Abstract
Enhancing stress resilience could protect against stress-induced psychiatric disorders in at-risk populations. We and others have previously reported that (R,S)-ketamine acts as a prophylactic against stress when administered 1 week before stress. While we have shown that the selective 5-hydroxytryptamine (5-HT) (serotonin) reuptake inhibitor (SSRI) fluoxetine (Flx) is ineffective as a prophylactic, we hypothesized that other serotonergic compounds such as serotonin 4 receptor (5-HT4R) agonists could act as prophylactics. We tested if three 5-HT4R agonists with varying affinity could protect against stress in two mouse strains by utilizing chronic corticosterone (CORT) administration or contextual fear conditioning (CFC). Mice were administered saline, (R,S)-ketamine, Flx, RS-67,333, prucalopride, or PF-04995274 at varying doses, and then 1 week later were subjected to chronic CORT or CFC. In C57BL/6N mice, chronic Flx administration attenuated CORT-induced weight changes and increased open-arm entries in the elevated plus maze (EPM). Chronic RS-67,333 administration attenuated CORT-mediated weight changes and protected against depressive- and anxiety-like behavior. In 129S6/SvEv mice, RS-67,333 attenuated learned fear in male, but not female mice. RS-67,333 was ineffective against stress-induced depressive-like behavior in the forced swim test (FST), but prevented anxiety-like behavior in both sexes. Prucalopride and PF-04995274 attenuated learned fear and decreased stress-induced depressive-like behavior. Electrophysiological recordings following (R,S)-ketamine or prucalopride administration revealed that both drugs alter AMPA receptor-mediated synaptic transmission in CA3. These data show that in addition to (R,S)-ketamine, 5-HT4R agonists are also effective prophylactics against stress, suggesting that the 5-HT4R may be a novel target for prophylactic drug development.
Similar content being viewed by others
Introduction
Stress exposure is a significant risk factor for the development of major depressive disorder (MDD) and post-traumatic stress disorder (PTSD). According to the National Comorbidity Study, ~60% of men and 51% of women have been exposed to one or more traumatic events during their lifetime. It is estimated that 7.8% of the overall population experiences PTSD at some point in their lives, with females (10.4%) experiencing the disorder at significantly higher rates than males (5.0%) [1]. Traditionally, affective disorders have been treated from a symptom-suppression approach. Existing drugs aim to mitigate the impact of these chronic diseases, but do not cure or prevent the disease itself. However, if drugs were developed that enhance stress resilience, they could potentially be used in at-risk populations to protect against stress-induced psychiatric disorders.
We and others have recently reported that (R,S)-ketamine acts as a resilience enhancing drug (e.g., prophylactic) against stress when administered 1 week before stress in mice [2,3,4,5,6]. In addition, limited data in human patients have demonstrated (R,S)-ketamine’s potential in preventing psychiatric disorders such as PTSD [7] and, perhaps in a dose-specific manner, postpartum depression (PPD) [8, 9]. Prophylactic drug efficacy has been limited to (R,S)-ketamine until recently when Gould et al. reported that group II metabotropic glutamate receptor (mGlu2/3) antagonists are also protective [10]. We have previously reported that the SSRI Flx is ineffective as a prophylactic, but it remains to be determined if other serotonergic drugs could be effective prophylactics and/or if the serotonergic system is involved in prophylactic efficacy.
Numerous studies have implicated the 5-HT4Rs as a target for treating depression and anxiety. 5-HT4Rs are metabotropic G-protein-coupled receptors that stimulate the Gs/cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway in response to 5-HT [11,12,13,14,15]. 5-HT4Rs are highly expressed in the periphery, including the heart and adrenal gland, as well as in the brain in areas, such as the amygdala (AMG), medial prefrontal cortex (mPFC), nucleus accumbens (NAc), and hippocampus (HPC) [16, 17]. 5-HT4R knockout mice display increased anxiety-like behavior and depressive-like behavior, while activation of 5-HT4Rs stimulates neurogenesis in the HPC and produces rapid-acting antidepressant-like effects [18,19,20,21]. However, if and how 5-HT4Rs are involved in stress resilience has yet to be determined.
Here, we hypothesized that since 5-HT4Rs have been heavily implicated in depression and anxiety, they may have a role in stress resilience. We focused our studies on three 5-HT4R agonists with varying affinity. First, RS-67,333 (1-(4-amino-5-chloro-2-methoxyphenyl)-3-[1(n-butyl)-4-piperidinyl]-1-propanone HCl) is a high-affinity 5-HT4R partial agonist [22]. This drug is effective in improving behavioral deficits, decreasing the number of amyloid plaques as well as level of amyloid beta (Aβ) species, and decreasing hippocampal astrogliosis and microgliosis in the 5xFAD mouse model of Alzheimer’s disease (AD) [23]. Second, prucalopride (4-amino-5-chloro-2,3-dihydro-N-[1-3-methoxypropyl)-4-piperidinyl]-7-benzofuran carboxamide monohydrochloride) is a selective, high-affinity 5-HT4R agonist [24]. In 2018, it was approved by the FDA for chronic constipation, and is currently being tested for chronic intestinal pseudo-obstruction. Prucalopride has also been tested in two separate clinical trials to investigate its effects on emotional processing in healthy volunteers after an acute (e.g., single dose) or chronic (e.g., 1 week) administration [25, 26]. Third, PF-04995274 (4-[4-[4-tetrahydrofuran-3-yloxy)-benzo[d]isoxazol-3-yloxymethyl]-piperidin-1-ylmethyl]-tetrahydropyran-4-ol) is a potent, partial 5-HT4R agonist [27]. A clinical trial was conducted to evaluate PF-04995274, alone or in combination with donepezil, on scopolamine-induced deficits in psychomotor and cognitive function in healthy adults; however, this trial was terminated, but not due to safety concerns [28]. Currently, a clinical trial is underway to test whether adjunctive administration of PF-04995247 has positive effects on emotional processing and neural activity in medicated, treatment-resistant (TRD) depressed patients compared with placebo [29].
To determine if 5-HT4R agonists may be potential prophylactics against stress, we utilized two different stress models (acute and chronic) in two different strains of mice (C57BL/6NTac and 129S6/SvEv). We found that RS-67,333, prucalopride, and PF-04995274 attenuate learned fear. RS-67,333 prevents depressive-like behavior when administered chronically, and stress-induced anxiety-like behavior in both sexes when administered acutely. Prucalopride and PF-04995274 decrease stress-induced depressive-like behavior in the FST. To investigate shared or distinct mechanisms of prophylactic (R,S)-ketamine and 5-HT4R agonists, we utilized slice electrophysiology to investigate spontaneous glutamatergic transmission in CA3. We found that (R,S)-ketamine and prucalopride attenuate bursts of large amplitude AMPA receptor-mediated synaptic currents. These data suggest that in addition to (R,S)-ketamine, 5-HT4R agonists are also effective prophylactics against stress and may alter AMPA-related glutamatergic transmission to enhance stress resilience.
Materials and methods
Mice
All mice were housed in a 12-h (06:00–18:00) light–dark colony room at 22 °C. Food and water were provided ad libitum. Behavioral testing was performed during the light phase.
C57BL/6NTac mice
Male C57BL/6NTac mice were purchased from Taconic Farms (Lille Skensved, Denmark) at 8 weeks of age, and were housed five per cage before the start of CORT treatment. All testing was conducted in compliance with the laboratory animal care guidelines and with protocols approved by the Institutional Animal Care and Use Committee (IACUC) (European Directive, 2010/63/EU for the protection of laboratory animals, permissions # 92-256B, authorization ethical committee CEEA n°26 2012_098).
129S6/SvEv mice
Male and female 129S6/SvEvTac mice were purchased from Taconic (Hudson, NY) at 7–8 weeks of age. The procedures described herein were conducted in accordance with the National Institutes of Health (NIH) regulations and approved by the IACUC of the New York State Psychiatric Institute (NYSPI).
Stress models
Corticosterone (CORT) model
In this model, glucocorticoid levels are exogenously increased in C57BL/6NTac mice. This chronic CORT elevation dysregulates the hypothalamic–pituitary–adrenal axis (HPA) in a manner similar to that observed in clinical depression. The dose and duration of CORT treatment were selected based on previous studies [20, 30]. CORT (35 μg/ml, equivalent to about 5 mg/kg/day) dissolved in 0.45% hydroxypropyl−β-cyclodextrin (β-CD) or vehicle (VEH) (0.45% β-CD) was available ad libitum in the drinking water in opaque bottles to protect it from light. VEH- and CORT-treated water was changed every 3 days to prevent possible degradation.
Contextual fear conditioning (CFC)
A 3-shock CFC procedure was administered, as previously published [31, 32]. Briefly, mice were placed into context A and administered 3 2-s shocks (0.75 mA) at 180 , 240, or 300 s following placement into context A. Mice were removed from the context 15 s following the termination of shock (at 317 s). For context retrieval, mice were placed back into context A for 300 s.
Electrophysiology
Electrophysiology was conducted as previously described [33]. Expanded methods are included in the Supplementary Information.
Statistical analysis
The results from data analyses are expressed as means ± SEM. Alpha was set to 0.05 for all analyses. The data were analyzed using GraphPad Prism v7.0 or v8.0. For all experiments, unless otherwise noted, one- or two-way ANOVAs with repeated measures were applied to the data as appropriate. Significant main effects and/or interactions were followed by Fisher’s PLSD post hoc analysis or unpaired t tests. All main effects, interactions, and p- values are listed in Supplementary Table S01.
Results
Chronic administration of RS-67,333 is prophylactic against stress in male mice
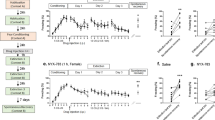
We have previously reported that chronic Flx administration (3 weeks of administration) is not prophylactic in 129S6/SvEv mice [3]. However, it remained to be determined if other serotonergic drugs could act as prophylactics. Here, we administered Flx (18 mg/kg/day) in the drinking water or RS-67,333 (1.5 mg/kg/day) in osmotic minipumps for 3 weeks prior to CORT administration in C57BL/6NTac male mice followed by a series of behavioral assays, including the EPM, novelty suppressed feeding (NSF), and sucrose splash test (ST) (Fig. 1a, b). CORT increased body weight over the 6-week behavioral protocol, as previously observed [30] (Fig. 1c, f), but this was attenuated by Flx and RS-67,333 administration.
RS-67,333 protects against depressive- and anxiety-like behavior induced with a neuroendocrine model in male C57BL/6NTac mice. a Experimental design. b Behavioral assays to test anxiety-like behavior (EPM, NSF) and depressive-like behavior (ST). c–f By Week 6, CORT administration resulted in increased body weight when compared with VEH administration. RS and Flx administration resulted in decreased body weight in CORT-treated mice. g All groups of mice exhibited comparable amounts of time in the open arms of the EPM. h CORT + Veh mice had significantly less entries into the open arms of the EPM when compared with VEH + Veh mice. RS, but not Flx, administration increased the number of entries into the open arms of the EPM in CORT-treated mice. i All groups of mice traveled a similar distance in the EPM. j, k CORT administration increased the latency to feed in the NSF when compared with the VEH administration. RS, but not Flx, administration decreased the latency to feed in CORT-treated mice. l CORT administration decreased grooming duration in the ST when compared with VEH administration. RS, but not Flx, administration increased the grooming duration in CORT-treated mice. (n = 9–14 male mice per group). Error bars represent ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. VEH vehicle, Veh vehicle, CORT corticosterone, Flx fluoxetine, RS RS-67,333, EPM elevated plus maze, NSF novelty suppressed feeding, ST splash test, sec seconds, no. number, cm centimeters, g grams
In the EPM, CORT + Veh, CORT + Flx, and CORT + RS-67,333 administration did not alter the time spent in the open arms when compared with VEH + Veh administration (Fig. 1g). However, CORT + Veh mice exhibited a significantly decreased number of entries into the open arms of the EPM when compared with VEH + Veh mice (Fig. 1h). CORT + Flx and CORT + RS-67,333 mice had significantly more entries into the open arms of the EPM when compared with CORT + Veh mice. The total distance traveled in the EPM did not differ between any of the groups (Fig. 1i).
Next, the NSF task was administered to assay anxiety-like behavior (Fig. 1j, k). CORT + Veh mice exhibited an increased latency to approach the food pellet when compared with VEH + Veh mice. CORT + RS-67,333, but not CORT + Flx mice, exhibited a significantly decreased latency to approach the pellet when compared with CORT + Veh mice.
Finally, in the ST, CORT + Veh mice exhibited decreased grooming duration when compared with VEH + Veh mice (Fig. 1l). CORT + RS-67,333, but not CORT + Flx mice, exhibited increased grooming duration when compared with CORT + Veh mice. These data suggest that chronic RS-67,333, but not chronic Flx administration, is prophylactic against a wide range of CORT-induced behavioral abnormalities.
A single, prophylactic injection of RS-67,333 attenuates learned fear and protects against stress-induced hypophagia in male mice
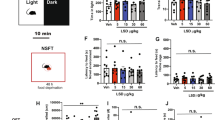
Previously, we have shown that a single injection of (R,S)-ketamine is prophylactic against stress-induced depressive-like behavior and attenuates learned fear in 129S6/SvEv mice [3]. Here, we sought to determine if a single injection of RS-67,333 could also prevent a variety of maladaptive behaviors following a single, acute stressor. Male 129S6/SvEv mice were injected with saline or RS-67,333 (1.5, 10, or 30 mg/kg) (Fig. 2a). One week later, mice were administered 3-shock CFC. Mice administered 30 mg/kg, but not 1.5 or 10 mg/kg, of RS-67,333 exhibited significantly less freezing during CFC training when compared with mice administered saline (Fig. 2b). Five days later, mice were re-exposed to the training context. Mice administered 1.5 or 10 mg/kg, but not 30 mg/kg, of RS-67,333 exhibited significantly less freezing when compared with mice administered saline (Fig. 2c, d).
A single, prophylactic injection of RS-67,333 attenuates learned fear and prevents novelty-induced hypophagia in male 129S6/SvEv mice. a Experimental design. b Mice administered 30 mg/kg, but not 1.5 or 10 mg/kg, of RS-67,333 exhibited significantly less freezing during CFC training when compared with mice administered saline. c, d Mice administered 1.5 or 10 mg/kg, but not 30 mg/kg, of RS-67,333 at exhibited significantly less freezing when compared with mice administered saline. e Mice administered 10 mg/kg, but not 1.5 or 30 mg/kg, of RS-67,333 exhibited reduced immobility when compared with mice administered saline during day 1 of the FST. f, g All groups of mice had comparable amounts of immobility during day 2 of the FST. h, i RS-67,333 (10 mg/kg) did not alter distance traveled or time spent in the center of the OF when compared to saline mice. j, k Both groups of mice had comparable time spent in the open arms and entries into the open arms of the EPM. l, m Mice administered RS-67,333 (10 mg/kg) exhibited a significantly reduced latency to feed when compared with saline mice. n Mice in both groups ate a comparable amount of food in the home cage following the NSF. o Following food deprivation, mice in both groups lost a comparable amount of weight. (n = 5–29 male mice per group). Error bars represent ± SEM. *p < 0.05; ***p < 0.001. Sal saline, CFC contextual fear conditioning, FST forced swim test, OF open field, EPM elevated plus maze, NSF novelty suppressed feeding, min minutes, sec seconds, g grams, mg milligram, kg kilogram, no. number, cm centimeter
Following CFC, mice were administered the FST. On day 1, mice administered 10 mg/kg, but not 1.5 or 30 mg/kg, of RS-67,333 were significantly less immobile when compared with saline mice (Fig. 2e). However, on day 2, immobility time was comparable between all groups (Fig. 2f, g).
Next, mice administered saline or RS-67,333 (10 mg/kg) were tested in the OF. Both groups of mice traveled a comparable distance (Fig. 2h) and spent a comparable amount of time in the center of the arena (Fig. 2i). Subsequently, mice were tested in the EPM, and neither in the open arms nor entries into the open arms of the maze, were significantly different between saline or RS-67,333 mice (Fig. 2j, k).
Finally, mice were administered the NSF. Mice given prophylactic RS-67,333 (10 mg/kg) exhibited a significantly reduced latency to approach the pellet (Fig. 2l, m). However, neither food eaten in the home cage nor weight loss following food deprivation differed between the groups (Fig. 2n, o). Together, these data indicate that a single injection of RS-67,333 is effective as a prophylactic in attenuating learned fear and preventing stress-induced hypophagia, but not depressive-like behavior, as measured by the FST, in male 129S6/SvEv mice.
A single, prophylactic injection of RS-67,333 protects against stress-induced hypophagia in female mice
We next sought to determine if a single injection of RS-67,333 could also be prophylactic in female mice. Female 129S6/SvEv mice were injected with saline or RS-67,333 (1.5 or 10 mg/kg) (Fig. 3a). One week later, mice were administered 3-shock CFC. All groups of mice exhibited comparable levels of freezing during CFC training (Fig. 3b). Five days later, mice were re-exposed to the training context. Again, all groups of mice exhibited comparable levels of freezing (Fig. 3c, d). Following CFC, mice were administered the FST. During days 1 (Fig. 3e) and 2 (Fig. 3f, g) of the FST, all groups of mice had comparable levels of immobility.
A single, prophylactic administration of RS-67,333 prevents novelty-induced hypophagia, but does not alter fear or depressive-like behavior, in female 129S6/SvEv mice. a Experimental design. b All mice exhibited comparable levels of freezing during CFC training. c, d All groups exhibited comparable levels of freezing during re-exposure. e All groups of mice had comparable amounts of immobility during day 1 of the FST. f, g All groups of mice had comparable amounts of immobility during day 2 of the FST. h, i RS-67,333 did not alter distance traveled or time spent in the center of the OF. j Time spent in the open arms of the EPM was comparable between all groups of mice. k Entries into the open arms of the EPM were comparable between all groups of mice. l, m A single, prophylactic dose of RS-67,333 (10 mg/kg) significantly reduced latency to feed in the NSF. n A single, prophylactic dose of RS-67,333 (10 mg/kg) did not alter the amount of food eaten in the home cage or (o) body weight loss. (n = 6–11 female mice per group). Error bars represent ± SEM. *p < 0.05; ***p < 0.001. Sal saline, CFC contextual fear conditioning, FST forced swim test, OF open field, EPM elevated plus maze, NSF novelty suppressed feeding, min minutes, sec seconds, cm centimeters, no number, g grams, mg milligram, kg kilogram
Next, mice were tested in the OF and the EPM. Mice in all groups traveled comparable distances in the OF and spent a comparable amount of time in the center of the arena (Fig. 3h, i). Similarly, in the EPM, mice spent a comparable amount of time in the open arms of the maze (Fig. 3j) and had a comparable number of entries into the open arms (Fig. 3k).
Finally, mice were assayed in the NSF paradigm. Prophylactic RS-67,333 (10 mg/kg), but not RS-67,333 (1.5 mg/kg), significantly reduced latency to feed (Fig. 3l, m). Neither food eaten in the home cage nor weight loss following food deprivation differed between the groups (Fig. 3n, o). Together, these data indicate that RS-67,333 does not attenuate learned fear or protect against stress-induced depressive-like behavior, but may prevent stress-induced hypophagia in the NSF in female 129S6/SvEv mice.
A single prophylactic injection of prucalopride or PF-04995274 is prophylactic against stress in male mice
We next sought to determine if other 5-HT4R agonists could also be prophylactic in male 129S6/SvEv mice. Male 129S6/SvEv mice were injected with saline, (R,S)-ketamine (30 mg/kg), prucalopride (3 or 10 mg/kg), or PF-04995274 (3 or 10 mg/kg) (Fig. 4a). One week later, mice were administered 3-shock CFC. All groups of mice exhibited comparable levels of freezing during CFC training (Fig. 4b). Five days later, mice were re-exposed to the training context. As we have previously published, (R,S)-ketamine attenuated learned fear (Fig. 4c, d). Interestingly, prucalopride at 3, but not 10 mg/kg, and PF-04995274 at 10, but not 3 mg/kg, attenuated learned fear when compared with saline administration.
A single, prophylactic administration of prucalopride or PF-04995274 attenuates learned fear and decreases depressive-like behavior in male 129S6/SvEv mice. a Experimental design. b All mice exhibited comparable levels of freezing during CFC training. c, d (R,S)-ketamine (30 mg/kg), prucalopride (3 mg/kg), and PF-04995274 (10 mg/kg), but not prucalopride (10 mg/kg) or PF-04995274 (3 mg/kg), administration attenuated learned fear when compared with saline administration. e All groups of mice had comparable amounts of immobility during day 1 of the FST. f, g (R,S)-ketamine (30 mg/kg), prucalopride (3 mg/kg), and PF-04995274 (10 mg/kg), but not prucalopride (10 mg/kg) or PF-04995274 (3 mg/kg), significantly decreased immobility time during day 2 of the FST. h All groups of mice traveled a comparable amount of distance in the OF. i All groups of mice spent a comparable amount of time in the open arms of the EPM. j All groups of mice had a comparable number of entries into the open arms of the EPM. k, l All groups of mice had a comparable latency to approach the pellet in the NSF. m All groups of mice lost a comparable amount of weight following food deprivation for the NSF. (n = 5–10 male mice per group). Error bars represent ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001. Sal saline, K (R,S)-ketamine, Prucal prucalopride, PF PF-04995274, CFC contextual fear conditioning, FST forced swim test, OF open field, EPM elevated plus maze, NSF novelty suppressed feeding, min minutes, sec seconds, cm centimeters, no number, mg milligram, kg kilogram
Following CFC, mice were administered the FST. During day 1, all groups of mice had comparable levels of immobility (Fig. 4e). During day 2, (R,S)-ketamine administration decreased immobility time when compared with saline administration (Fig. 4f, g). Moreover, prucalopride at 3, but not 10 mg/kg, and PF-04995274 at 10, but not 3 mg/kg, decreased immobility time when compared with saline administration.
Stress-induced anxiety-like behavior was next quantified. In the OF, all groups of mice traveled a comparable distance (Fig. 4h). In the EPM, all groups of mice spent comparable time in the open arms (Fig. 4i) and entered into the open arms a comparable number of times (Fig. 4j). In the NSF paradigm, all groups of mice approached the pellet in a comparable amount of time (Fig. 4k, l). Finally, all mice lost a comparable amount of weight during the NSF paradigm (Fig. 4m). In summary, these data indicate that a single injection of prucalopride or PF-04995274 results in prophylactic efficacy by attenuating learned fear and decreasing stress-induced depressive-like behavior. However, these drugs are not prophylactic against stress-induced anxiety-like behavior.
(R,S)-ketamine and prucalopride exhibit a common mechanism by reducing bursts of large AMPA receptor-driven synaptic currents in CA3
We next sought to elucidate potential common mechanisms between (R,S)-ketamine and a 5-HT4R agonist such as prucalopride. Specifically, we hypothesized that there may be similarities between the effects of (R,S)-ketamine and prucalopride on glutamatergic transmission in CA3 since we previously reported that prophylactic (R,S)-ketamine alters activity in ventral CA3 (vCA3), but not in the DG [6]. To test this, mice were injected with saline, (R,S)-ketamine (30 mg/kg), or prucalopride (3 mg/kg), and were euthanized 1 week later (Fig. 5a). We performed whole-cell voltage clamp recordings of spontaneous excitatory postsynaptic currents (EPSCs) in CA3 pyramidal cells. We found there were no differences in the average EPSC amplitude (Fig. 5b) or the number of EPSCs (Fig. 5c) between the groups. However, we did find that saline-treated mice typically displayed large bursts of EPSCs (−590.8 ± 13.85 pA), which were completely blocked by the AMPA receptor blocker NBQX (Fig. 5d). These large AMPA receptor-mediated signals were not present in either (R,S)-ketamine- (Fig. 5e) or prucalopride-treated mice (Fig. 5f), suggesting that although these drugs target different receptors, they both alter AMPA-mediated synaptic transmission in a similar manner.
(R,S)-ketamine and prucalopride exhibit a common mechanism by reducing large AMPA-driven synaptic bursts in CA3. a Experimental design. b The average EPSC amplitude did not differ between the groups. c The average number of EPSCs (within a 20-s recording window) did not differ between the groups. d Saline-treated mice typically displayed large bursts of EPSCs (−590.8 ± 13.85 pA), which were blocked by the AMPA receptor antagonist NBQX. These large AMPA receptor-mediated signals were not present in either e (R,S)-ketamine- or f prucalopride-treated mice. (n = 5–7 mice per group). Error bars represent ± SEM. Sal saline, K (R,S)-ketamine, Prucal prucalopride, CA3 Cornu Ammonis 3, pA picoamps, EPSCs excitatory postsynaptic currents, no. number, NBQX 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline, mg milligram, kg kilogram, ms millisecond
Discussion
Here, we hypothesized that 5-HT4R agonists could be prophylactic against fear, depressive-like, and/or anxiety-like behavior. We tested if three 5-HT4R agonists with varying affinity could protect against stress. Chronic administration of RS-67,333 was prophylactic against CORT stress. A single injection of RS-67,333 attenuated learned fear in male, but not female, 129S6/SvEv mice and prevented stress-induced hypophagia in the NSF in both sexes. Acute administration of RS-67,333 was ineffective against stress-induced depressive-like behavior. A single injection of either prucalopride or PF-04995274 attenuated learned fear and decreased depressive-like behavior, but had no effect on anxiety-like behavior. Moreover, a single injection of (R,S)-ketamine or prucalopride reduced large, spontaneous AMPA receptor-driven bursts in CA3, indicating a common mechanism by which either drug may protect against stress-induced maladaptive behavior.
The three 5-HT4R agonists chosen in this study have differential affinity to the 5-HT4R (Supplementary Fig. S1). RS-67,333 and PF-04995274 are high-affinity 5-HT4R partial agonists, whereas prucalopride is a selective, high-affinity 5-HT4R agonist. RS-67,333 attenuated learned fear and protected against novelty-induced hypophagia, but did not decrease stress-induced depressive-like behavior. Prucalopride and PF-04995274 attenuated learned fear and decreased depressive-like behavior, but had no effect on various measures of anxiety-like behavior. These data suggest that the unique combination of high pKi and partial selectivity for the 5-HT4R exhibited by RS-67,333 is sufficient to prevent against anxiety-like behavior, whereas the differential activity of prucalopride and PF-04995274 at the 5-HT4R protect against stress-induced depressive-like behavior. Further study is necessary to determine if and how the 5-HT4R may contribute to the neurobiological mechanisms underlying stress resilience.
The expression and activity of 5-HT4Rs within the central nervous system (CNS) and periphery may provide insight into these mechanisms. In the brain, 5-HT4Rs are expressed in areas of the brain involved in processing emotion, including the HPC, AMG, and PFC [11, 16, 21, 34, 35]. In addition to a multitude of other functions, such as modulating dopamine and acetylcholine release [35], as well as facilitating synaptic plasticity [35], 5-HT4Rs are known to interact with the calcium effector protein p11 [36]. 5-HT4Rs are highly co-expressed with p11, which increases surface expression of the receptor in the HPC and AMG, facilitates its downstream signaling pathways, and is necessary for the antidepressant effects of 5-HT4R stimulation [36, 37]. Levels of p11 are correlated with measures of suicidality and PTSD, indicating its potential as a biomarker for suicidal ideation and PTSD [38,39,40]. In addition, 5-HT4R expression and activity in the PFC are regulated by casein kinase 2 (CK2), which may be an important modulator of depressive- and anxiety-like behaviors [41]. Further studies examining 5-HT4R agonists and their effects on these cellular regulators of 5-HT4R expression and activity could yield further insight into prophylactic efficacy.
Because all three 5-HT4R agonists exhibited prophylactic properties similar to (R,S)-ketamine, we investigated whether these compounds had comparable effects on neural activity in CA3. We found that a single injection of (R,S)-ketamine or prucalopride eliminated large bursts of AMPA receptor-mediated synaptic currents typically seen in saline controls without significantly altering the overall amplitude or number of EPSCs. Therefore, although these compounds target distinct receptors, they may achieve similar behavioral effects by altering AMPA receptor-dependent glutamatergic transmission in a convergent manner. Although (R,S)-ketamine is known to inhibit NMDA receptors [42,43,44], emerging evidence indicates that (R,S)-ketamine may also act on AMPA receptors to exert its antidepressant effects [45, 46]. Our results are congruent with these data and suggest that (R,S)-ketamine’s actions on AMPA receptor-mediated glutamatergic activity may contribute to the compound’s prophylactic effects. In addition, previous studies show that pharmacological activation of 5-HT4Rs results in the long-term potentiation (LTP) of CA3-CA1 synapses along the Schaffer collaterals [47]. In combination with these data, our results suggest that (R,S)-ketamine and 5-HT4R agonists, by attenuating large, spontaneous AMPA receptor-driven synaptic events in the CA3 autoassociative network, may reduce overall noise in the hippocampal circuit which may allow for a greater signal-to-noise ratio of relevant stimuli [48]. However, further research is necessary to confirm this hypothesis and to examine whether this potential mechanism directly contributes to enhanced resilience.
In addition to the actions of 5-HT4R agonists within the brain, it is likely that these compounds exert additional changes within the periphery. 5-HT4Rs are expressed in the periphery, such as the enteric nervous system (ENS), adrenal glands, and heart [17]. Importantly, 5-HT4Rs play a major role in maintaining communication along the gut–brain axis. Recent data indicate that microbiota in the ENS communicate with the CNS by stimulating 5-HT4Rs present throughout the gut to stimulate serotonin release in the brain [49]. Concurrently, numerous previous studies have shown that activation of 5-HT4Rs is neuroprotective against oxidative stress, reduces inflammation, and stimulates neurogenesis in the brain and ENS [49,50,51]. Our manipulations may have stimulated gut–brain communication to promote neuroprotection and neurogenesis, and thereby enhance resilience against stress. We hypothesize that this action may have had an additive effect on the numerous, well-characterized consequences of 5-HT4R stimulation in the brain, such as increasing neuronal firing in the medial PFC (mPFC) and enhancing mitogenesis in the HPC [19, 34], although this remains to be determined.
To develop safe and efficacious pharmacological methods of enhancing stress resilience, it will be necessary to determine the toxicity of 5-HT4R agonists. Because 5-HT4Rs are so widely expressed throughout the periphery, chronic exposure to these drugs could result in negative outcomes [17]. We found that chronic administration of RS-67,333 did not result in adverse side effects. However, because we did not conduct additional assays, such as assessing changes in cardiovascular activity or liver toxicity, it is impossible to know if chronic 5-HT4R administration would negatively impact peripheral organs. Nonetheless, the drugs that we tested were efficacious in enhancing stress resilience even after a single dose, obviating chronic administration.
Utilization of numerous mouse strains is essential in determining drug efficacy. While C57BL/6 mice are the most widely used inbred strain, these mice may not be optimized to model susceptibility to stress. Numerous previous reports have shown opposing conclusions if mice of varying genetic backgrounds are tested in the same behavioral assays [52]. These studies suggest that phenotypic relationships cannot be inferred by studying a single genetic background. Here, we utilized two strains—C57BL/6NTac and 129S6/SvEv—in order to validate our effects of RS,67-333 in both a neuroendocrine model of stress and a fear-based stressor. In the C57BL/6NTac mice, we found that prophylactic RS-67,333 was effective at decreasing depressive- and anxiety-like behavior, whereas in the 129S6/SvEv mice, we found prophylactic RS-67,333 was effective at attenuating learned fear and preventing anxiety-like behavior in the NSF, but not decreasing depressive-like behavior. Moving forward, numerous strains should be utilized in stress studies in order to infer drug-behavioral relationships.
Utilization of both sexes is also essential in determining drug efficacy. Previous research examining 5-HT4R agonists as rapid-acting antidepressants have exclusively used male subjects [19, 20, 53]. However, previous studies indicate that antidepressant (R,S)-ketamine exhibits sex-specific behavioral and neurobiological effects. Across the estrous cycle, the efficacy of antidepressant (R,S)-ketamine varies in female mice, and this variability may be attributed to changing levels of neurotrophic factors (e.g., BDNF) or changes in NMDA receptor activity across the estrous cycle [54, 55]. In addition, acute (R,S)-ketamine administration may lead to a sustained increase in GluR1 and GluR2 AMPA receptor subunits in the mPFC and HPC of male, but not female mice [44, 45, 56, 57]. Despite numerous studies showing prophylactic efficacy in male rodents, only one study to date has examined female rodents [58]. Maier et al. showed that prophylactic (R,S)-ketamine reduced stress-induced activation of the dorsal raphe nucleus (DRN) and eliminated DRN-dependent social exploration deficits in female rats. However, this study did not measure fear, depressive-like, and anxiety-like behavior as done here. Nonetheless, we show that RS-67,333 does not attenuate learned fear or prevent depressive-like behavior, but does protect against stress-induced anxiety-like behavior in female 129S6/SvEv mice. Thus, our data indicate that 5-HT4R agonists may exclusively target the neural circuits underlying anxiety-like, but not depressive- or fear-related, behaviors in female mice. We did not utilize female C57BL/6NTac mice, as a previous study of our own has shown that female C57BL/6NTac mice are insensitive to chronic CORT [59]. Future studies are necessary to determine the sex- and dose-specific effects of prophylactic compounds, as women are 2–3 times more at risk of suffering from stress-related anxiety or depressive disorders than men [60].
Overall, this study has identified three novel compounds to be effective prophylactics against two types of stress and in both sexes. These data suggest that the 5-HT4R may be a novel target for prophylactic development, and future studies may lead to novel insights on how 5-HT4R agonists administered prior to a stressor result in stress resiliency.
Funding and disclosure
BKC, IM-D, CF, DJD, AMG, and CAD are named on provisional patent applications for the prophylactic use of (R,S)-ketamine and other compounds against stress-related psychiatric disorders. VML has no competing interests. BKC was supported by a T32 training grant. IM-D was supported by a National Alliance for Research on Schizophrenia and Depression (NARSAD) 2017 Young Investigator Award from the Brain & Behavior Research Foundation and by the Deniker Foundation. VML was supported by a K01AG054765-03. CAD was supported by a retention package from the NYSPI.
References
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60.
Amat J, Dolzani SD, Tilden S, Christianson JP, Kubala KH, Bartholomay K, et al. Previous ketamine produces an enduring blockade of neurochemical and behavioral effects of uncontrollable stress. J Neurosci. 2016;36:153–61.
Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, et al. Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol Psychiatry. 2016;79:776–86.
McGowan JC, LaGamma CT, Lim SC, Tsitsiklis M, Neria Y, Brachman RA, et al. Prophylactic ketamine attenuates learned fear. Neuropsychopharmacology. 2017;42:1577–89.
McGowan JC, Hill C, Mastrodonato A, LaGamma CT, Kitayev A, Brachman RA, et al. Prophylactic ketamine alters nucleotide and neurotransmitter metabolism in brain and plasma following stress. Neuropsychopharmacology. 2018;43:1813–21.
Mastrodonato A, Martinez R, Pavlova IP, LaGamma CT, Brachman RA, Robison AJ, et al. Ventral CA3 activation mediates prophylactic ketamine efficacy against stress-induced depressive-like behavior. Biol Psychiatry. 2018;84:846–56.
McGhee LL, Maani CV, Garza TH, Slater TM, Petz LN, Fowler M. The intraoperative administration of ketamine to burned U.S. service members does not increase the incidence of post-traumatic stress disorder. Mil Med. 2014;179:41–6.
Ma J-H, Wang S-Y, Yu H-Y, Li D-Y, Luo S-C, Zheng S-S, et al. Prophylactic use of ketamine reduces postpartum depression in Chinese women undergoing cesarean section. Psychiatry Res. 2019;279:252–8.
Xu Y, Li Y, Huang X, Chen D, She B, Ma D. Single bolus low-dose of ketamine does not prevent postpartum depression: a randomized, double-blind, placebo-controlled, prospective clinical trial. Arch Gynecol Obstet. 2017;295:1167–74.
Highland JN, Zanos P, Georgiou P, Gould TD. Group II metabotropic glutamate receptor blockade promotes stress resilience in mice. Neuropsychopharmacology. 2019;44:1788–96.
Bockaert J, Claeysen S, Compan V, Dumuis A. 5-HT4 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:39–51.
Bockaert J, Claeysen S, Compan V, Dumuis A. 5-HT(4) receptors: history, molecular pharmacology and brain functions. Neuropharmacology. 2008;55:922–31.
Dumuis A, Sebben M, Bockaert J. The gastrointestinal prokinetic benzamide derivatives are agonists at the non-classical 5-HT receptor (5-HT4) positively coupled to adenylate cyclase in neurons. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:403–10.
Dumuis A, Sebben M, Bockaert J. BRL 24924: a potent agonist at a non-classical 5-HT receptor positively coupled with adenylate cyclase in colliculi neurons. Eur J Pharmacol. 1989;162:381–4.
Faye C, Hen R, Guiard BP, Denny CA, Gardier AM, Mendez-David I, et al. Rapid anxiolytic effects of RS67333, a serotonin type 4 receptor agonist, and diazepam, a benzodiazepine, are mediated by projections from the prefrontal cortex to the dorsal raphe nucleus. Biol Psychiatry. 2019. https://www.sciencedirect.com/science/article/abs/pii/S000632231931621X.
Bonaventure P, Hall H, Gommeren W, Cras P, Langlois X, Jurzak M, et al. Mapping of serotonin 5-HT(4) receptor mRNA and ligand binding sites in the post-mortem human brain. Synapse. 2000;36:35–46.
Hegde SS, Eglen RM. Peripheral 5-HT4 receptors. FASEB J. 1996;10:1398–407.
Amigo J, Diaz A, Pilar-Cuellar F, Vidal R, Martin A, Compan V, et al. The absence of 5-HT4 receptors modulates depression- and anxiety-like responses and influences the response of fluoxetine in olfactory bulbectomised mice: adaptive changes in hippocampal neuroplasticity markers and 5-HT1A autoreceptor. Neuropharmacology. 2016;111:47–58.
Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, et al. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007;55:712–25.
Mendez-David I, David DJ, Darcet F, Wu MV, Kerdine-Romer S, Gardier AM, et al. Rapid anxiolytic effects of a 5-HT(4) receptor agonist are mediated by a neurogenesis-independent mechanism. Neuropsychopharmacology. 2014;39:1366–78.
Samuels BA, Mendez-David I, Faye C, David SA, Pierz KA, Gardier AM, et al. Serotonin 1A and serotonin 4 receptors: essential mediators of the neurogenic and behavioral actions of antidepressants. Neuroscientist. 2016;22:26–45.
Eglen RM, Bonhaus DW, Johnson LG, Leung E, Clark RD. Pharmacological characterization of two novel and potent 5-HT4 receptor agonists, RS 67333 and RS 67506, in vitro and in vivo. Br J Pharmacol. 1995;115:1387–92.
Giannoni P, Gaven F, de Bundel D, Baranger K, Marchetti-Gauthier E, Roman FS, et al. Early administration of RS 67333, a specific 5-HT4 receptor agonist, prevents amyloidogenesis and behavioral deficits in the 5XFAD mouse model of Alzheimer’s disease. Front Aging Neurosci. 2013;5:96.
Prins NH, Van Haselen JF, Lefebvre RA, Briejer MR, Akkermans LM, Schuurkes JA. Pharmacological characterization of 5-HT4 receptors mediating relaxation of canine isolated rectum circular smooth muscle. Br J Pharmacol. 1999;127:1431–7.
Morris PJ, Moaddel R, Zanos P, Moore CE, Gould TD, Zarate CA Jr., et al. Synthesis and N-Methyl-d-aspartate (NMDA) receptor activity of ketamine metabolites. Org Lett. 2017;19:4572–5.
Zanos P, Gould TD. Intracellular signaling pathways involved in (S)- and (R)-ketamine antidepressant actions. Biol Psychiatry. 2018;83:2–4.
Grimwood S, Drummond E, Zasadny K, Skaddan M, Brodney M, Coffman K, et al. Translational receptor occupancy for the 5-HT4 partial agonist PF-04995274 in rats, non-human primates and healthy volunteers. Alzheimer’s Dement: J Alzheimer’s Assoc. 2011;7:S653.
Zanos P, Highland JN, Liu X, Troppoli TA, Georgiou P, Lovett J, et al. R)-Ketamine exerts antidepressant actions partly via conversion to (2R,6R)-hydroxynorketamine, while causing adverse effects at sub-anaesthetic doses. Br J Pharmacol. 2019;176:2573–92.
Morris PJ, Moaddel R, Zanos P, Moore CE, Gould TD, Zarate CA Jr., et al. Correction to “synthesis and N-methyl-d-aspartate (NMDA) receptor activity of ketamine metabolites”. Org Lett. 2017;19:5494.
David DJ, Samuels BA, Rainer Q, Wang J-W, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–93.
Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2012;22:1188–201.
Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–54.
Luna VM, Anacker C, Burghardt NS, Khandaker H, Andreu V, Millette A, et al. Adult-born hippocampal neurons bidirectionally modulate entorhinal inputs into the dentate gyrus. Science. 2019;364:578.
Lucas G, Compan V, Charnay Y, Neve RL, Nestler EJ, Bockaert J, et al. Frontocortical 5-HT4 receptors exert positive feedback on serotonergic activity: viral transfections, subacute and chronic treatments with 5-HT4 agonists. Biol Psychiatry. 2005;57:918–25.
Eglen RM, Wong EH, Dumuis A, Bockaert J. Central 5-HT4 receptors. Trends Pharm Sci. 1995;16:391–8.
Warner-Schmidt JL, Flajolet M, Maller A, Chen EY, Qi H, Svenningsson P, et al. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J Neurosci. 2009;29:1937.
Egeland M, Warner-Schmidt J, Greengard P, Svenningsson P. Co-expression of serotonin 5-HT1B and 5-HT4 receptors in p11 containing cells in cerebral cortex, hippocampus, caudate-putamen and cerebellum. Neuropharmacology. 2011;61:442–50.
Su T-P, Zhang L, Chung M-Y, Chen Y-S, Bi Y-M, Chou Y-H, et al. Levels of the potential biomarker p11 in peripheral blood cells distinguish patients with PTSD from those with other major psychiatric disorders. J Psychiatr Res. 2009;43:1078–85.
Zhang L, Su T-P, Choi K, Maree W, Li C-T, Chung M-Y, et al. P11 (S100A10) as a potential biomarker of psychiatric patients at risk of suicide. J Psychiatr Res. 2011;45:435–41.
Zhang L, Ursano RJ, Li H. P11: a potential biomarker for posttraumatic stress disorder. Methods Mol Biol. 2012;829:453–68.
Castello J, LeFrancois B, Flajolet M, Greengard P, Friedman E, Rebholz H. CK2 regulates 5-HT4 receptor signaling and modulates depressive-like behavior. Mol Psychiatry. 2018;23:872–82.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5.
Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33:6990–7002.
Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6.
Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546:E1–e3.
Teixeira CM, Rosen ZB, Suri D, Sun Q, Hersh M, Sargin D, et al. Hippocampal 5-HT input regulates memory formation and Schaffer collateral excitation. Neuron. 2018;98:992–1004.e4.
Rebola N, Carta M, Mulle C. Operation and plasticity of hippocampal CA3 circuits: implications for memory encoding. Nat Rev Neurosci. 2017;18:208–20.
De Vadder F, Grasset E, Manneras Holm L, Karsenty G, Macpherson AJ, Olofsson LE, et al. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci USA. 2018;115:6458–63.
Bianco F, Bonora E, Natarajan D, Vargiolu M, Thapar N, Torresan F, et al. Prucalopride exerts neuroprotection in human enteric neurons. Am J Physiol Gastrointest Liver Physiol. 2016;310:G768–75.
Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–99.
Sittig LJ, Carbonetto P, Engel KA, Krauss KS, Barrios-Camacho CM, Palmer AA. Genetic background limits generalizability of genotype-phenotype relationships. Neuron. 2016;91:1253–9.
Darcet F, Gardier AM, David DJ, Guilloux JP. Chronic 5-HT4 receptor agonist treatment restores learning and memory deficits in a neuroendocrine mouse model of anxiety/depression. Neurosci Lett. 2016;616:197–203.
Dossat AM, Wright KN, Strong CE, Kabbaj M. Behavioral and biochemical sensitivity to low doses of ketamine: influence of estrous cycle in C57BL/6 mice. Neuropharmacology. 2018;130:30–41.
Picard N, Takesian AE, Fagiolini M, Hensch TK. NMDA 2A receptors in parvalbumin cells mediate sex-specific rapid ketamine response on cortical activity. Mol Psychiatry. 2019;24:828–38.
Thelen C, Flaherty E, Saurine J, Sens J, Mohamed S, Pitychoutis PM. Sex differences in the temporal neuromolecular and synaptogenic effects of the rapid-acting antidepressant drug ketamine in the mouse brain. Neuroscience. 2019;398:182–92.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64.
Dolzani SD, Baratta MV, Moss JM, Leslie NL, Tilden SG, Sorensen AT, et al. Inhibition of a descending prefrontal circuit prevents ketamine-induced stress resilience in females. eNeuro. 2018;5:pii: ENEURO.0025-18.2018.
Mekiri M, Gardier AM, David DJ, Guilloux JP. Chronic corticosterone administration effects on behavioral emotionality in female c57bl6 mice. Exp Clin Psychopharmacol. 2017;25:94–104.
Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19.
Acknowledgements
We thank members of the laboratory for insightful comments on this project and paper. In addition, we thank V Domergue and the staff of the animal care facility of the SFR-UMS Institut Paris-Saclay Innovation Thérapeutique for their technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, B.K., Mendez-David, I., Luna, V.M. et al. Prophylactic efficacy of 5-HT4R agonists against stress. Neuropsychopharmacol. 45, 542–552 (2020). https://doi.org/10.1038/s41386-019-0540-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-019-0540-3
This article is cited by
-
Concurrent anxiety in patients with major depression and cerebral serotonin 4 receptor binding. A NeuroPharm-1 study
Translational Psychiatry (2022)
-
Déjà-vu? Neural and behavioural effects of the 5-HT4 receptor agonist, prucalopride, in a hippocampal-dependent memory task
Translational Psychiatry (2021)
-
Sex-specific neurobiological actions of prophylactic (R,S)-ketamine, (2R,6R)-hydroxynorketamine, and (2S,6S)-hydroxynorketamine
Neuropsychopharmacology (2020)