table 2d, Fig. 1e). No significant associations were found between change in FC and QoL pre-to-post treatment with Venlafaxine-XR (Table 3d).
Escitalopram responders vs. non-responders
There were no significant between-group differences in NAc FC between escitalopram responders and non-responders.
Discussion
This study is the first to examine ADM treatment change in reward circuit FC with outcome measures of symptom response and QoL in MDD. Symptom responders demonstrated a greater increase in NAc-dACC FC compared to non-responders, which was significantly associated with improvement in physical QoL, and decreased NAc-IPL FC relative to controls. Improvement in QoL was associated with differential increases in striatal–thalamic–cortical FC: physical QoL with increased NAc-thalamus FC, social QoL with increased NAc-paracingulate FC, and environmental QoL with increased NAc-vACC FC. Lastly, we found distinct changes in reward circuit FC associated with symptom response to sertraline and venlafaxine-XR, but not escitalopram.
Symptom response to ADM treatment was associated with increased NAc-dACC FC. The dACC is a cortical brain region implicated in supporting reward valuation and is activated during choice selection and anticipation of rewards [33, 34]. Activation of the ACC has shown robust functional coupling with the NAc during processing of differential magnitude of gains and missed rewards [35]. Several studies have demonstrated decreased FC between striatal and frontal reward-related regions in individuals with MDD (e.g., [36] and at familial risk (e.g., [37]) for MDD. While we are unaware of other studies that have found increased NAc-dACC FC with ADM treatment, fluoxetine and venlafaxine-XR were found to increase FC between the NAc and frontal cortical regions in patients with MDD during an emotion processing task in association with an increase in positive affect [38]. Thus, increased NAc-dACC FC may contribute to clinical symptom reduction by altering fronto–striatal-based reward processing.
Symptom responders also exhibited reduced NAc-IPL FC compared to controls. The IPL is involved in downregulating reward expectancy [39], and reduced connectivity between these regions could represent reduced downregulation and a consequent increase in the ability to experience reward among treatment responders. The IPL is also a central component of the default mode network (DMN), posited to underlie rumination and negative self-appraisal in depression [40, 41]. Indeed, hyperconnectivity of the DMN is one of the most robust FC findings in depression [22]. Greater FC between the NAc and DMN may thereby impair reward processing due to excessive focus on internalizing thoughts at the expense of attending to and experiencing external rewards. This suggest that reductions in inter-network hyperconnectivity between the DMN and reward circuitry may contribute to greater likelihood of treatment response. A reduction in FC between reward circuitry and the DMN in treatment responders may thus be a compensatory mechanism that counterweights DMN hyperconnectivity in depression.
Distinct pre-to-post treatment increases in cortico-striatal FC of reward circuitry were associated with dimensional improvement in environmental QoL (NAc-vACC FC) and categorical response versus non-response in social (NAc-paracingulate gyrus FC) and physical (NAc-thalamus FC) QoL. Pre-to-post treatment increases in NAc associated with improvement in environmental and physical QoL are particularly noteworthy as they were independent of clinical symptom improvement. Improved physical QoL was also significantly associated with increased NAc-dACC FC in relation to symptom response. Conversely, change in reward circuitry FC did not relate to psychological QoL, consistent with the notion that the absence of depressive symptoms is not equivalent to psychological health [42]. PET and FMRI studies have highlighted the critical importance of these cortical brain regions, the vACC in particular, for proper reward circuit functioning [43]. The paralimbic belt of the ACC (i.e., paracingulate cortex) represents a cingulo-frontal transition area, given its reciprocal connections with prefrontal cortical regions, and is referred to as the ‘cognitive’ division of the ACC [44]. Along with the dACC, these regions are posited to work in concert to compare valued options, choose among them, and channel that choice into a course of action that promotes acquiring the most rewarding outcome [43, 45], functions that must work properly in order to have a satisfactory QoL. Studies examining neural correlates of happiness and wellbeing have similarly reported the critical importance of proper functioning of the NAc and frontal brain regions including the ACC and insular cortex [46,47,48]. Our findings suggest that, as striatal–cortical FC within reward circuitry improves, patients may increase their activity levels and engagement with life activities, resulting in improved QoL. Furthermore, differential changes in reward circuit FC with ADM may help identify neurobiologically based correlates of treatment response with respect to QoL.
Symptom response to sertraline and venlafaxine-XR was associated with differential changes in reward circuit FC; a decrease in NAc-insula FC for sertraline and an increase in NAc-inferior temporal gyrus FC for venlafaxine-XR. This differential change accords with prior findings from the iSPOT-D trial observed during inhibition task-related FC, in which a decrease in precentral-superior temporal FC was associated with symptom response for sertraline, and an increase in orbitofrontal–subcortical FC was associated with symptom response for venlafaxine-XR [49]. These opposing profiles in relation to reward circuitry might in part reflect the action of sertraline and venlafaxine-XR at the receptor level, in particular the action of sertraline in inhibiting dopamine [50, 51] and the role of venlafaxine-XR as an inhibitor of the reuptake of dopamine and noradrenaline as well as serotonin [52, 53]. Our observation that the NAC was associated with opposing directions of effect in connection with distinct cortical regions (insula for sertraline and temporal gyrus for venlafaxine-XR) further suggests distinct mechanisms by which these antidepressants may exert their effects on reward circuitry.
We note study limitations: iSPOT-D was designed as a practical trial comparing first-line ADMs in clinical settings, and thus no placebo comparison was incorporated. While study findings inform the use of change in reward circuitry FC as a potential biomarker of ADM response with implications for mechanisms, trials designed to incorporate a placebo arm are needed to probe mechanistic change in a direct and causal manner. Recent findings from the EMBARC study report a moderating effect of ventral striatal activity on sertraline treatment response versus placebo following 8-weeks of treatment [54]. Although iSPOT-D was powered to compare three different ADM arms, larger samples with imaging measures may be needed to detect differential reward-related biomarkers that distinguish among SSRI and SNRI categories. While rigorous motion artifact correction was applied, artifact always remains a potential confound when interpreting fMRI data [17]. In the present study, we used an a priori seed-based approach given our specific focus on change in reward circuit FC. Given the promising findings for both symptom and QoL response it would be important in future studies to extend this approach to data-driven connectomic analyses. For example, such future work might investigate pre-treatment predictive models and additional outcome measures, thereby expanding on complementary iSPOT-D findings for pretreatment functional connectivity prediction of treatment response and remission [55].
In conclusion, quality of life is a vital and integral component of health, and many patients with MDD continue to experience markedly diminished quality of life even with “successful” treatment response in terms of traditionally-defined clinical criteria. Study findings highlight changes in FC within reward neurocircuitry that may be potential neural substrates that mechanistically contribute to improvement with ADM treatment. Furthermore, distinct findings with respect to clinical symptoms and quality of life highlight the importance of assessing quality of life outcomes that are not currently assessed in the majority of clinical trials and neuroimaging studies of depression. By characterizing differential profiles of change in reward circuit FC in relation to improvement in symptoms and quality of life, this work has the potential to contribute to a more nuanced assessment, mechanistic interpretation and definition of treatment response with ADM.
Funding and disclosures
ASF, BHG, SLF, LMH, TBM, AFS, and LMW have no financial disclosures related to the work reported in this manuscript. The International Study to Predict Optimized Treatment in Depression (iSPOT-D) was sponsored by Brain Resource Ltd (NCT00693849). ASF was supported by NIMH T32-MH019938, TMB by NIMH K23-MH113708, and LMW by R01 MH101496. In the past year AFS has received consulting fees from Alkermes, Avanir, Bracket, Epiodyne, Jazz, Lundbeck/Takeda, McKinsey, Myriad Genetics, Neuronetics, Owl Biomedical and Sage, has equity in Corcept (co-founder), Epiodyne, Gilead, Incyte, Intersect ENT, Merck, Owl Biomedical, Seattle Genetics, Titan and Xhale, has received research funding from Janssen and is named inventor on pharmacogenetic and antiglucocorticoid use patents on prediction of ADM response. In the past year, LMW has received scientific advisory board fees from Psyberguide of the One Mind Institute and is named as inventor on an image processing system patent for depression and anxiety. In the past year, SLF has received consulting fees from Youper. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources; the funding agencies played no role in the study design, collection, management, analysis, interpretation, preparation, review, interpretation, or submission of this article.
References
National Institutes of Health (NIH). Prevalence of major depressive episode among adults. 2019. https://www.nimh.nih.gov/health/statistics/major-depression.shtml#part_155029.
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence,prevalence, and years lived with disability for diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602.
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40.
Rhebergen D, Beekman AT, de Graaf R, Nolen WA, Spijker J, Hoogendijk WJ, et al. Trajectories of recovery of social and physical functioning in major depression, dysthymic disorder and double depression: a 3-year follow-up. J Affect Disord. 2010;124:148–56.
Chevance A, Ravaud P, Tomlinson A, Le Berre C, Teufer B, Touboul S, et al. Identifying outcomes for depression that matter to patients, informal caregivers, and health-care professionals: qualitative content analysis of a large international online survey. Lancet Psychiatry. 2020;7:692–702.
Dzevlan A, Redzepagic R, Hadzisalihovic M, Curevac A, Masic E, Alisahovic-Gelo E, et al. Quality of life assessment in antidepressant treatment of patients with depression and/or anxiety disorder. Mater Sociomed. 2019;31:14–18.
Williams LM, Rush AJ, Koslow SH, Wisniewski SR, Cooper NJ, Nemeroff CB, et al. International study to predict optimized treatment for depression (iSPOT-D), a randomized clinical trial: rationale and protocol. Trials. 2011;12:4.
Grieve SM, Korgaonkar MS, Etkin A, Harris A, Koslow SH, Wisniewski S, et al. Brain imaging predictors and the international study to predict optimized treatment for depression: study protocol for a randomized controlled trial. Trials. 2013;14:224.
Ng TH, Alloy LB, Smith DV. Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl Psychiatry. 2019;9:293.
Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12.
Greenberg T, Fournier JC, Stiffler R, Chase HW, Almeida JR, Aslam H, et al. Reward related ventral striatal activity and differential response to sertraline versus placebo in depressed individuals. Mol Psychiatry. 2019;25:1526–36.
Lener MS, Iosifescu DV. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Ann N Y Acad Sci. 2015;1344:50–65.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. quiz 34-57.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–5.
Hotopf M, Mayou R, Wadsworth M, Wessely S. Temporal relationships between physical symptoms and psychiatric disorder. Results from a national birth cohort. Br J Psychiatry. 1998;173:255–61.
Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41.
Goldstein-Piekarski AN, Staveland BR, Ball TM, Yesavage J, Korgaonkar MS, Williams LM. Intrinsic functional connectivity predicts remission on antidepressants: a randomized controlled trial to identify clinically applicable imaging biomarkers. Transl Psychiatry. 2018;8:57.
Elliott ML, Knodt AR, Cooke M, Kim MJ, Melzer TR, Keenan R, et al. General functional connectivity: shared features of resting-state and task fMRI drive reliable and heritable individual differences in functional brain networks. Neuroimage. 2019;189:516–32.
Cole MW, Ito T, Schultz D, Mill R, Chen R, Cocuzza C. Task activations produce spurious but systematic inflation of task functional connectivity estimates. Neuroimage. 2019;189:1–18.
Korgaonkar MS, Ram K, Williams LM, Gatt JM, Grieve SM. Establishing the resting state default mode network derived from functional magnetic resonance imaging tasks as an endophenotype: a twins study. Hum Brain Mapp. 2014;35:3893–902.
Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76.
Friston KJ, Worsley KJ, Frackowiak RS, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–20.
Grigoriadis S, Kennedy SH, Bagby RM. A comparison of antidepressant response in younger and older women. J Clin Psychopharmacol. 2003;23:405–7.
Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–52.
Murphy E, Hou L, Maher BS, Woldehawariat G, Kassem L, Akula N, et al. Race, genetic ancestry and response to antidepressant treatment for major depression. Neuropsychopharmacology. 2013;38:2598–606.
Ghio L, Gotelli S, Marcenaro M, Amore M, Natta W. Duration of untreated illness and outcomes in unipolar depression: a systematic review and meta-analysis. J Affect Disord. 2014;152-154:45–51.
Chen CH, Ridler K, Suckling J, Williams S, Fu CH, Merlo-Pich E, et al. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol Psychiatry. 2007;62:407–14.
Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–58.
Holck A, Wolkowitz OM, Mellon SH, Reus VI, Nelson JC, Westrin A, et al. Plasma serotonin levels are associated with antidepressant response to SSRIs. J Affect Disord. 2019;250:65–70.
Andreescu C, Lenze EJ, Dew MA, Begley AE, Mulsant BH, Dombrovski AY, et al. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: controlled study. Br J Psychiatry. 2007;190:344–9.
Darlington RB, & Hayes, AF. Regression analysis and linear models: concepts, applications, and implementation. Guilford Publications; 2016.
Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, et al. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–97.
Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55:594–602.
Pedroni A, Koeneke S, Velickaite A, Jancke L. Differential magnitude coding of gains and omitted rewards in the ventral striatum. Brain Res. 2011;1411:76–86.
Furman DJ, Hamilton JP, Gotlib IH. Frontostriatal functional connectivity in major depressive disorder. Biol Mood Anxiety Disord. 2011;1:11.
Fischer AS, Ellwood-Lowe ME, Colich NL, Cichocki A, Ho TC, Gotlib IH. Reward-circuit biomarkers of risk and resilience in adolescent depression. J Affect Disord. 2019;246:902–09.
Heller AS, Johnstone T, Light SN, Peterson MJ, Kolden GG, Kalin NH, et al. Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. Am J Psychiatry. 2013;170:197–206.
Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11:880–1.
Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47:1434–48.
Viviani R. Emotion regulation, attention to emotion, and the ventral attentional network. Front Hum Neurosci. 2013;7:746.
Organization WH. Promoting mental health. Concepts, emerging evidence, practice. Geneva: World Health Organization; 2004.
Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26.
Fornito A, Yucel M, Wood S, Stuart GW, Buchanan JA, Proffitt T, et al. Individual differences in anterior cingulate/paracingulate morphology are related to executive functions in healthy males. Cereb Cortex. 2004;14:424–31.
Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7.
Berridge KC, Kringelbach ML. Building a neuroscience of pleasure and well-being. Psychol Well Being. 2011;1:1–3.
Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–78.
Suardi A, Sotgiu I, Costa T, Cauda F, Rusconi M. The neural correlates of happiness: A review of PET and fMRI studies using autobiographical recall methods. Cogn Affect Behav Neurosci. 2016;16:383–92.
Tozzi L, Goldstein-Piekarski AN, Korgaonkar MS, Williams LM. Connectivity of the cognitive control network during response inhibition as a predictive and response biomarker in major depression: evidence from a randomized clinical trial. Biol Psychiatry. 2020;87:462–72.
Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline: are they all alike? Int Clin Psychopharmacol. 2014;29:185–96.
Nemeroff CB, Owens MJ. Pharmacologic differences among the SSRIs: focus on monoamine transporters and the HPA axis. CNS Spectr. 2004;9:23–31.
Wellington K, Perry CM. Venlafaxine extended-release: a review of its use in the management of major depression. CNS Drugs. 2001;15:643–69.
Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry. 2000;61:5–12.
Greenberg T, Fournier JC, Stiffler R, Chase HW, Almeida JR, Aslam H, et al. Reward related ventral striatal activity and differential response to sertraline versus placebo in depressed individuals. Mol Psychiatry. 2020;25:1526–36.
Korgaonkar MS, Goldstein-Piekarski AN, Fornito A, Williams LM. Intrinsic connectomes are a predictive biomarker of remission in major depressive disorder. Mol Psychiatry. 2020;25:1537–49.
Acknowledgements
We thank the study participants for participating in this study. We gratefully acknowledge the contributions of the co-investigators at the Sydney site where imaging data were acquired. Specifically, we thank Dr. Anthony Harris and Dr. Tim Usherwood who were the clinical PIs, and Dr. Claire Day who was the Global Study coordinator. We thank Dr. Stuart Grieve, Dr. Lavier Gomes, Ms. Sheryl Foster, and the Department of Radiology at Westmead Hospital for their substantial contributions to MRI data acquisition and other contributions to the imaging component of the iSPOT-D study. Clinical trials registration details are as follows: Trial registration: International Study to Predict Optimized Treatment in Depression (iSPOT-D); registry name: Clinical-Trials.gov; Registration number: NCT00693849.
Author information
Authors and Affiliations
Contributions
LMW designed the umbrella iSPOT-D study. ASF, BHG, SLF, and LMW conceptualized and designed the analytical plan. ASF, BHG, and SLF analyzed the data. ASF, BHG, SLF, TMB, LMH, AFS, and LMW wrote the paper. All authors approve the final version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fischer, A.S., Holt-Gosselin, B., Fleming, S.L. et al. Intrinsic reward circuit connectivity profiles underlying symptom and quality of life outcomes following antidepressant medication: a report from the iSPOT-D trial. Neuropsychopharmacol. 46, 809–819 (2021). https://doi.org/10.1038/s41386-020-00905-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-020-00905-3
This article is cited by
-
Sexual dysfunction worsens both the general and specific quality of life of women with irritable bowel syndrome. A cross-sectional study
BMC Women's Health (2023)
-
Plasticity of synapses and reward circuit function in the genesis and treatment of depression
Neuropsychopharmacology (2023)
-
Association of executive function with suicidality based on resting-state functional connectivity in young adults with subthreshold depression
Scientific Reports (2023)
-
Brain connectivity in major depressive disorder: a precision component of treatment modalities?
Translational Psychiatry (2023)
-
Optimizing psychedelic compounds for neuropsychiatric therapy
Neuropsychopharmacology (2021)