Abstract
Cocaine-associated contextual cues can trigger relapse behavior by recruiting the hippocampus. Extinction of cocaine-associated contextual memories can reduce cocaine-seeking behavior, however the molecular mechanisms within the hippocampus that underlie contextual extinction behavior and subsequent reinstatement remain poorly understood. Here, we extend our previous findings for a role of Cav1.2 L-type Ca2+ channels in dopamine 1 receptor (D1R)-expressing cells in extinction of cocaine conditioned place preference (CPP) in adult male mice. We report that attenuated cocaine CPP extinction in mice lacking Cav1.2 channels in D1R-expressing cells (D1cre, Cav1.2fl/fl) can be rescued through chemogenetic activation of D1R-expressing cells within the dorsal dentate gyrus (dDG), but not the dorsal CA1 (dCA1). This is supported by the finding that Cav1.2 channels are required in excitatory cells of the dDG, but not in the dCA1, for cocaine CPP extinction. Examination of the role of S1928 phosphorylation of Cav1.2, a protein kinase A (PKA) site using S1928A Cav1.2 phosphomutant mice revealed no extinction deficit, likely due to homeostatic scaling up of extinction-dependent S845 GluA1 phosphorylation in the dDG. However, phosphomutant mice failed to show cocaine-primed reinstatement which can be reversed by chemogenetic manipulation of excitatory cells in the dDG during extinction training. These findings outline an essential role for the interaction between D1R, Cav1.2, and GluA1 signaling in the dDG for extinction of cocaine-associated contextual memories.
Similar content being viewed by others
Introduction
Cocaine addiction is characterized by compulsive drug intake that can be triggered by learned associations between contextual cues and the rewarding properties of the drug [1,2,3]. In humans and rodents, the hippocampus plays a large role in establishing and maintaining associations between a drug and the environmental context in which the drug is taken. In particular, the dorsal hippocampus (dHPC), which is heavily implicated in contextual memory, can form cocaine-context associations and trigger drug seeking upon reexposure to the drug-paired context [2]. Furthermore, subregions of the hippocampus are differentially implicated in contextual associations. The dorsal dentate gyrus (dDG) subregion of the dHPC, which is the primary input to the hippocampal circuit, generates contextual memories associated with cocaine conditioned place preference (CPP) [4], in part through dopamine 1 receptor (D1R)-dependent mechanisms [5, 6]. Accordingly, the dDG is activated following reexposure to a cocaine-paired context [7] and has been recently implicated in extinction of morphine CPP [8] and cue-induced reinstatement of methamphetamine self-administration [9]. Cocaine also recruits the dorsal CA1 (dCA1) subregion of the dHPC during cocaine CPP [10, 11].
Our previous work has found that extinction of cocaine CPP involves Cav1.2 L-type Ca2+ channel (LTCC) mechanisms within the dHPC [12]. Cav1.2 is well known for its role in hippocampal-dependent learning and memory [13, 14]. The Cav1.2 gene CACNA1C has been identified as a major candidate risk gene for multiple neuropsychiatric disorders that are highly comorbid with substance abuse and addiction [13, 14] with risk alleles being associated with alcohol dependence and life-time cocaine abuse in bipolar patients [15] as well as altered reward processing in healthy individuals [16, 17]. We reported that Cav1.2 channels in D1R-expressing cells are required for extinction of cocaine CPP and extinction-dependent changes in hippocampal protein levels [12], suggesting that D1Rs and Cav1.2 may work in tandem within the hippocampus to produce extinction behavior. D1Rs play a crucial role in memory processes including extinction of learned behaviors such as cocaine CPP [18]. However, the hippocampal subregion and cell type in which D1Rs and Cav1.2 channels interact to produce extinction behavior and their subsequent impact on cocaine-primed reinstatement (CPR) remains unknown.
Cav1.2 channels can be regulated in an activity-dependent manner by protein kinase A (PKA) [19, 20], potentially via phosphorylation at serine 1928 (S1928) [19, 21]. PKA, activated downstream of D1Rs [22], also phosphorylates GluA1 AMPA receptor at serine 845 (S845) [23] which is shown to be involved in spatial memory [24, 25] and cocaine self-administration [26]. PKA is localized to Cav1.2 [20, 27, 28] and GluA1 [20, 23, 29] by A-kinase anchor protein 150 (AKAP150), which was identified in an unbiased proteomic screen to be significantly increased in the nucleus accumbens following cocaine extinction and is required for cocaine-induced reinstatement behavior via recruitment of GluA1 AMPA receptor mechanisms [30]. This PKA–AKAP interaction is also required within hippocampal synapses for mechanisms underlying hippocampal-dependent contextual learning and memory [31].
Here, using a combination of genetic, chemogenetic, and molecular experiments, we provide evidence that excitatory cells of the dDG recruit Cav1.2 channel signaling within D1R-expressing cells that is necessary for extinction and cocaine-induced reinstatement of CPP behavior.
Materials and methods
Animals
Adult (>postnatal day 60) male wildtype mice (Jackson Laboratories) and all mutant mice used for experiments were bred on the C57BL/6J background. Mice with conditional knockout of Cav1.2 in dopamine D1 receptor (D1R) expressing cells (D1cre, Cav1.2fl/fl) and their wildtype littermates (D1cre, Cav1.2+/+) were generated by breeding male and female D1cre, Cav1.2+/− mice. Viral vector mediated deletion of Cav1.2 was achieved using Cav1.2fl/fl mice as previously published [12]. Mice expressing a serine to alanine knock-in (KI) substitution at position serine 1928 of Cav1.2 (S1928A Cav1.2 KIs) were generated as described previously [32]. Mice expressing a serine to alanine KI substitution at position serine 845 of GluA1 (S845A GluA1 KIs) were generated as described previously [33]. Mutant mice were indistinguishable from wildtype mice in weight, development, and general health. Mice were maintained under climate-controlled conditions on a 12 h light/dark cycle with ad-libitum access to food and water. All procedures were approved by the Weill Cornell Medicine Institutional Animal Care and Use Committee and conducted in accordance with the 2011 Eighth Edition of the National Institutes of Health guidelines for the Care and Use of Laboratory Animals.
Drugs
Cocaine Hydrochloride (Sigma Aldrich) was dissolved in 0.9% saline at a concentration of 1 mg/mL and injected intraperitoneally at 0.01 ml/g body weight for a final dose of 10 mg/kg. Clozapine-N-oxide (CNO, Enzo Life Sciences) was dissolved in saline at a concentration of 0.3 mg/ml and injected 0.01 ml/g body weight for a final dosage of 3 mg/kg.
Chemogenetic manipulation
DREADDs (Designer Receptor Exclusively Activated by Designer Drug) were utilized to manipulate cells during behavioral testing. Adult male mice underwent bilateral stereotaxic surgery as previously described [12] using coordinates adopted from the Mouse Brain Atlas [34]. A Cre-dependent adeno-associated viral (AAV) vector expressing hM3Dq excitatory DREADD (AAV8-hSyn-DIO-HA-hM3DGq-IRES-mCitrine, Duke University Viral Vector Core) [35] was injected into bilateral dDG (1.8 mm anterior, 2.5 mm ventral, and ±1.2 mm lateral to bregma, 400 nl/side) or bilateral dCA1 (1.8 mm anterior, 2.0 mm ventral, and ±1.2 mm lateral to bregma, 500 nl/side) of D1cre, Cav1.2fl/fl mice. Experimental mice received CNO (3 mg/kg, i.p.) 45 min prior to each extinction training session. The control group consisted of two types of surgeries. Mice in the first control group were injected with a Cre-dependent AAV expressing the hM3Dq excitatory DREADD into the dDG (200 nl/side) and dCA1 (300 nl/side) and received saline injection (0.01 ml/g body weight; i.p.) 45 min prior to each extinction training session. Mice in the second control group were injected with a Cre-dependent virus lacking the hM3Dq isoform (AAV8-EF1a-DIO-eYFP) into the dDG and dCA1 and received CNO (3 mg/kg, i.p.) 45 min prior to each extinction training session. Fluorescence immunohistochemistry was performed to confirm injection placement.
A CaMK2-driven excitatory DREADD (AAV8-CaMK2a-hM3D(Gq)-mCherry, addgene #50476) [35] was used to excite CaMK2-expressing cells of the dDG in S1928A Cav1.2 KI mice during extinction training. Adult male mice were bilaterally injected into the dDG as described above. For control mice, an AAV expressing eGFP under the CaMK2 promoter (AAV8-CaMK2a-eGFP) was injected into bilateral dDG. Behavioral experiments began after a minimum of 3 weeks to allow for maximal DREADD virus expression. All mice received CNO (3 mg/kg, i.p.) 45 min prior to each extinction training session but did not receive CNO prior to the cocaine-reinstatement test. Fluorescence immunohistochemistry was performed to confirm injection placement.
Focal deletion of Cav1.2
An AAV expressing Cre recombinase under the CaMK2 promoter (AAV2/2-CaMK2-Cre-eGFP) was bilaterally delivered into either the dDG or dCA1 of cacna1c floxed mice (Cav1.2fl/fl mice) using coordinates and volumes listed above. For control mice, an AAV expressing GFP under the CaMK2 promoter (AAV2/2-CaMK2-eGFP) was delivered into the dDG (200 nl/side) and dCA1 (300 nl/side). GFP fluorescence immunohistochemistry was performed to confirm injection placement.
Fluorescence immunohistochemistry
Brain sections were incubated in anti-chicken GFP (1:5000; Aves Labs, cat # GFP-1010) and anti-rabbit mCherry (1:5000; Abcam, cat # ab167453) as previously described [12] and imaged using an epifluorescent microscope (Leica DM550B with Leica Application Suite Advanced Fluorescence 3.0.0 build 8134 software, Leica Microsystems).
CPP behavioral protocol
Cocaine CPP and extinction was performed as previously published [12] using a biased protocol with a three-chamber place preference apparatus (Med Associates Inc., St. Albans, VT, USA). During the baseline test, mice were given free access to all three chambers for 20 min, and time spent in each chamber was recorded. During the acquisition training period (days 2–4), in the morning session, each mouse was given a systemic injection of saline (0.01 ml/g body weight) and confined for 20 min to the most-preferred chamber. Six hours later (afternoon session), each mouse was given a systemic injection of cocaine (10 mg/kg i.p.) and confined to the opposite chamber for 20 min. For experiments involving a saline control group, mice were injected with saline prior to being placed into both the morning-assigned chamber and afternoon-assigned chamber. For the acquisition test session (day 5), mice were allowed free access to all three chambers for 20 min, and cocaine preference score (in seconds) was calculated as time spent in the cocaine-paired chamber minus time spent in the saline-paired chamber.
To assess extinction, mice were allowed free access to all three chambers for 20 min (beginning day 6) every 24 h and cocaine preference was evaluated as above. For experiments that compared extinction scores between groups, all mice were run for 4 days of extinction training and preference scores on the final day of extinction training were reported. For experiments that included a CPR test, mice were injected with cocaine (10 mg/kg, i.p.) and allowed free access to all three chambers for 20 min. Cocaine preference was evaluated as time spent in cocaine-paired chamber minus time spent in saline-paired chamber.
Locomotion
Horizontal distance traveled as measured by number of beam breaks was recorded in the three-chamber place preference apparatus (Med Associates Inc., St. Albans, VT, USA) during baseline CPP testing, extinction CPP testing, and cocaine-reinstatement testing using computer-assisted activity monitoring software (Med Associates) for 20 min.
Protein lysate preparation
Protein lysates were prepared as previously published [36]. One hour following the final extinction trial, mice were rapidly decapitated and whole brains were quickly removed. Fresh brains were sectioned in the coronal plane using a brain block (Zivic instruments), and the dDG and dCA1 were bilaterally dissected. Tissue was homogenized using a Teflon pestle (Pyrex) in cold sucrose buffer containing protease and phosphatase inhibitors. After spinning the homogenate at 1000 × g for 10 min, the supernatant was collected and used for western immunoblotting.
Western immunoblotting
Western immunoblotting was performed as previously described [12]. Twenty micrograms of protein/sample were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes, which were blocked in 5% nonfat dry milk and then incubated 24–48 h at 4 °C in a primary antibody prior to being incubated in peroxidase-linked IgG conjugated secondary antibody at 1:5000 at room temperature for 1 h. Blots were incubated with the following primary antibodies at a dilution of 1:1000; Cav1.2 (Alomone Labs Cat# AGP-001), S1928 P-Cav1.2 (Signalway Antibody, Cat # 12674), AKAP150 (Santa Cruz, Cat # sc-10765), PKA (BD Biosciences Cat # 610980), GluA1 (Millipore Cat# AB1504), S845 P-GluA1 (Abcam Cat # ab3901), and GAPDH (Abcam Cat# ab22555). The Cav1.2 antibody used has been validated for its specificity [36]. Proteins were quantified at the following molecular weights: Cav1.2 and P-Cav1.2 at 250 kDa, PKA at 45 kDa, AKAP150 at 150 kDa, GluA1 and P-GluA1 at 100 kDa, and GAPDH at 36 kDa. Protein bands were quantified as mean optical intensity using ImageJ software (NIH) and normalized to GAPDH. Normalized optical density values from each sample were used to calculate the fold change for each treatment group compared with the control group (set to 1.0) on the same blot.
Statistics
All CPP behavioral data were analyzed by repeated measures two-way ANOVA followed by Bonferroni–Dunn post-hoc test to compare main effects of treatment and testing session between groups. Other data were first analyzed for normality using the Shapiro–Wilk normality test. All normally distributed experimental data were analyzed by a parametric independent samples t-test. Nonnormally distributed data were analyzed with a nonparametric test (Mann–Whitney). Statistical analyses were conducted using GraphPad Prism version 6.0 for Mac (GraphPad Software, La Jolla, CA) and considered to be statistically significant for values of p ≤ 0.05.
Results
Activation of dopamine D1R-expressing cells in the dorsal DG is sufficient to rescue the extinction deficit in D1cre, Cav1.2fl/fl mice
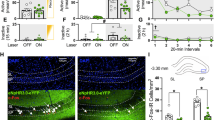
We previously demonstrated using a conditional knockout mouse line in which Cav1.2 was globally deleted from dopamine D1 receptor (D1R)-expressing cells (D1cre, Cav1.2fl/fl) that Cav1.2 channels were required in this cell type for extinction of cocaine CPP and for extinction-associated protein changes in the hippocampus [12]. To further determine the hippocampal subregion involved in this extinction deficit, we used chemogenetics to test whether excitation of dopamine D1R-expressing cells lacking Cav1.2 in the dDG or dCA1 during extinction was sufficient to rescue the extinction deficit previously observed in D1cre, Cav1.2fl/fl mice compared with D1cre, Cav1.2+/+ mice [12]. Adult male D1cre, Cav1.2fl/fl mice received bilateral stereotaxic injections of a Cre-dependent excitatory DREADD (AAV8-hSyn-DIO-HA-hM3DGq-IRES-mCitrine) into the dDG or dCA1 (Fig. 1a). Control groups included D1cre, Cav1.2fl/fl mice that were either injected with the Cre-dependent excitatory hM3DGq DREADD in the dHPC and received saline during extinction training to control for viral expression (hM3Dq + Saline) or injected with the control AAV8-EF1a-DIO-eYFP in the dHPC and received CNO during extinction training to control for off-target behavioral effects (eYFP + CNO). The spread of injections targeting the dDG or dCA1 is displayed in Supplementary Fig. 1.
a D1cre, Cav1.2fl/fl mice were injected with a Cre-dependent AAV expressing hM3D(Gq) into either bilateral dDG or bilateral dCA1 in order to express hM3Dq in D1R-expressing cells that lacked Cav1.2. Representative images of brain sections from D1cre, Cav1.2fl/fl mice displaying mCitrine-positive cells expressing AAV8-hSyn-DIO-HA-hM3D(Gq)-IRES-mCitrine in either the dDG or dCA1. b Experimental timeline of the CPP acquisition and extinction protocol and CNO treatment. Mice were injected with CNO 45 min prior to extinction training. c All groups of D1cre, Cav1.2fl/fl mice acquired cocaine CPP prior to any CNO treatment (*p < 0.0001, Bonferroni post-hoc baseline vs. acquisition). Both groups of D1cre, Cav1.2fl/fl control mice (hM3Dq + Saline [n = 7] and eGFP + CNO [n = 9]) failed to extinguish cocaine CPP (*p < 0.0001, Bonferroni post-hoc baseline vs. extinction). D1cre, Cav1.2fl/fl mice injected with excitatory DREADDs in the dDG (n = 8) extinguished cocaine CPP (#p < 0.001, Bonferroni post-hoc acquisition vs. extinction) while D1cre, Cav1.2fl/fl mice injected with excitatory DREADDs in the dCA1 (n = 8) continued to show an extinction deficit (*p < 0.0001, Bonferroni post-hoc baseline vs. extinction).
Mice underwent cocaine CPP training and testing as outlined in Fig. 1b. As expected from our prior study [12], all groups acquired cocaine CPP. Following acquisition of cocaine CPP, mice underwent extinction training. Forty-five minutes prior to each extinction session, mice were injected with CNO (hM3DGq + CNO and eYFP + CNO) or saline (hM3DGq + Saline). On the final extinction session, both groups of D1cre, Cav1.2fl/fl control mice continued to show an extinction deficit (Fig. 1c), as previously published in naive D1cre, Cav1.2fl/fl mice compared with D1cre, Cav1.2+/+ mice [12]. In contrast, mice injected with the excitatory DREADD into the dDG, but not the dCA1 were able to fully extinguish their cocaine preference (Fig. 1c; main effect of test day, F(2, 56) = 64.83, p < 0.0001, no main effect of experimental group, F(3, 28) = 1.501, p = 0.2358, no significant interaction, test day × region, F(6, 56) = 1.364, p = 0.2453). These results demonstrated that chemogenetic activation of the D1R-expressing cells of the dDG, but not the dCA1 was sufficient to rescue the extinction deficit in D1cre, Cav1.2fl/fl mice.
Cav1.2 channels are required in excitatory cells of the dorsal DG for extinction of cocaine CPP
Given our findings that activating dDG D1R-expressing cells of D1cre, Cav1.2fl/fl mice was sufficient to reverse the extinction deficit, we next sought to examine the dDG cell type in which Cav1.2 channels play a role. Previous work has shown that D1Rs are expressed predominantly within excitatory neurons of the dDG [37, 38] whereas in the dCA1 they are predominantly expressed in inhibitory neurons [38, 39]. Using morphology as a cursory readout of Cre-dependent expression of mCitrine in D1R-expressing cells in the dDG and dCA1, our data supported these previous findings. Cells within the dDG revealed strong labeling (Fig. 2a) with the observed morphology and localization of tightly packed, elliptical cells within the granule cell (GC) layer with a cone-shaped tree of apical dendrites extending throughout the molecular layer (Fig. 2a′), matching characteristics of excitatory cells [40] and in agreement with D1R-expressing cells within this layer being mostly excitatory [38]. Immunohistochemistry within the dCA1 revealed sparse labeling of D1R-expressing cells (Fig. 2b) suggestive of previously reported inhibitory cell types [39] that matched the dendritic morphology of inhibitory cells [41] as well as biased labeling of cells in the stratum oriens (Or) and stratum radiatum (Rad) (Fig. 2b′) that mostly express inhibitory cell types [42].
a Representative images of brain sections from D1cre, Cav1.2fl/fl mice displaying mCitrine-positive D1R-expressing cells in the dDG. Labeled cells are expressed most densely in the granule cell (GC) layer of the dDG and display morphological characteristics of principal excitatory cells (A′). b Representative images of brain sections from D1cre, Cav1.2fl/fl mice displaying mCitrine-positive D1R-expressing cells in the dCA1. Labeled cells are sparsely expressed throughout the stratum radiatum (Rad) and stratum oriens (Or) with almost a complete lack of expression in the stratum pyramidale (Pyr) (B′). Labeled cells display morphological characteristics of inhibitory neurons. c Left, Cav1.2fl/fl mice were injected with an AAV expressing Cre under the CaMK2 promoter into either bilateral dDG or bilateral dCA1 in order to selectively delete Cav1.2 in excitatory cells of these subregions. Right, Representative images of brain sections from Cav1.2fl/fl mice displaying eGFP-positive cells expressing AAV2-CaMK2-eGFP-cre in either the dDG or dCA1. d Experimental timeline of surgery and the CPP extinction protocol. e Control mice [eYFP (dDG + dCA1); n = 15] acquired (*p < 0.0001, Bonferroni post-hoc baseline vs. acquisition) and extinguished (#p < 0.0001, Bonferroni post-hoc acquisition vs. extinction) cocaine CPP. Mice with Cav1.2 deleted in excitatory cells of the dDG (n = 8) acquired (*p < 0.0001, Bonferroni post-hoc baseline vs. acquisition) but did not extinguish (*p < 0.0001, Bonferroni post-hoc baseline vs. extinction) cocaine CPP. Mice with Cav1.2 deleted in excitatory cells of the dCA1 (n = 6) acquired (*p < 0.001, Bonferroni post-hoc baseline vs. acquisition) and extinguished (#p < 0.001, Bonferroni post-hoc acquisition vs. extinction) cocaine CPP.
Given the presence of D1Rs primarily in excitatory cells in the dDG based on published work [37, 38] and morphological characteristics as described above, we next sought to determine if Cav1.2 was required in this dDG cell population for cocaine CPP extinction. We generated focal deletion of Cav1.2 in excitatory dDG or dCA1 cells in adult male Cav1.2fl/fl mice by bilateral stereotaxic delivery of AAV2/2-CaMK2-eGFP-Cre (Fig. 2c), with viral spread similar to that displayed in Supplementary Fig. 1. Control mice were injected with AAV2/2-CaMK2-eGFP into the dHPC. We have previously demonstrated efficient knockdown of Cav1.2 mRNA and protein using this identical Cre recombinase strategy in Cav1.2fl/fl mice [43,44,45,46,47,48]. Mice underwent cocaine CPP training and testing (Fig. 2d). All groups of injected mice acquired cocaine CPP (Fig. 2e), demonstrating that Cav1.2 channels in excitatory cells of the dDG or the dCA1 is not required for the acquisition of cocaine CPP. However, compared with control and dCA1 injected mice, AAV2/2-CaMK2-Cre dDG injected mice showed a significant deficit in extinction of cocaine CPP (Fig. 2e; main effect of test day, F(2, 52) = 47.13, p < 0.0001, no main effect of region, F(2, 26) = 0.6672, p = 0.5217, significant interaction, test day × region, F(4, 52) = 4.02, p = 0.0065). Taken together, the findings that chemogenetic excitation of Cav1.2-deficient D1R-expressing cells within the dDG rescues the extinction deficit (Fig. 1c) and that D1Rs are expressed primarily in excitatory dDG cells (Fig. 2a) [37, 38] suggest that Cav1.2 channels within D1R-expressing excitatory cells of the dDG may be functionally involved in extinction-induced plasticity.
Extinction of cocaine CPP induces D1R-dependent molecular changes in the dorsal DG
Next to begin to explore the signaling mechanisms downstream of D1Rs and Cav1.2 channels in the dDG, we first examined molecular changes in C57BL/6J mice that underwent either saline or cocaine CPP training and extinction (Fig. 3a, b; main effect of test day, F(2, 28) = 9.862, p = 0.0004, no main effect of treatment, F(1, 14) = 1.543, p = 0.2346, significant interaction, test day × treatment, F(2, 28) = 10.6, p = 0.0004). We compared protein levels from mice that underwent saline and cocaine extinction as our previous study found that Cav1.2 levels were altered only following extinction, but not acquisition of cocaine CPP nor a similar period of withdrawal following cocaine CPP [12].
a Experimental timeline of the saline and cocaine extinction protocol in C57BL/6J mice. b Cocaine (n = 8), but not saline-treated mice (n = 8) demonstrate acquisition (*p < 0.0001, Bonferroni post-hoc baseline vs. acquisition) and extinction (#p < 0.0001, Bonferroni post-hoc acquisition vs. extinction) of cocaine CPP. c Extinction of cocaine CPP significantly increased protein levels of Cav1.2, S1928 P-Cav1.2/GAPDH, S845 P-GluA1/GAPDH, S845 P-GluA1/GluA1, and PKA but not S1928 P-Cav1.2/ Cav1.2, GluA1, or AKAP150 in the dDG of cocaine extinguished mice (n = 7–8) compared with saline controls (n = 8). Representative bands from each group run on the same blot are shown. d Experimental timeline of the CPP extinction protocol in D1cre, Cav1.2fl/fl mice. e Both D1cre, Cav1.2+/+ mice (n = 8) and D1cre, Cav1.2fl/fl mice (n = 9) acquired cocaine CPP (*p < 0.0001, Bonferroni post-hoc baseline vs. acquisition). D1cre, Cav1.2+/+ mice extinguished cocaine CPP (#p < 0.001, Bonferroni post-hoc acquisition vs. extinction), while D1cre, Cav1.2fl/fl mice demonstrated an extinction deficit (*p < 0.0001, Bonferroni post-hoc baseline vs. extinction). f Following extinction training, the dDG of D1cre, Cav1.2+/+ (n = 6–8) and D1cre, Cav1.2fl/fl mice (n = 6–8) was analyzed for protein changes. Compared with D1cre, Cav1.2+/+ mice that successfully extinguished cocaine CPP, extinction-resistant D1cre, Cav1.2fl/fl mice showed significant decreases in Cav1.2, S845 P-GluA1/GAPDH, and AKAP150 and no differences in S1928 P-Cav1.2/GAPDH, S1928 P-Cav1.2/Cav1.2, S845 P-GluA1/GluA1, GluA1, and PKA. Representative bands from each genotype run on the same blot are shown.
After the last extinction session, total protein lysates from dDG were used for western blots to examine changes in protein levels of Cav1.2, S1928 P-Cav1.2, GluA1, S845 P-GluA1, AKAP150, and PKA. The S1928 P-Cav1.2 antibody was validated for specificity using protein lysates from mice lacking the S1928 phosphorylation site (S1928A Cav1.2 KI mice) [32] that demonstrated significantly lower levels of S1928 P-Cav1.2 levels compared with wildtype littermates (Supplementary Fig. 2; t(9) = 6.933; p < 0.0001). Quantitative analysis revealed that cocaine CPP extinction significantly increased Cav1.2 levels in the dDG (p = 0.0003, U = 0), similar to our previous report using total hippocampal lysates [12]. Examination of S1928 P-Cav1.2 was higher when normalized to GAPDH (Fig. 3c; p = 0.0104, U = 8), however this increase was absent when normalized to total Cav1.2 levels (Fig. 3c; t(13) = 1.872; p = 0.0839). Protein levels of S845 P-GluA1 were higher following extinction when normalized to GAPDH (Fig. 3c; t(14) = 2.491; p = 0.0259) and total GluA1 (Fig. 3c; p = 0.0012, U = 2). PKA levels were also higher (t(13) = 2.476; p = 0.0278; Fig. 3c). No change in levels of total GluA1 (t(14) = 1.575; p = 0.1377) or AKAP150 (t(13) = 0.9432; p = 0.3628) was observed (Fig. 3c).
Next to determine whether the observed molecular changes in the dDG could be attributed to D1R-expressing cells, D1cre, Cav1.2+/+ mice and D1cre, Cav1.2fl/fl mice were examined for protein changes following cocaine CPP extinction (Fig. 3d). Replicating our previous findings [12], D1cre, Cav1.2fl/fl mice did not extinguish cocaine CPP (Fig. 3e; main effect of test day, F(2, 30) = 33.37, p < 0.0001, no main effect of genotype, F(1, 15) = 0.5573, p = 0.4669, significant interaction, test day × genotype, F(2, 30) = 5.182, p = 0.0117). As expected based on our previous report using hippocampus tissue [12], Cav1.2 was significantly reduced in the dDG of D1cre, Cav1.2fl/fl mice compared with D1cre, Cav1.2+/+ mice (Fig. 3f; t(11) = 2.224; p = 0.0480). Protein levels of S1928 P-Cav1.2 were unaltered in D1cre, Cav1.2fl/fl mice compared with D1cre, Cav1.2+/+ mice when normalized to GAPDH (Fig. 3f; t(12) = 1.13; p = 0.2805) or total Cav1.2 (Fig. 3f; t(11) = 0.6954; p = 0.5013). Protein levels of total GluA1 were unaltered between genotypes (Fig. 3f; t(11) = 1.552; p = 0.1490) while S845 P-GluA1 was significantly lower when normalized to GAPDH (Fig. 3f; t(11) = 3.203; p = 0.0084) but not total GluA1 (Fig. 3f; t(11) = 1.542; p = 0.1514). AKAP150 levels were significantly lower in D1cre, Cav1.2fl/fl mice compared with D1cre, Cav1.2+/+ mice (Fig. 3f; t(11) = 2.703; p = 0.0205) while PKA (Fig. 3f; t(12) = 0.4727; p = 0.6449) was unaltered between genotypes.
S1928A Cav1.2 KI mice demonstrate an attenuated response to cocaine-primed reinstatement (CPR)
Next, given the inconclusive findings regarding the contribution of S1928 P-Cav1.2 to extinction using western analysis (Fig. 3c, f), we next sought to complement these findings by examining global S1928A Cav1.2 phosphomutant KI mice [32] and their WT littermates in cocaine CPP (Fig. 4a). KI mice showed no gross abnormalities [19, 32] and no difference in basal locomotion (Supplementary Fig. 3A; t(36) = 0.436; p = 0.6655). Both S1928A Cav1.2 KI mice and WT littermates acquired cocaine CPP (Fig. 4b), and both groups of mice were able to fully extinguish their cocaine preference (Fig. 4b). Given these findings, we next sought to determine the necessity of S1928 P-Cav1.2 phosphorylation for CPR. Following extinction, mice were tested in CPR following a priming injection of cocaine. WT littermates, but not S1928A Cav1.2 KI mice, showed successful reinstatement of cocaine CPP (Fig. 4b; main effect of test day, F(3, 63) = 21.67, p < 0.0001, no main effect of genotype, F(1, 21) = 2.629, p = 0.1199, significant interaction, test day × genotype, F(3, 63) = 4.8, p = 0.0045). The lack of reinstatement was not due to altered cocaine-induced locomotor activity during the CPR test. Cocaine significantly increased locomotor activity in both genotypes, with significantly higher cocaine-induced locomotor response in KI versus WT mice (Supplementary Fig. 3B; main effect of test day, F(1,26) = 42.41, p < 0.0001, main effect of genotype, F(1,26) = 20.64, p = 0.0001, no significant interaction, test day × genotype F(1, 26) = 1.933, p = 0.176).
a Experimental timeline of the CPP acquisition, extinction, and cocaine-primed reinstatement (CPR) protocol. b Both WT littermates (n = 13) and S1928A Cav1.2 KI (n = 10) mice demonstrate acquisition (*p < 0.001, Bonferroni post-hoc baseline vs. acquisition) and extinction (#p < 0.01, Bonferroni post-hoc acquisition vs. extinction) of cocaine CPP. WT littermates reinstated cocaine CPP following a cocaine-priming injection (^p < 0.0001, Bonferroni post-hoc extinction vs. CPR), whereas S1928A Cav1.2 KIs demonstrated a significantly attenuated reinstatement response (#p < 0.001, Bonferroni post-hoc acquisition vs. CPR). c Left, S1928A Cav1.2 KI mice were injected with AAV2-CaMK2-hM3D(Gq)-mCherry in the dDG in order to express the hM3Dq in excitatory cells of the dDG. Right, Representative image of brain sections from S1928A Cav1.2 KI mice displaying mCherry-positive cells expressing AAV8-CaMK2a-hM3D(Gq)-mCherry in the dDG. d Experimental timeline of CPP acquisition, extinction and CPR protocol and CNO treatment. CNO was injected 45 min prior to extinction training but not prior to CPR. e Both S1928A Cav1.2 KI mice with (n = 7) and without (n = 6) hM3Dq in the absence of CNO treatment acquired cocaine CPP (*p < 0.01, Bonferroni post-hoc baseline vs. acquisition). hM3Dq-mediated activation of dDG excitatory cells of S1928A Cav1.2 KI mice had no impact on extinction behavior as both hM3Dq-injected KI and control KI mice showed significantly lower preference score on extinction compared with acquisition test day (#p < 0.01, Bonferroni post-hoc acquisition vs. extinction). On CPR test day in the absence of CNO treatment, however, S1928A Cav1.2 KI control mice did not demonstrate CPR (#p < 0.001, Bonferroni post-hoc acquisition vs. CPR), while S1928A Cav1.2 KI mice with hM3Dq manipulation during extinction demonstrated CPR (^p < 0.0001, Bonferroni post-hoc extinction vs. CPR). f Both S1928A Cav1.2 KI mice with (n = 7) and without (n = 6) the excitatory DREADD showed similar rates of extinction as demonstrated by no difference in the number of days to reach criterion for extinction.
Activation of excitatory cells in the dDG of S1928A Cav1.2 KI mice is sufficient to reverse CPR
Given the surprising findings of a lack of cocaine-induced reinstatement in S1928A Cav1.2 KI mice, we next sought to test the involvement of the dDG during extinction on the lack of CPR in these global KI mice. Using chemogenetics, we tested whether enhancing activity of excitatory cells of the dDG of S1928A Cav1.2 KI mice during extinction training would be sufficient to reverse their lack of cocaine-induced reinstatement.
S1928A Cav1.2 KI mice were injected bilaterally in the dDG with an excitatory DREADD under the CaMK2 promoter (AAV8-CaMK2a-hM3D(Gq)-mCherry) or a control virus (AAV8-CaMK2a-eGFP; Fig. 4c), with typical viral spread similar to that displayed in Supplementary Fig. 1, and subjected to cocaine CPP (Fig. 4d). Both groups acquired cocaine CPP as expected (Fig. 4e). During extinction training, all mice were injected with CNO 45 min prior to placement into the CPP chamber. hM3Dq-mediated activation of dDG excitatory cells of S1928A Cav1.2 KI mice had no impact on extinction behavior as both hM3Dq-injected and control KI mice showed significantly lower preference score on extinction compared with acquisition day (Fig. 4e). In addition, both groups extinguished cocaine CPP within a similar number of days (Fig. 4f; t(11) = 0.2269; p = 0.8247), demonstrating that activation of excitatory cells in the dDG of these mice did not change the rate of extinction. Once extinguished, mice received a cocaine-priming challenge without CNO administration and were tested for reinstatement. S1928A Cav1.2 KI control mice continued to show a lack of reinstatement (Fig. 4e) as previously seen (Fig. 4b). S1928A Cav1.2 KI hM3Dq-injected mice, however, demonstrated a significant reinstatement of cocaine preference in response to the cocaine challenge, (Fig. 4e; main effect of test day, F(3, 33) = 16.40, p < 0.0001, trending significant main effect of genotype, F(1, 11) = 4.073, p = 0.0686, significant interaction, test day × viral treatment, F(3, 33) = 8.429, p = 0.0003), indicating that activation of excitatory dDG cells in S1928A Cav1.2 KI mice during extinction learning was sufficient to induce reinstatement.
S1928A Cav1.2 KI mice demonstrate enhanced basal levels of S845 P-GluA1 that is required for extinction of cocaine CPP
To further explore the lack of the extinction deficit in S1928A Cav1.2 KI mice, we next examined if the chronic developmental loss of phosphorylation at S1928, and potentially chronic developmental loss of Cav1.2 channel activity, could induce compensatory molecular changes in the dDG. Western blot analysis of dDG protein lysates found no basal difference in Cav1.2 levels in S1928A Cav1.2 KI mice compared with WT littermates (Fig. 5a; t(12) = 1.204; p = 0.2516), demonstrating that S1928A mutation does not alter stability of Cav1.2 protein. Although GluA1 protein levels were lower (Fig. 5a; t(12) = 3.02; p = 0.0107), S845 P-GluA1/GAPDH levels were higher in S1928A Cav1.2 KI mice compared with WT littermates (Fig. 5a; t(12) = 1.86; p = 0.0876), resulting in overall higher level of S845 P-GluA1 compared with total GluA1 (Fig. 5a; t(12) = 4.354; p = 0.0009), reminiscent of homeostatic plasticity-induced increase in S845 P-GluA1 [23] as a result of hypoactivity of LTCCs [49, 50]. Interestingly, PKA levels were lower (Fig. 5a; p = 0.0379, U = 8) with no change in AKAP levels (Fig. 5a; t(12) = 0.1034; p = 0.9194).
a Basally, there were no differences in protein levels of Cav1.2 in S1928A Cav1.2 KI (n = 7) compared with WTs (n = 7). S1928A Cav1.2 KI mice had significantly lower protein levels of GluA1 and trending higher protein levels of S845 P-GluA1/GAPDH compared with WTs, resulting in significantly higher protein levels of S845 P-GluA1/GluA1. There was no difference in AKAP150 but significantly lower PKA in S1928A Cav1.2 KI compared with WTs. Representative bands from each genotype are shown from the same blot. b Experimental timeline of the CPP extinction protocol. c Both WT littermates (n = 18) and S845A GluA1 KI mice (n = 18) acquired cocaine CPP (*p < 0.0001, Bonferroni post-hoc baseline vs. acquisition). WTs extinguished cocaine CPP (#p < 0.0001, Bonferroni post-hoc acquisition vs. extinction), whereas S845A GluA1 KI mice did not extinguish (*p < 0.0001, Bonferroni post-hoc baseline vs. extinction) cocaine CPP.
Given the higher basal S845 P-GluA1 in the dDG of S1928A Cav1.2 KI mice that successfully extinguished cocaine CPP (Fig. 5a) and the observed increase in S845 P-GluA1 levels following extinction in C57BL/6J mice (Fig. 3c), we next sought to examine the necessity of S845 GluA1 phosphorylation in extinction of cocaine CPP by testing global KI mice lacking the S845 GluA1 phosphorylation site (S845A GluA1 KI mice) [33]. S845A GluA1 KI and WT mice were subjected to cocaine CPP followed by extinction (Fig. 5b). Although S845A GluA1 KI mice acquired cocaine CPP to a similar degree as WT littermates, S845A GluA1 KI mice were unable to extinguish cocaine CPP (Fig. 5c; main effect of test day, F(2, 48) = 41.57, p < 0.0001, main effect of genotype, F(1, 24) = 6.444, p = 0.0180, trending interaction, test day × genotype, F(2, 48) = 2.072, p = 0.1370), as demonstrated by their sustained high preference score during the extinction test.
Discussion
In this study, we provide genetic and chemogenetic evidence that supports contribution of Cav1.2 channels and D1R-expressing excitatory cells of the dDG in extinction of cocaine CPP. Examination of mice globally deficient in phosphorylation of Cav1.2 at S1928, a PKA site, revealed that these mice do not have a deficit in extinction. However, these S1928 Cav1.2 phosphomutant mice failed to reinstate cocaine CPP following a cocaine challenge. Studies exploring the possible lack of an extinction deficit in S1928 Cav1.2 phosphomutant mice revealed higher basal levels of S845 phosphorylation of GluA1 subunit of AMPA receptors in the dDG, suggesting homeostatic adaptation. Testing mice globally deficient in S845 GluA1 revealed that these mice have an extinction deficit. These findings illustrate involvement of Cav1.2 channels within the dDG in extinction of cocaine CPP, with data supporting a role in D1R-expressing subpopulation of neurons within this region and a role for S1928 P-Cav1.2 in cocaine-induced reinstatement of cocaine CPP.
Dopamine D1Rs, dHPC, Cav1.2 channels, and extinction of cocaine-associated behaviors
Although the role of dopamine and D1Rs in the dHPC during cocaine-associated behaviors, particularly extinction, remains underexplored, studies in humans find that dopamine release in the hippocampus in response to a drug-associated stimuli can enhance hippocampus-dependent memory formation [7] and is shown in rodents to regulate extinction of drug-associated CPP [5, 7], with a role for D1Rs [18]. Our chemogenetic manipulation during extinction training wherein we activated D1R-expressing cells in the dDG of D1cre, Cav1.2fl/fl mice that are primarily excitatory based on previous studies in Drd1a-EGFP mice [37, 38] and observed cell morphology in this study, was sufficient to rescue their extinction deficit. Cre recombinase-mediated knockout of Cav1.2 in excitatory cells of the dDG attenuated cocaine CPP extinction, suggesting that Cav1.2 channels in D1R cells of the dDG are required for extinction. Although Cav1.2 channels have not been studied in the dDG in extinction of drug-related behaviors, our findings are supported by previous literature examining cellular mechanisms of learning in both rat and human dDG cells. These studies demonstrate that activation of voltage-dependent calcium channels is essential for dopamine-induced long term potentiation (LTP) [51, 52], a mechanism associated with fear extinction [53].
However, based on the experimental design utilized in this study, it remains unknown whether Cav1.2 channels promote extinction learning or if DREADD-induced activation of D1R-expressing cells lacking Cav1.2 acutely affects expression of the extinction behavior. Future experiments will examine the consequence of activating dDG D1R-expressing cells during extinction learning by examining extinction behavior in the absence of CNO. We additionally cannot rule out nonspecific hM3Dq expression in D1R-negative neurons of the dDG, however numerous studies have demonstrated the selectivity of hM3Dq expression in Cre-expressing cells using viral brain-specific injections [54, 55]. Our finding that Cav1.2 channels are not required in excitatory cells of the dCA1 that do not express D1Rs [39] (Fig. 2b), supports an important role of synergistic activity of dopamine (via D1Rs) and Cav1.2 channels in the dDG in cocaine CPP extinction learning.
GCs within the dDG, which express D1Rs and do not express D2Rs [38], are considered to be the “gatekeepers” of the hippocampus [56] through their excitatory projections to pyramidal cells in the dCA3 [57]. Overall, dopamine input into the dDG reduces excitation of excitatory GCs [58]. Therefore, activation of D1R-expressing cells in the dDG during extinction learning could potentially inhibit the original cocaine-associated contextual memory that is thought to require activity of the dCA3 region for extinction of cocaine self-administration [59]. Lower activation of D1R-expressing cells in the dDG as a result of Cav1.2 knockout could therefore result in lack of inhibition of dCA3 and thus lead to the observed extinction deficit.
Another potential mechanism by which dopamine can impact extinction is through D1R-mediated enhancement of the differentiation and survival of newborn adult hippocampal neurons, shown to be required for extinction of cocaine CPP and self-administration [7]. As newborn neurons are more excitable and modulate network activity in the dDG, any alterations in neurogenesis, which is dependent on Cav1.2 channels [43, 60, 61], would also impact final output activity of the dHPC [57] and potentially alter neurogenesis-dependent behaviors including extinction learning.
Dopamine D1R, Cav1.2 channels, and dorsal hippocampal signaling mechanisms in extinction of cocaine-associated behaviors
Despite the fact that hippocampal dopamine signaling mechanisms involved in cocaine extinction learning remain poorly understood, rodent studies demonstrate that D1R activation upregulates protein synthesis in hippocampal neurons, a mechanism that contributes to extinction of cocaine CPP [18]. Given that LTCCs [62, 63] and Cav1.2 channels [46] regulate protein synthesis, it is highly plausible that Cav1.2 channel-induced increase in protein synthesis within D1R-expressing cells of the dDG contribute to mechanisms underlying learning of cocaine CPP extinction, a question for future studies. Although our previous publication suggests that the observed protein changes are specific to extinction [12], future studies will also compare protein levels following acquisition and withdrawal of cocaine CPP.
D1R-activated PKA has been implicated in hippocampal-dependent processes and memories [20] including extinction of contextual fear memories [18], although its impact on Cav1.2 channels remains unknown. Neuronal activity localizes PKA to Cav1.2 at hippocampal synapses through the tightly coordinated interaction with AKAP150 [20, 64], phosphorylating Cav1.2 at S1928 [19], a mechanism recently shown to be essential for hippocampal LTP [19]. However our experiment to test the necessity of S1928 using global S1928A Cav1.2 phosphomutant KI mice revealed that these mice successfully extinguished CPP but did not reinstate in response to a cocaine challenge.
There are a few possibilities for the lack of an extinction deficit in S1928A Cav1.2 KI mice. One, it is possible that phosphorylation of Cav1.2 at S1928 in D1R-expressing cells of the dDG is not required for extinction, given the lack of a decrease in S1928 P-Cav1.2 levels in the dDG of D1creCav1.2fl/fl mice. Thus, other D1R-mediated mechanisms regulating Cav1.2 channels may be recruited in the dDG for cocaine CPP extinction.
Another possibility for the lack of an extinction deficit could be a compensatory increase in S845 P-GluA1 in the dDG that we find in S1928A Cav1.2 KI mice using western blot analysis. S845 P-GluA1 has been shown to promote GluA1 targeting to the cell surface, promoting Ca2+-permeable AMPAR formation and hippocampal plasticity [20, 23]. Our findings are reminiscent of another study demonstrating higher levels of Ca2+-permeable AMPARs in excitatory cells of the amygdala of Cav1.2 knockout mice [65], a compensatory adaptation attributed to lack of fear conditioning in these mice. Additional support for this possibility comes from studies in hippocampal neurons demonstrating homeostatic scaling up of S845 P-GluA1 as a result of chronic hypoactivity of LTCCs [49, 50]. Given that extinction increased S845 P-GluA1 in the dDG and global S845A GluA1 phosphomutant mice did not extinguish cocaine CPP, it is plausible that the lack of an extinction deficit in S1928A Cav1.2 KI mice may be due to this compensatory increase in S845 P-GluA1 that is able to override the loss of S1928 P-Cav1.2.
It is also possible that a small subset of D1R dDG cells are recruited during extinction (discussed below), which may explain why we do not see a decrease in S1928 P-Cav1.2 in D1creCav1.2fl/fl mice (Fig. 3f). Future studies using conditional S1928A Cav1.2 KI mice [66] manipulating S1928 in the dDG in a cell type-specific manner, without compensatory adaptations, would be needed to directly test the requirement of S1928 Cav1.2 phosphorylation in the dDG for extinction. The use of viral tools to directly manipulate dDG D1R-expressing cells will additionally address the role of S1928 in this subpopulation of the dDG.
Dopamine D1Rs, Cav1.2 channels, dHPC, and reinstatement of cocaine-associated behaviors
Our cocaine CPP extinction and reinstatement experiments in global S1928A Cav1.2 phosphomutant KI mice revealed that reinstatement requires S1928 Cav1.2 phosphorylation. Use of chemogenetics to activate excitatory cells of the dDG of S1928A KI mice during extinction demonstrated that manipulation of the dDG during extinction impacts cocaine-induced reinstatement of cocaine CPP. This is consistent with findings that neuronal mechanisms underlying extinction learning can impact future reinstatement behavior [1] and that the dHPC is necessary for context-induced reinstatement of cocaine self-administration [59, 67]. Even though our studies do not directly test dopamine D1R signaling or S1928 Cav1.2 phosphorylation in dDG D1R-expressing cells in reinstatement, dopamine action in the dDG through D1Rs is shown to promote reinstatement of morphine-associated CPP [68, 69] and context-induced reinstatement of cocaine self-administration [6]. As the dDG is the input point for the hippocampus, the dDG has a large role in distinguishing specific characteristics of contextual cues to retrieve previously stored memories [4]. In addition, stimulation of the dDG increases dopamine release in the NAc [70] a key region involved in reinstatement of cocaine seeking [3].
It remains unknown whether the same D1R-expressing dDG cells and Cav1.2 channel mechanisms that are recruited during extinction are also recruited during cocaine-induced reinstatement. It is highly possible that separate subpopulations of dDG D1R-expressing cells are driving extinction versus reinstatement. In support of this, a recent contextual fear-conditioning study reported that separate populations (engrams) of dDG neurons drive fear extinction behavior versus relapse of contextual fear behavior [71]. Thus, it is possible that following a cocaine-priming injection, D1R-mediated phosphorylation of Cav1.2 within a subpopulation of D1R-expressing dDG cells are recruited for reinstatement. This is supported by studies showing that reinstatement of cocaine self-administration requires D1Rs in the dHPC [6] and that cocaine-induced reinstatement of self-administration recruits mechanisms involving D1Rs, LTCCs, and GluA1 [72, 73]. As next steps to this study, future studies will directly address this question and the respective cell type-specific contribution of Cav1.2 and S1928 Cav1.2 phosphorylation to extinction versus reinstatement of cocaine CPP.
In summary, we have provided evidence that Cav1.2 channels are required in excitatory cells of the dDG for extinction of cocaine CPP and that this may occur through its actions in D1R-expressing cells of the dDG. As genetic factors account for two thirds of the risk for cocaine addiction [74] and risk alleles in CACNA1C have been linked to life-time cocaine abuse [15], these findings provide a framework for future studies addressing genetic and molecular mechanisms underlying extinction and reinstatement of cocaine-associated behaviors.
Funding and disclosure
This work was supported by NIH grants R01 DA029122 (AMR), T32DA039080–01 (CEB and CCB), the Frank and Blanche Mowrer Memorial Fellowship (CEB and CCB), TL1TR002386 grant from the National Center for Advancing Translational Sciences (CCB), T32 HL135465 Hunter-Weill T32 Transdisciplinary Research Training (AM-R), R01 NS078792 (JWH), R01 AG055357 (JWH), R01 NS036715 (RLH), and The Paul Fund (AMR). The authors declare no competing financial interests.
Change history
24 May 2020
This article has been updated to change the Figures to colour.
References
Farrell MR, Schoch H, Mahler SV. Modeling cocaine relapse in rodents: behavioral considerations and circuit mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87:33–47.
Kutlu MG, Gould TJ. Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learn Mem. 2016;23:515–33.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
Hernandez-Rabaza V, Hontecillas-Prieto L, Velazquez-Sanchez C, Ferragud A, Perez-Villaba A, Arcusa A, et al. The hippocampal dentate gyrus is essential for generating contextual memories of fear and drug-induced reward. Neurobiol Learn Mem. 2008;90:553–9.
Katebi N, Farahimanesh S, Fatahi Z, Zarrabian S, Haghparast A. Involvement of D1- and D2-like dopamine receptors in the dentate gyrus in the acquisition, expression, and extinction of the morphine-induced conditioned place preference in rats. Behav Brain Res. 2018;353:185–93.
Xie X, Wells AM, Fuchs RA. Cocaine seeking and taking: role of hippocampal dopamine D1-like receptors. Int J Neuropsychopharmacol. 2014;17:1533–8.
Castilla-Ortega E, Serrano A, Blanco E, Araos P, Suarez J, Pavon FJ, et al. A place for the hippocampus in the cocaine addiction circuit: potential roles for adult hippocampal neurogenesis. Neurosci Biobehav Rev. 2016;66:15–32.
Rivera PD, Simmons SJ, Reynolds RP, Just AL, Birnbaum SG, Eisch AJ. Image-guided cranial irradiation-induced ablation of dentate gyrus neurogenesis impairs extinction of recent morphine reward memories. Hippocampus. 2019;29:726–35.
Galinato MH, Takashima Y, Fannon MJ, Quach LW, Morales Silva RJ, Mysore KK, et al. Neurogenesis during abstinence is necessary for context-driven methamphetamine-related memory. J Neurosci. 2018;38:2029–42.
Meyers RA, Zavala AR, Neisewander JL. Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport. 2003;14:2127–31.
Trouche S, Perestenko PV, van de Ven GM, Bratley CT, McNamara CG, Campo-Urriza N, et al. Recoding a cocaine-place memory engram to a neutral engram in the hippocampus. Nat Neurosci. 2016;19:564–7.
Burgdorf CE, Schierberl KC, Lee AS, Fischer DK, Van Kempen TA, Mudragel V, et al. Extinction of contextual cocaine memories requires Cav1.2 within D1R-expressing cells and recruits hippocampal Cav1.2-dependent signaling mechanisms. J Neurosci. 2017;37:11894–911.
Kabir ZD, Martinez-Rivera A, Rajadhyaksha AM. From gene to behavior: L-type calcium channel mechanisms underlying neuropsychiatric symptoms. Neurotherapeutics. 2017;14:588–613.
Kabir ZD, Lee AS, Rajadhyaksha AM. L-type Ca(2+) channels in mood, cognition and addiction: integrating human and rodent studies with a focus on behavioural endophenotypes. J Physiol. 2016;594:5823–37.
Mosheva M, Serretti A, Stukalin Y, Fabbri C, Hagin M, Horev S, et al. Association between CANCA1C gene rs1034936 polymorphism and alcohol dependence in bipolar disorder. J Affect Disord. 2019;261:181–6.
Lancaster TM, Heerey EA, Mantripragada K, Linden DE. CACNA1C risk variant affects reward responsiveness in healthy individuals. Transl Psychiatry. 2014;4:e461.
Wessa M, Linke J, Witt SH, Nieratschker V, Esslinger C, Kirsch P, et al. The CACNA1C risk variant for bipolar disorder influences limbic activity. Mol Psychiatry. 2010;15:1126–7.
Abraham AD, Neve KA, Lattal KM. Activation of D1/5 dopamine receptors: a common mechanism for enhancing extinction of fear and reward-seeking behaviors. Neuropsychopharmacology. 2016;41:2072–81.
Qian H, Patriarchi T, Price JL, Matt L, Lee B, Nieves-Cintron M, et al. Phosphorylation of Ser1928 mediates the enhanced activity of the L-type Ca2+ channel Cav1.2 by the beta2-adrenergic receptor in neurons. Sci Signal. 2017. https://doi.org/10.1126/scisignal.aaf9659.
Woolfrey KM, Dell’Acqua ML. Coordination of protein phosphorylation and dephosphorylation in synaptic plasticity. J Biol Chem. 2015;290:28604–12.
Nystoriak MA, Nieves-Cintron M, Patriarchi T, Buonarati OR, Prada MP, Morotti S, et al. Ser1928 phosphorylation by PKA stimulates the L-type Ca2+ channel CaV1.2 and vasoconstriction during acute hyperglycemia and diabetes. Sci Signal. 2017. https://doi.org/10.1126/scisignal.aaf9647.
Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–47.
Diering GH, Huganir RL. The AMPA receptor code of synaptic plasticity. Neuron. 2018;100:314–29.
Ferretti V, Perri V, Cristofoli A, Vetere G, Fragapane P, Oliverio A, et al. Phosphorylation of S845 GluA1 AMPA receptors modulates spatial memory and structural plasticity in the ventral striatum. Brain Struct Funct. 2015;220:2653–61.
Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–43.
Choi KH, Edwards S, Graham DL, Larson EB, Whisler KN, Simmons D, et al. Reinforcement-related regulation of AMPA glutamate receptor subunits in the ventral tegmental area enhances motivation for cocaine. J Neurosci. 2011;31:7927–37.
Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, McKnight GS, et al. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry. 2007;46:1635–46.
Davare MA, Dong F, Rubin CS, Hell JW. The A-kinase anchor protein MAP2B and cAMP-dependent protein kinase are associated with class C L-type calcium channels in neurons. J Biol Chem. 1999;274:30280–7.
Sanderson JL, Scott JD, Dell’Acqua ML. Control of homeostatic synaptic plasticity by AKAP-anchored kinase and phosphatase regulation of Ca(2+)-permeable AMPA receptors. J Neurosci. 2018;38:2863–76.
Reissner KJ, Uys JD, Schwacke JH, Comte-Walters S, Rutherford-Bethard JL, Dunn TE, et al. AKAP signaling in reinstated cocaine seeking revealed by iTRAQ proteomic analysis. J Neurosci. 2011;31:5648–58.
Wild AR, Sinnen BL, Dittmer PJ, Kennedy MJ, Sather WA, Dell’Acqua ML. Synapse-to-nucleus communication through NFAT is mediated by L-type Ca(2+) channel Ca(2+) spike propagation to the soma. Cell Rep. 2019;26:3537–50.e4.
Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, et al. Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in Cav1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283:34738–44.
Lee HK, Takamiya K, He K, Song L, Huganir RL. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. J Neurophysiol. 2010;103:479–89.
Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. Houston, Texas: Gulf Publishing Company; 2004.
Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Investig. 2011;121:1424–8.
Bavley CC, Fischer DK, Rizzo BK, Rajadhyaksha AM. Cav1.2 channels mediate persistent chronic stress-induced behavioral deficits that are associated with prefrontal cortex activation of the p25/Cdk5-glucocorticoid receptor pathway. Neurobiol Stress. 2017;7:27–37.
Shuto T, Kuroiwa M, Sotogaku N, Kawahara Y, Oh YS, Jang JH, et al. Obligatory roles of dopamine D1 receptors in the dentate gyrus in antidepressant actions of a selective serotonin reuptake inhibitor, fluoxetine. Mol Psychiatry. 2018. https://doi.org/10.1038/s41380-018-0316-x.
Gangarossa G, Longueville S, De Bundel D, Perroy J, Herve D, Girault JA, et al. Characterization of dopamine D1 and D2 receptor-expressing neurons in the mouse hippocampus. Hippocampus. 2012;22:2199–207.
Puighermanal E, Cutando L, Boubaker-Vitre J, Honore E, Longueville S, Herve D, et al. Anatomical and molecular characterization of dopamine D1 receptor-expressing neurons of the mouse CA1 dorsal hippocampus. Brain Struct Funct. 2017;222:1897–911.
Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res. 2007;163:3–22.
Booker SA, Vida I. Morphological diversity and connectivity of hippocampal interneurons. Cell Tissue Res. 2018;373:619–41.
Bezaire MJ, Soltesz I. Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus. 2013;23:751–85.
Lee AS, De Jesus-Cortes H, Kabir ZD, Knobbe W, Orr M, Burgdorf C, et al. The neuropsychiatric disease-associated gene cacna1c mediates survival of young hippocampal neurons. eNeuro. 2016;3:pii: ENEURO.0006-16.2016.
Lee AS, Gonzales KL, Lee A, Moosmang S, Hofmann F, Pieper AA, et al. Selective genetic deletion of cacna1c in the mouse prefrontal cortex. Mol Psychiatry. 2012;17:1051.
Lee AS, Ra S, Rajadhyaksha AM, Britt JK, De Jesus-Cortes H, Gonzales KL, et al. Forebrain elimination of cacna1c mediates anxiety-like behavior in mice. Mol Psychiatry. 2012;17:1054–5.
Kabir ZD, Che A, Fischer DK, Rice RC, Rizzo BK, Byrne M, et al. Rescue of impaired sociability and anxiety-like behavior in adult cacna1c-deficient mice by pharmacologically targeting eIF2alpha. Mol Psychiatry. 2017;22:1096–109.
Kabir ZD, Lee AS, Burgdorf CE, Fischer DK, Rajadhyaksha AM, Mok E, et al. Cacna1c in the prefrontal cortex regulates depression-related behaviors via REDD1. Neuropsychopharmacology. 2017;42:2032–42.
Bavley CC, Fetcho RN, Burgdorf CE, Walsh AP, Fischer DK, Hall BS, et al. Cocaine- and stress-primed reinstatement of drug-associated memories elicit differential behavioral and frontostriatal circuit activity patterns via recruitment of L-type Ca(2+) channels. Mol Psychiatry. 2019. https://doi.org/10.1038/s41380-019-0513-2.
Gong B, Wang H, Gu S, Heximer SP, Zhuo M. Genetic evidence for the requirement of adenylyl cyclase 1 in synaptic scaling of forebrain cortical neurons. Eur J Neurosci. 2007;26:275–88.
Frank CA. How voltage-gated calcium channels gate forms of homeostatic synaptic plasticity. Front Cell Neurosci. 2014;8:40.
Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25:9883–92.
Han YY, Wang XD, Liu L, Guo HM, Cong W, Yan WW, et al. L-type VDCCs participate in behavioral-LTP and memory retention. Neurobiol Learn Mem. 2017;145:75–83.
Menezes J, Alves N, Borges S, Roehrs R, de Carvalho Myskiw J, Furini CR, et al. Facilitation of fear extinction by novelty depends on dopamine acting on D1-subtype dopamine receptors in hippocampus. Proc Natl Acad Sci USA. 2015;112:E1652–8.
Campbell EJ, Marchant NJ. The use of chemogenetics in behavioural neuroscience: receptor variants, targeting approaches and caveats. Br J Pharmacol. 2018;175:994–1003.
Zhu H, Aryal DK, Olsen RH, Urban DJ, Swearingen A, Forbes S, et al. Cre-dependent DREADD (designer receptors exclusively activated by designer drugs) mice. Genesis. 2016;54:439–46.
Hamilton TJ, Wheatley BM, Sinclair DB, Bachmann M, Larkum ME, Colmers WF. Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells. Proc Natl Acad Sci USA. 2010;107:18185–90.
Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2012;22:106–16.
Mu Y, Zhao C, Gage FH. Dopaminergic modulation of cortical inputs during maturation of adult-born dentate granule cells. J Neurosci. 2011;31:4113–23.
Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–7.
De Jesus-Cortes H, Rajadhyaksha AM, Pieper AA. Cacna1c: protecting young hippocampal neurons in the adult brain. Neurogenesis. 2016;3:e1231160.
Temme SJ, Bell RZ, Fisher GL, Murphy GG. Deletion of the mouse homolog of CACNA1C disrupts discrete forms of hippocampal-dependent memory and neurogenesis within the dentate gyrus. eNeuro. 2016;3:pii: ENEURO.0118-16.2016.
Panja D, Bramham CR. BDNF mechanisms in late LTP formation: a synthesis and breakdown. Neuropharmacology. 2014;76:664–76.
Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76:639–56.
Patriarchi T, Buonarati OR, Hell JW. Postsynaptic localization and regulation of AMPA receptors and Cav1.2 by beta2 adrenergic receptor/PKA and Ca(2+)/CaMKII signaling. EMBO J. 2018;37:e99771.
Langwieser N, Christel CJ, Kleppisch T, Hofmann F, Wotjak CT, Moosmang S. Homeostatic switch in hebbian plasticity and fear learning after sustained loss of Cav1.2 calcium channels. J Neurosci. 2010;30:8367–75.
Katchman A, Yang L, Zakharov SI, Kushner J, Abrams J, Chen BX, et al. Proteolytic cleavage and PKA phosphorylation of alpha1C subunit are not required for adrenergic regulation of CaV1.2 in the heart. Proc Natl Acad Sci USA. 2017;114:9194–9.
McGlinchey EM, Aston-Jones G. Dorsal hippocampus drives context-induced cocaine seeking via inputs to lateral septum. Neuropsychopharmacology. 2018;43:987–1000.
Norozpour Y, Zarrabian S, Rezaee L, Haghparast A. D1- and D2-like receptors in the dentate gyrus region of the hippocampus are involved in the reinstatement induced by a subthreshold dose of morphine and forced swim stress in extinguished morphine-CPP in rats. Behav Neurosci. 2019;133:545–55.
Khakpour-Taleghani B, Reisi Z, Haghparast A. The blockade of D1/D2-like dopamine receptors within the dentate gyrus of hippocampus decreased the reinstatement of morphine-extinguished conditioned place preference in rats. Basic Clin Neurosci. 2015;6:73–82.
Tritschler L, Kheirbek MA, Dantec YL, Mendez-David I, Guilloux JP, Faye C, et al. Optogenetic activation of granule cells in the dorsal dentate gyrus enhances dopaminergic neurotransmission in the Nucleus Accumbens. Neurosci Res. 2018;134:56–60.
Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, et al. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat Neurosci. 2019;22:753–61.
Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–53.
Guercio LA, Hofmann ME, Swinford-Jackson SE, Sigman JS, Wimmer ME, Dell’Acqua ML, et al. A-kinase anchoring protein 150 (AKAP150) promotes cocaine reinstatement by increasing AMPA receptor transmission in the accumbens shell. Neuropsychopharmacology. 2018;43:1395–404.
Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160:687–95.
Acknowledgements
We would like to acknowledge Emmanuel Valjent for his hippocampal cell morphology and anatomy expertise.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Burgdorf, C.E., Bavley, C.C., Fischer, D.K. et al. Contribution of D1R-expressing neurons of the dorsal dentate gyrus and Cav1.2 channels in extinction of cocaine conditioned place preference. Neuropsychopharmacol. 45, 1506–1517 (2020). https://doi.org/10.1038/s41386-019-0597-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-019-0597-z
This article is cited by
-
Locus coeruleus input-modulated reactivation of dentate gyrus opioid-withdrawal engrams promotes extinction
Neuropsychopharmacology (2023)
-
Statistical power and false positive rates for interdependent outcomes are strongly influenced by test type: Implications for behavioral neuroscience
Neuropsychopharmacology (2023)