Abstract
To date, neurons have been the primary focus of research on the role of glucocorticoids in the regulation of brain function and pathological behaviors, such as addiction. Astrocytes, which are also glucocorticoid-responsive, have been recently implicated in the development of drug abuse, albeit through as yet undefined mechanisms. Here, using a spectrum of tools (whole-transcriptome profiling, viral-mediated RNA interference in vitro and in vivo, behavioral pharmacology and electrophysiology), we demonstrate that astrocytes in the nucleus accumbens (NAc) are an important locus of glucocorticoid receptor (GR)-dependent transcriptional changes that regulate rewarding effects of morphine. Specifically, we show that targeted knockdown of the GR in the NAc astrocytes enhanced conditioned responses to morphine, with a concomitant inhibition of morphine-induced neuronal excitability and plasticity. Interestingly, GR knockdown did not influence sensitivity to cocaine. Further analyses revealed GR-dependent regulation of astroglial metabolism. Notably, GR knockdown inhibited induced by glucocorticoids lactate release in astrocytes. Finally, lactate administration outbalanced conditioned responses to morphine in astroglial GR knockdown mice. These findings demonstrate a role of GR-dependent regulation of astrocytic metabolism in the NAc and a key role of GR-expressing astrocytes in opioid reward processing.
Similar content being viewed by others
Introduction
Alterations in function of glucocorticoid receptors (GRs) are listed among the key factors underlying vulnerability to addiction [1, 2]. Drugs of abuse activate the hypothalamic pituitary-adrenal (HPA) axis to release glucocorticoids [3,4,5], endogenous ligands of the GRs. Likewise, glucocorticoid administration increases drug self-administration [6,7,8], facilitates psychomotor stimulant effects of cocaine and morphine [9], and promotes reinstatement [7, 10]. Although opioids and psychostimulants induce common behavioral responses, such as motor sensitization or conditioned reward, it was recently argued that they engage different molecular pathways and distinct cellular circuits [11]. It was pointed out that GR in dopaminoceptive neurons selectively modulates behavioral responses to cocaine and amphetamine, but not morphine [12, 13]. Nevertheless, both molecular and behavioral data suggest that GR is involved in opiate-related behavior. Indeed, morphine regulates a large network of genes controlled by the GR in the striatum [14], and GR antagonist administration was shown to alter acute locomotor and conditioned place preference to morphine [15, 16]. Thus, the exact mechanism by which the GR contributes to opiate responses remains unexplored.
A majority of research so far has addressed the neuronal actions of glucocorticoids as an important contribution to the development of addictive behaviors [12, 13, 17]. However, emerging evidence continues to support the potentially significant role of glial cells. Previous findings showed that glucocorticoids regulate distinct neuron- and astrocyte-enriched mRNAs in vitro and in vivo [18, 19]. Moreover, recent studies have shown that part of the GR-dependent transcriptome alterations induced by morphine in the striatum occurs in astrocytes [20, 21]. Collectively, these data suggest that GR in the brain regulates gene expression in a cell type-dependent fashion, implying that neurons and astrocytes might contribute to glucocorticoid-dependent behavioral alterations through distinct mechanisms.
A number of studies support the hypothesis that functional alterations of astrocytes in reward circuitry might be partly responsible for the development of drug addiction. An increase of GFAP (marker of astrocyte activation) has been found following administration of both opiates and psychostimulants [22,23,24]. Recent studies using animal models have identified suppression of astroglial-specific glutamate transporters as one of the key mechanisms mediating cocaine reinstatement [25, 26] and shown that disrupting astrocyte-neuron lactate transfer persistently reduces conditioned responses to cocaine [27]. These reports suggest that metabolic interplay between astrocytes and neurons might be important in the mediation of rewarding effects of drugs of abuse.
In the brain, lactate is formed predominantly in astrocytes, from either glucose or glycogen and transferred to neurons in response to their energetic needs [28]. One recent study has demonstrated that lactate shuttling from astrocytes to neurons modulates neuronal excitability in the hippocampus [29]. Glucocorticoids might be involved in the regulation of lactate synthesis and release, since it has been previously shown that exposure to dexamethasone, a GR agonist, reduces glycogen synthesis in cultured astrocytes [30]. However, how glucocorticoid-mediated changes in astrocytes may impact brain function and subsequent behavior, as well as the precise mechanism involved, is still unknown.
In this study, we set out to investigate the functional contribution of astroglial GR to striatal reward circuitry. Cell-type specific GR-dependent transcriptional profiling in the nucleus accumbens (NAc) pointed to astrocytes as a potential mediator of glucocorticoid action. Therefore, we selectively targeted GR in Aldh1l1-positive astrocytes, using viral-mediated RNA interference. Astroglial GR knockdown enhanced conditioned responses to morphine, but not cocaine, and promoted morphine-induced inhibition of neural excitability and plasticity in the NAc. GR knockdown resulted in inhibition of glucocorticoid-induced lactate release in astrocytes, whereas systemic lactate administration balanced the increase of conditioned response to morphine observed in astroglial GR knockdown mice. Thus, our findings reveal a critical role of astrocytic GR in the modulation of reward-related behavior and synaptic transmission, possibly via GR-mediated regulation of metabolic processes in astrocytes.
Materials and methods
Animals
Transgenic mice expressing the Cre recombinase under aldehyde dehydrogenase 1 family promoter (Tg(Aldh1l1-cre)PB1Gsat, purchased from Mutant Mouse Regional Resource Center; backcrossed to C57BL/6J background for nine generations before the onset of experiments) or C57BL/6J mice were bred and housed 3–5 per cage in the animal house of Maj Institute of Pharmacology, PAS, Krakow, Poland. Mice had free access to food and water, and were kept under a 12-h light/dark cycle (lights on at 7 a.m.) in temperature- and humidity-controlled room. All animal experiments were performed according to the requirements of the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the II Local Bioethics Commission (permission numbers 1152/2015 and 84/2018, Krakow, Poland).
Lentiviral vectors
To produce the lentiviral vectors, shRNA sequences: control (nonmammalian targeting) and targeting the GR (TRC library clone ID NM_008173.3-2119s21c1) were cloned into pSico lentiviral plasmid (a gift from Tyler Jacks [31]; Addgene plasmid #11578). Titers of both viruses were comparable and ranged between 1.13 × 108 and 2.19 × 108 transducing U ml−1. Virus suspensions were stored at −70 °C until use, and were kept on ice immediately before injection. For in vitro validation of LV-pSico-shGR, astrocytes were cotransduced with lentiviral vectors expressing Cre recombinase (LV-Cre pLKO.1, Addgene plasmid #25997) in the presence of 4 µg/ml polybrene (Sigma). Ninety-six hours post infection, cells were stimulated with 100 nM dexamethasone for 4 h. For investigation of metabolic activity in vitro, astrocytes were transduced with Cre-independent LV (LeGO-G-shRNA) harboring the same control or shGR sequences.
Stereotactic surgery
Aldh1l1-Cre transgenic mice (≥8 weeks old) were anesthetized intraperitoneally (ip) with ketamine (7.5 mg/kg) and xylazine (1 mg/kg) before being placed in a stereotactic frame. Viral vectors were injected bilaterally into the NAc (anterior + 1.0 mm, lateral: ±1.5 mm, ventral: −4.2 mm, relative to Bregma), with an injection volume of 1 µl. Syringe was left in place for additional 10 min after each injection to ensure complete diffusion of the vector. Mice were injected with LV-pSico-shGR or LV-pSico-shGFP (control construct), and tested at least 3 weeks post surgery. The transduction site of viral vectors in the NAc of mice used in the experiments was verified by immunohistochemistry (described in Supplement 1). In case when the brain slices were used for gene expression profiling or electrophysiology, a visual inspection using fluorescent microscope system (Nikon, Japan) was performed.
Behavioral procedures
Mice were handled by experimenters for 3 days before any behavioral test. All behavioral tests were analyzed by automated cages or video-recorded and assessed by an experimenter unaware of genotype and treatment of the mice.
Conditioned place preference (CPP) procedure was performed in place preference boxes with three distinct, neutral compartments (Med Associates, GA, USA). On days 1 and 5, during the pre- and postconditioning tests, mice were placed in the center compartment and allowed to explore the apparatus freely for 20 min. Assignment of mice to the compartments was unbiased. On days 2–4, drug-paired animals received saline injection in each morning session, and morphine (5 or 10 mg/kg, subcutaneously (sc)) or cocaine (5 or 10 mg/kg, ip) in each afternoon session. Saline-paired animals received saline injections during both sessions. Each conditioning session lasted 40 min. The CPP scores are presented as the increase of time spent in the paired chamber between post- and preconditioning tests. For lactate pretreatment experiments, we have administered lactate in a dose of 1 g/kg (ip), as it was previously presented that peripheral administration at this dose increases extracellular lactate concentration in the mouse brain that lasts about 40 min [32].
Locomotor activity was measured in activity chambers (10 × 20 × 10 cm) equipped with photocells (custom made for the Maj Institute of Pharmacology PAS). Horizontal (ambulatory counts) activity was measured for each mouse to assess locomotor activity. All mice were habituated to the locomotor activity chambers for 2 h, 1 day before the onset of the experiments. For the measurement of morphine-induced locomotor sensitization, mice received daily injections of saline or morphine (5 mg/kg, sc) in 2 h sessions for 6 days. Expression of morphine (5 mg/kg, sc) sensitization was tested 7 days following the cessation of morphine administration. For a detailed description of other behavioral methods used in the present paper, please see Supplement 1.
Gene expression profiling
Microarray experiments were performed using MouseWG-6 v2 BeadChip arrays (Illumina Inc., CA, USA). Detailed procedures for tissue collection, RNA preparation, microarray hybridization and quantitative PCR are provided in Supplement 1.
Fluorescence in situ hybridization (FISH) and immunohistochemistry
Standard protocols were used for FISH and immunohistochemical labeling. Detailed procedural and analytic methods are described in Supplement 1.
Electrophysiological recordings
Astroglial GR knockdown and control mice were habituated to saline injections for 3 days before the experiment. Mice received single saline or morphine (5 mg/kg, sc) injection 40 min before decapitation. Whole-cell patch-clamp recordings and extracellular recordings in slices were performed as previously described [33] (for detailed procedures, see Supplement 1).
Cell culture
Brain hemispheres were dissected from 5–6-day-old C57BL/6J mice and dissociated into single-cell suspension using Neural Tissue Dissociation Kit (T) (Miltenyi Biotec, Germany) according to the manufacturer’s protocol. Astrocytes were isolated by magnetic sorting using Anti-Glast Microbead Kit and MS Columns (Miltenyi Biotec). Cells were cultured on polyornithine-coated dishes (Sigma, MO, USA) in DMEM (Life Technologies, CA, USA), supplemented with 10% fetal bovine serum (Life Technologies) and penicillin/streptomycin (Life Technologies) under standard conditions.
In vitro assays
Glucose uptake was measured by the colorimetric assay of accumulation of 2-deoxyglucose-6-phosphate (2-DG6P) in cells using Glucose Uptake Assay Kit (Abcam, UK), accumulation of l-Lactate in cell culture medium was measured using colorimetric l-Lactate Assay Kit (Abcam), glycogen content in cultured cells was assessed using Glycogen Assay Kit (Abcam), following protocols of the supplier. Fluorescence was measured using Tecan Infinite m1000 Pro microplate reader.
Statistical analysis
Statistical analysis of gene expression profiling was performed by dChip software using compare-samples module and followed by correction for multiple testing. Hierarchical clustering was performed using the measure of Euclidian distance and average distance linkage methods. Cluster visualization was performed using dChip software. Statistical analysis of biochemical, behavioral, and electrophysiological data was done by GraphPad Prism version 7.0 or R version 3.3.1. Three-way ANOVA was used in morphine-induced locomotor sensitization to analyze three parameters (treatment × genotype × time). Two-way ANOVA was used in biochemical, behavioral, and electrophysiological studies that analyze two parameters (treatment × genotype), followed by Bonferroni’s post hoc tests where appropriate. Student’s t-test was used to compare biochemical and behavioral data from two groups. Data are presented on graphs as the means of absolute values ± SEMs. Statistically significant differences between tested groups are marked with the symbols “*” and “#” (*,#p < 0.05; **,##p < 0.01; ***,###p < 0.001).
Results
Astrocytes are the main target for GR-activated gene expression in the NAc
To study cell-specific GR-dependent gene expression in the NAc, we used whole-transcriptome microarrays. We measured the effects of dexamethasone (4 mg/kg, ip), a selective GR agonist, on gene expression 4 h after acute administration. We identified 83 transcripts regulated by dexamethasone (Fig. 1a, Supplement 2) in the tissue, with false discovery rate empirically estimated at 7.2% (at p < 0.001). Further, the identified profile of transcriptional alterations was compared between neurons and astrocytes that were separated from the NAc using magnetic cell sorting (Fig. S1). We found that 67.5% of identified GR-dependent transcriptome alterations in the NAc were regulated in astrocytes when compared with neurons (Fig. 1a). Next, we investigated to what extent GR-dependent transcriptional alterations are induced by morphine (20 mg/kg, ip) in NAc, as it was previously shown that opioids influence striatal transcriptome through the activation of HPA axis and the release of glucocorticoids [20]. Morphine administration resulted in significant transcriptional changes of 48.2% of genes regulated by dexamethasone in the NAc (Fig. 1a, right panel). A number of studies have indicated that the repositioning of chromatin fragments, including genes movement within the space of the cell nucleus, is an important feedback regulatory mechanism of transcriptional response [34, 35]. Therefore, we aimed to observe whether GR-dependent mRNA expression levels induced by morphine are linked to GR gene location studied by DNA FISH, supported by morphometric analysis. Accordingly, we have examined the effects of morphine administration in vivo on distribution of the GR gene (Nr3c1) in chromatin of neuronal and astrocytic nuclei in the NAc (Fig. 1b–d). Our results demonstrated that under control conditions (saline administration) the distribution of GR gene alleles was similar in condensed and noncondensed chromatin compartments of astrocytic and neuronal nuclei (Fig. 1c; two-way ANOVA, interaction effect: F(2,18) = 0.16, p = 0.86). Morphine administration resulted in GR gene translocation toward condensed chromatin in both cell types, however, the effect was much more evident in astrocytes (Fig. 1d; two-way ANOVA, interaction effect: F(2,18) = 50.47, p < 0.001). Thus, our results show that glucocorticoid-induced response in the brain is partly managed by nonneuronal cells and confirm that GR-dependent component of morphine-induced signaling in the NAc occurs in astrocytes.
GR-dependent transcriptome alterations in vivo are largely confined to astrocytes. a Dexamethasone (GR agonist) administration caused changes in the mRNA abundance levels of 83 transcripts in the accumbal tissue, astrocytes, and neurons (full list of genes in Supplement 2). Morphine administration resulted in significant transcriptional changes of 48.2% genes regulated by the GR in the NAc. Microarray results include genes with genome-wide significance filtered out using Student’s t-test p < 0.001 and fold change >0.3-log2 fold change of control, presented as a heat map. The colored rectangles represent transcript abundance at 4 h after saline (Sal) or dexamethasone (Dex; 4 mg/kg, ip) administration in different brain compartments (tissue, astrocytes, and neurons) for the genes labeled on the right. The intensity of color is proportional to the standardized values for each microarray, as indicated in the legend. The panel on the right presents morphine (Morph)-induced regulation of GR-dependent genes in the NAc. Genes regulated at p < 0.05 are marked with green rectangles. b Confocal images showing GR gene (Nr3c1, green) in the astroglial (GFAP, red) nuclei (Hoechst 33342, blue) of saline- or morphine-treated mice. Scale bars: 5 µm. Distribution of GR gene alleles in different chromatin compartments of astroglial and neuronal nuclei after c saline administration was similar, however, d morphine-induced GR gene translocation toward condensed chromatin was significantly more pronounced in astrocytes. Exact n listed in Table S1. Significant differences between cell types (Astro vs. Neuro) marked with ***p < 0.001. Data presented as mean ± SEM
Molecular effects of GR knockdown in astrocytes in vitro and in vivo
To gain a better understanding of the role of astrocytic GR in the NAc we have selectively downregulated the expression of GR mRNA and protein levels in astrocytes. For in vitro experiments, primary murine astrocytes were sequentially double-transduced with LV-Cre and LV-shGR (vector harboring Cre-dependent shRNA expression cassette for GR silencing), or LV-shGFP (control vector). Ninty-six hours post transduction, cells were stimulated with dexamethasone (100 nM) for 4 h. Expression of GR (Nr3c1) in LV-shGR transfected astrocytes was reduced by 70% when compared with control vector (Fig. 2a; Student’s t-test, t(2) = 72.12, p < 0.001). Additionally, the induction of expression of Fkbp5, a GR-dependent gene, after dexamethasone stimulation was reduced by 75% in LV-shGR astrocytes when compared with LV-shGFP astrocytes, although significant increase of gene expression was observed in both control and LV-shGR transfected cultures when compared with saline-treated astrocytes (Fig. 2a; two-way ANOVA, interaction effect: F(1,4) = 615.91, p < 0.001).
GR knockdown in astrocytes in vitro and in vivo. a Suppressed expression of the GR (Nr3c1) and induction of Fkbp5 by dexamethasone (Dex) was observed in LV-pSico-shGR transduced astrocytes in vitro. b Representative immunofluorescent images of lentiviral transduced area. White traces mark the NAc. c Fraction of s100β-positive astrocytes expressing the GR was significantly reduced in GR knockdown mice compared with control group. d Immunofluorescence labeling for Hoechst (nuclei), GFP (viral vector), GR, and s100β (astroglial marker), presenting silencing of the GR expression in astrocytes of GR knockdown mice when compared with control mice. White boxes indicate the area of magnification (presented as bottom panels). The white arrows denote s100β-positive cells. Scale bars: top panels 100 µm, bottom panels 20 µm. e Fraction of neurons expressing the GR was similar in control and GR knockdown mice (representative images presented in Fig. S2). f mRNA expression levels of GR-dependent genes: Cdkn1a, Ddit4, and Arrdc2 in the NAc after dexamethasone administration (4 mg/kg, ip) was suppressed by astrocytic GR knockdown. g mRNA expression levels of Camk1g (gene regulated in neurons, see: Fig. 1a) in the NAc after dexamethasone administration (4 mg/kg, ip) was not altered by astroglial GR knockdown. h Dexamethasone caused similar suppression of blood plasma corticosterone levels in both control and astrocytic GR knockdown mice. Exact n listed in Table S1. Where appropriate, tests were followed by Bonferroni post hoc analysis. Significant differences in treatment (Sal vs. Dex) marked with *p < 0.05, **p < 0.01, ***p < 0.001, significant differences between genotypes (control vs. GR knockdown) marked with #p < 0.05, ##p < 0.01, ###p < 0.001. Data presented as mean ± SEM
For in vivo studies we have used Cre-mediated GR targeting shRNAs in transgenic mice that express the Cre recombinase under aldehyde dehydrogenase 1 family member L1 promoter (Aldh1l1-Cre), specific for astrocytes. As control vector, we have used scrambled shRNA in the same mice. Transduction site was verified in brain slices (representative immunofluorescent images shown in Fig. 2b), based on which we have estimated the spread of viral vector in the NAc, relative to Bregma: anterior: +1.0 ± 0.5 mm, bilateral: 1.5 ± 0.2 mm, ventral: −4.4 ± 0.4 mm. It has to be noted that expression of the viral vector was detected in the dorsal striatum along the needle track. We further analyzed GR protein expression in astrocytes in the transduction site with immunofluorescent labeling. The GR, nuclear marker (Hoechst) and astroglial marker (s100β) were clearly colocalized in control mice, while negligible colocalization of the aforementioned markers has confirmed efficient silencing of the GR in astrocytes of GR knockdown mice (Fig. 2d). Semiquantification of GR/s100β colocalization revealed that 81.3% of astrocytes expressed GR in control mice, while fraction of GR-positive astrocytes was reduced to 22.3% in GR knockdown mice (Fig. 2c; Student’s t-test, t(4) = 11.91, p < 0.001). Further, to assess the specificity of GR elimination we checked for a fraction of NeuN-positive cells expressing the GR and detected no changes between control and astroglial GR knockdown mice (Fig. S2), with 99.3% and 95.6% of GR-positive neurons, respectively (Fig. 2e; Student’s t-test, t(6) = 2.4, p = 0.06). To test for functional molecular effects of astrocytic GR knockdown we have analyzed mRNA expression levels of the selected GR-dependent genes in the NAc, after acute saline or dexamethasone (4 mg/kg, ip) administration in vivo. The genes were chosen based on the results of gene expression profiling, form the cluster of genes upregulated specifically in astrocytes after dexamethasone injection (see: Fig. 1a). Results show that induced by dexamethasone mRNA expression of Cdkn1a, Ddit4, and Arrdc2 was significantly reduced by astroglial GR knockdown by about 30% (Fig. 2f; two-way ANOVA, interaction effect: Cdkn1a F(1,18) = 8.79, p = 0.008, Ddit4 F(1,18) = 7.41, p = 0.01, Arrdc2 F(1,18) = 6.24, p = 0.02). No significant differences in mRNA expression of abovementioned genes were noted between groups after saline administration (Fig. 2f). Additionally, no differences in mRNA expression of Camk1g, a gene regulated in neurons after dexamethasone administration (see: Fig. 1a) were noted between both groups of mice (Fig. 2g; two-way ANOVA, interaction effect: F(1,18) = 1, p = 0.33). Finally, we measured blood plasma corticosterone levels after saline or dexamethasone administration. Basal levels and levels suppressed by dexamethasone corticosterone were comparable in both groups (Fig. 2h; two-way ANOVA, interaction effect: F(1,18) = 0.29, p = 0.59), indicating that astroglial GR knockdown in the NAc did not affect the general HPA axis activity of the mice. Together, these results indicate that our approach led to efficient knockdown of the GR in the NAc astrocytes.
Astroglial GR knockdown in the NAc selectively increases sensitivity to opioid reward
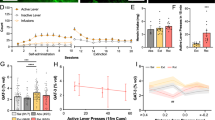
A number of studies have shown a relationship between GR-mediated effects in the brain and susceptibility to drug reward and addiction-like behavior [15, 36]. Various drugs of abuse upregulate transcription of a considerable number of GR-dependent genes that are largely assigned to astrocytic compartment of the brain [14]. We therefore hypothesized, that knockdown of GR from accumbal astrocytes would affect reward-related behavior. To test that, we analyzed morphine- and cocaine-induced CPP, as well as morphine-induced locomotor sensitization. CPP procedure consisted of pre test, six consecutive 40-min conditioning sessions, and post test (Fig. 3a). Before conditioning, control and GR knockdown mice spent a similar amount of time in each of the two distinct compartments of the apparatus, indicating that the procedure was unbiased (Fig. S3). Both morphine (5 mg/kg and 10 mg/kg, sc) and cocaine (5 mg/kg and 10 mg/kg, ip) administration induced CPP when compared with saline in both groups (Fig. 3b, c), however GR knockdown mice presented considerably greater preference to morphine-paired compartment than control mice (Fig. 3b; two-way ANOVA, interaction effect: F(2,66) = 3.382, p = 0.04; genotype effect: F(1,66) = 6.421, p = 0.01. Bonferroni post hoc analysis: 5 mg/kg morphine: p = 0.002; 10 mg/kg morphine: p = 0.14). Interestingly, conditioned response to cocaine was similar in both groups of animals (Fig. 3c; two-way ANOVA, interaction effect: F(2,52) = 0.04, p = 0.96, genotype effect not significant). Next, to confirm the involvement of endogenous corticosteroids in the effects of both drugs, we treated C57BL/6J mice with either saline, morphine, or cocaine at the doses of 5 and 10 mg/kg, and measured blood corticosterone at 40 min after administration (time point corresponding to the CPP procedure). Morphine in both doses significantly elevated plasma corticosterone levels when compared with saline, while cocaine administration had no such effect (Fig. S4). This demonstrates that opioid reward sensitivity and expression of morphine-associated memory is possibly mediated through the activation of the HPA axis and consequent regulation of the GR-dependent pathway in the NAc astrocytes.
Astroglial GR selectively regulates sensitivity to opioid reward. a Experimental schedule of conditioned place preference (CPP). The experiment consisted of preconditioning test on day 1, six alternating saline and drug-paired conditioning sessions on days 2–4 and postconditioning test on day 5. Drug-paired mice received saline (Sal) in the morning sessions and morphine (Morph; 5 or 10 mg/kg) or cocaine (Coc; 5 or 10 mg/kg) in the afternoon sessions. Saline-paired mice received saline injections during both sessions. b Both groups of animals have acquired conditioned response to morphine, however, astrocytic GR knockdown resulted in escalation of morphine-induced preference when compared with control mice. c Both control and astroglial GR knockdown mice presented similar conditioned response to cocaine. d Experimental schedule of morphine-induced locomotor sensitization. Animals received daily injections of saline or morphine (5 mg/kg) in 2 h sessions for 6 days. The challenge morphine injection was performed 7 days later. e Astrocytic GR knockdown had no effect on acquisition or f expression of morphine-induced sensitization. Exact n listed in Table S1. Significant differences in treatment (Sal vs. Morph/Sal vs. Coc) marked with *p < 0.05, **p < 0.01, ***p < 0.001, significant genotype effect (control vs. GR knockdown) marked with ##p < 0.01. Data presented as mean ± SEM
Further, we have studied the development of sensitization to morphine-induced hyperlocomotion. After adaptation to locomotor activity chambers, mice received alternate daily injections of saline or morphine (5 mg/kg, sc) for 6 days. Expression of locomotor sensitization was tested 7 days following cessation of morphine administration (Fig. 3d). Astrocytic GR knockdown did not influence acquisition (Fig. 3e; three-way ANOVA, F(1,270) = 175.64, p < 0.001 with significant treatment × time interaction: F(5,270) = 10.36, p < 0.001, other effects insignificant) nor expression (Fig. 3f; two-way ANOVA, interaction effect: F(1,53) = 1.27, p = 0.26, treatment effect: F(1,53) = 75.98, p < 0.001, genotype effect insignificant) of morphine-induced locomotor sensitization.
Taking into consideration previous reports about the participation of the GR in opiate withdrawal [37, 38], we have tested mice for signs of naloxone-precipitated physical dependence after chronic morphine treatment. No significant differences were observed in the number of jumps between astrocytic GR knockdown and control mice, indicating similar withdrawal intensity in both groups (Fig. S5a). Altogether, our results show that astrocytic GR in the NAc selectively modulates sensitivity to opioid reward, without changing other opioid-related addictive behaviors, such as locomotor sensitization and withdrawal.
Astroglial GR knockdown in the NAc does not affect stress-related behavior
GR is thought to be an important factor in the development of stress-related disorders, such as depression and posttraumatic stress disorder [2, 39, 40]. Previous studies in animal models showed involvement of the ventral striatum in these disorders [41, 42], therefore we have assessed the behavior of astrocytic GR knockdown mice in a series of tests that measure depression-like behavior, anxiety as well as stress-related memory formation and expression. No significant differences were observed between both groups of animals in saccharin preference (Fig. S5b), latency to immobility and time spent immobile in tail suspension test (Fig. S5c, d), indicating that core symptoms of depressive-like behavior, anhedonia and learned helplessness, were unaffected by the astrocytic GR knockdown. Similarly, no significant differences were observed in anxiety levels measured as novel object exploration time (Fig. S5e), latency to enter the anxiolytic, illuminated compartment of the light-dark box, as well as total time spent in this compartment (Fig. S5f, g). To measure stress-induced memory formation and expression we have behaviorally challenged the mice in fear conditioning paradigm using foot shocks (5 × 0.4 mA) as aversive stimuli. Both groups presented similar acquisition and expression of conditioned fear (Fig. S5h–j). Behavioral profile of the mice indicates that astrocytic GR knockdown in the NAc does not cause alterations in basal stress-related behavioral traits, such as anhedonia, learned helplessness, anxiety, and fear memory.
GR knockdown in astrocytes alters morphine-induced effects on synaptic transmission and plasticity in the NAc
Silencing of GR in astrocytes in NAc has caused a selective increase of sensitivity to opioid reward. To further examine possible neural correlates of these behavioral changes, we aimed to study the effect of astrocytic GR knockdown on electrophysiological characteristics of NAc medium spiny neurons as well as long-term potentiation (LTP) of the excitatory input of NAc in the slices prepared after either saline or morphine (5 mg/kg, sc) administration in vivo. Resting membrane potential and resistance of the recorded neurons were similar in control and astrocytic GR knockdown mice and remained unchanged regardless of treatment (Fig. S6a, b). Saline administration did not alter amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) recorded in slices originating from control and GR knockdown mice (Fig. 4a; two-way ANOVA, interaction effect: F(49,1127) = 0.26, p = 1). Interestingly, while there was evident increase in the mean sEPSCs amplitude after morphine administration (compared with saline) in control group, such change was not observed in slices from astrocytic GR knockdown mice (Fig. 4b; two-way ANOVA, interaction effect: F(49,1519) = 5.19, p < 0.001; additional two-way ANOVA used to compare within-group effects of saline and morphine treatments is presented in Supplement 3). The sEPSCs frequency remained unchanged in both groups after both treatments (Fig. S6c, d). Saline administration did not influence the excitability of NAc neurons in slices from GR knockdown mice (Fig. 4c; two-way ANOVA, interaction effect: F(15,360) = 0.73, p = 0.75), however, after morphine administration the excitability of the cells was markedly decreased (Fig. 4d; two-way ANOVA, interaction effect: F(15,450) = 4.22, p < 0.001). Consistently with other results, we observed no significant differences in the magnitude of LTP in the NAc between control and GR knockdown mice after saline injection (Fig. 4e; left panel: two-way ANOVA, interaction effect: F(10,130) = 1.67, p = 0.1; right panel: Student’s t-test, t(13) = 1.11, p = 0.15), but LTP induction after morphine treatment was blocked in astroglial GR knockdown mice (Fig. 4f; left panel: two-way ANOVA, interaction effect: F(10,160) = 3.8, p < 0.001; right panel: Student’s t-test, t(17) = 2.01, p = 0.03). Altogether, obtained results show that, similarly to behavioral changes, GR knockdown in astrocytes does not influence basal responses of the neurons but alters the effect of morphine on synaptic plasticity. Observed changes in sEPSCs amplitudes in single cells as well as in stimulus-induced field potential amplitudes suggest that astroglial GR knockdown resulted in alterations of postsynaptic properties of medium spiny neurons in the NAc.
GR knockdown in astrocytes alternates morphine-induced synaptic plasticity. a–d Whole-cell patch-clamp analysis of the NAc cells. a Basal (Sal) sEPSCs amplitude and frequency (Fig. S6c) were comparable in both groups. b Morphine (Morph) treatment resulted in increased neuronal sEPSCs amplitude in control mice that was attenuated by astrocytic GR knockdown. No differences were observed in frequency (Fig. S6d). a, b Left: representative sEPSCs traces. Scale bars: 10 pA/100 ms. Right: mean cumulative probability of sEPSCs amplitude. c No significant differences in the stimulus-induced excitability of medium spiny neurons were observed between groups under basal conditions, but d astrocytic GR knockdown resulted in reduced neuronal excitability after morphine administration. c, d Left: representative voltage responses to hyperpolarizing (−80 pA) and depolarizing (320 pA) current injections in MSN cells. Scale bars: 50 pA/200 ms. Right: relation between the stimulus intensity and the number of generated spikes. e Basal LTP in the NAc was comparable in both groups of animals, however, f astroglial GR knockdown caused significant alteration of LTP after morphine administration. e, f Left panels: relative amplitude values of the field potentials (FP). The use of amplitude for NAc was necessitated by the small potential amplitudes and the resulting low signal-to-noise ratio. The arrow indicates the time point at which stimulus was applied. Middle: representative evoked potentials in control and astrocytic GR knockdown mice before and after stimulation, as indicated by the numbers 1–4. Scale bars: 0, 1 mV/ 5 ms. Right: mean averaged values 45–60 min after the stimulus. Exact n listed in Table S1. Where appropriate, tests were followed by Bonferroni post hoc analysis. Significant differences between genotypes (control vs. GR knockdown) marked with *p < 0.05, **p < 0.01, ***p < 0.001. Data presented as mean ± SEM
Astroglial GR modulates sensitivity to opioid reward via local regulation of metabolic processes
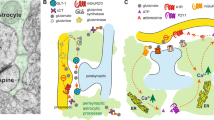
Functional analysis of the GR-dependent genes in astrocytes (see: Fig. 1a) pointed out to several processes that can influence neural plasticity and possibly underlie changes in animal behavior. These processes include regulation of cellular metabolism at various levels: glycogenolysis (Pdk4, Sult1a1, Map3k6, Nt5c3), glucose transport (Slc2a1) and signaling (Sgk3), glutamate uptake (Slc7a11) and control of negative feedback on the action of steroids (Fkbp5; Fig. 5e). We therefore investigated the role of the GR in the regulation of astrocyte metabolism in vitro. Dexamethasone stimulation (100 nM, 24 h) caused significant increase of glucose uptake and decrease of glycogen content in astrocytes that was associated with enhanced release of lactate (Fig. 5a–c). Our results suggest there exists a GR-dependent metabolic switch that increases astrocytic trophic support for neurons providing them with lactate as an energetic substrate. GR knockdown in astrocytes inhibited dexamethasone-induced effects on glucose uptake (Fig. 5a; Student’s t-test, control t(6) = 2.41, p = 0.03, GR knockdown t(6) = 0.49, p = 0.64) and lactate release (Fig. 5b; Student’s t-test, control t(6) = 2.02, p = 0.04, GR knockdown t(6) = 0.28, p = 0.39) and reversed the decrease in glycogen content (Fig. 5c; Student’s t-test, control t(6) = 4.36, p = 0.005, GR knockdown t(6) = 0.74, p = 0.13), suggesting that observed behavioral alterations and synaptic plasticity may result from the GR-mediated modulation of astrocytic lactate release. To test that hypothesis, we systemically administered saline or lactate (1 g/kg, ip), that was previously shown to increase extracellular lactate in the hippocampus and cortex in vivo [32, 43], 15 min prior to each morphine (5 mg/kg, sc) conditioning session (experimental schedule like in Fig. 3a). Firstly, we have confirmed that lactate administration in the chosen dose does not cause conditioned preference nor aversion (Fig. S7a, b). During pretest control and GR knockdown mice spent similar amount of time in each of the two distinct compartments of the apparatus (Fig. S7c). Saline pretreated mice recapitulated enhanced conditioned response to morphine in GR knockdown mice compared with controls (Fig. 5d). While lactate pretreatment did not alter morphine-induced place preference in control group, it caused a significant reduction of place preference in GR knockdown mice (Fig. 5d; Two-way ANOVA, interaction effect: F(1,23) = 5.04, p = 0.03). To sum up, lactate supplementation prevented the increased morphine CPP observed in astrocytic GR knockdown mice. This suggests that glucocorticoids act locally in the NAc through astroglial GR to provide neurons with metabolic support, that, when unbalanced, may modulate animal behavior and synaptic activity.
Astroglial GR regulates opioid reward via metabolic processes. GR knockdown in astrocytes in vitro resulted in inhibition of dexamethasone-induced (Dex; 100 nM, 24 h) a increase in glucose uptake and b lactate release as well as c reversal of decrease in glycogen content. d Pretreatment with lactate (1 g/kg, ip) suppressed escalation of morphine-induced (Morph; 5 mg/kg, sc) place preference mediated by astrocytic GR knockdown. e Schematic diagram presenting the possible involvement of astrocytic GR in the regulation of opioid reward. Elevations in glucocorticoid levels caused by morphine induce gene transcription in astrocytes that control metabolic processes (Pdk4, Sult1a1, Map3k6, Nt5c3), glucose transport (Slc2a1, Sgk3), glutamate uptake (Slc7a11), and negative feedback on the action of steroids (Fkbp5). Induced by glucocorticoids release of lactate from astrocytes provides trophic support for neurons, thus modulating neuronal plasticity and excitability that contributes to morphine reward sensitivity. Exact n listed in Table S1. Test was followed by Bonferroni post hoc analysis. Significant differences between treatments (Sal vs. Dex/Sal vs. Lactate) marked with *p < 0.05, **p < 0.01. Data presented as mean ± SEM
Discussion
Glucocorticoid action in astrocytes
In this study we demonstrate the involvement of astroglial GR in the opioid reward processing and synaptic plasticity in the NAc. Our results point to astrocytes as important target of glucocorticoid action in the reward circuitry: in vivo, GR-dependent transcriptional changes in the NAc occur largely in this type of glial cells. These results are in concordance with previous assumptions that astroglia account for a substantial proportion of the transcriptional response to glucocorticoids in the brain [14, 18, 44]. We also show that morphine administration induced GR-dependent transcriptional alterations that coincided with localization of the GR gene in the chromatin of the NAc astrocytes. Since condensed and noncondensed chromatin acts as an inhibitory or permissive environment for gene transcription [34, 35], our observation support the view that astrocytes are an important target of morphine-induced glucocorticoid actions in the NAc.
Previous work suggested that astrocytes are involved in processes that can influence neural plasticity and may thus contribute to drug addiction [45]. Our own functional analysis of genes regulated by dexamethasone and morphine in astrocytes indicated a role for GR in the regulation of astrocyte metabolism; we identified the genes encoding ATP binding (Pdk4, Entpd2, Sgk3), glycolysis (Slc2a1), glucose transport and signaling (Slc2a1, Sgk3) and other metabolic pathway-related molecules (Sult1a1, Nt5c3, Pigh) as being regulated by both the glucocorticoid (dexamethasone) and the opioid (morphine). Accordingly, we here also confirmed that dexamethasone stimulation alters glucose uptake, glycogen content, and lactate release in astrocytes in vitro; lactate is an end-product of metabolism that provides trophic support for neurons. Since all of the latter metabolic effects were absent in GR knockdown astrocytes, these results confirm a vital role for GR in the regulation of metabolic processes in astrocytes, and are consistent with a recent report that glucocorticoids enhance metabolic substrates release from spinal astrocytes [46].
Behavioral consequences of astroglial GR knockdown in the NAc
Mice with selective knockdown of the GR in the NAc astrocytes showed a striking increase of opioid reward sensitivity, presented as expression of morphine-associated memory in the CPP procedure. Importantly, astrocytic GR knockdown-associated enhancement of the conditioned response to morphine was reversed by systemic administration of lactate. It should be noted that the effect of lactate in the NAc is hypothetical in our approach, as systemic lactate injection may have altered a number of other brain regions and peripheral functions. Nevertheless, these observations are consistent with our results from this study, that lactate release is reduced in GR knockdown astrocytes as well as with the recent demonstration that expression and retrieval of addictive drug memories requires astrocyte-derived lactate [27, 47]. Thus, we propose that drug-induced lactate release in astrocytes is regulated by the GR.
Mice with an astrocyte-specific deletion of the GR did not differ from controls in terms of acquisition and expression of locomotor sensitization after the repeated morphine administration. Recent literature suggests that CPP and locomotor sensitization rely on different neural substrates [48, 49]. Briefly, while the circuitry for morphine-induced locomotor sensitization includes dopamine projections from the VTA to the NAc [50, 51], recent studies reported that dopamine is not required for morphine-induced CPP [49, 52].
Interestingly, the enhanced conditioned response to morphine in mice bearing a knockdown of the GR in astrocytes, did not generalize to another drug of abuse, cocaine. The reinforcing properties of cocaine are known to involve activation of mesolimbic dopaminergic pathways [53]. Strikingly, genetic ablation of the GR in dopaminoceptive neurons results in insensitivity to the psychomotor actions of cocaine and diminishes cocaine-conditioned behaviors [17]. Moreover, GR inactivation in dopaminoceptive neurons leads to altered molecular and behavioral responses to psychostimulants, but not opiates [12, 13]. These observations suggesting that cocaine and morphine engage different molecular pathways and cellular substrates provide a tenable explanation for why GR knockdown in astroglia specifically impact dopamine-independent NAc-mediated opioid reward processing. However, our data also suggest an alternative explanation. We show that, in time corresponding to CPP procedure, morphine administration increased blood corticosterone, while cocaine treatment had no such effect, indicating that both of these drugs might engage HPA axis and recruit glucocorticoid signaling in a different manner. It is possible that the behavioral effects of astroglial GR knockdown in our approach may depend on drug-induced corticosterone release. Therefore, the increase in sensitivity to reward in these mice might be also elicited by other drugs of abuse in doses that activate the HPA axis and possibly by higher doses of cocaine that increase glucocorticoids levels.
Our study show that control and astroglial GR knockdown mice presented comparable responses to stressful and aversive stimuli. As we have silenced the GR specifically within the NAc, glial GR signaling in that brain region appears to be dispensable for acute stress processing. Nonetheless, our recently published results show that astrocyte-specific ablation of the GR in other brain structures, including the hippocampus and amygdala, resulted in impaired aversive memory expression in mice [54], implicating that the astroglial GR may be an important player in the central effects of stress.
Effects of astroglial GR knockdown on synaptic transmission and plasticity in the NAc
Our analysis included an investigation of the possible neural correlates of the observed increased behavioral sensitivity to morphine reward. We found that GR knockdown in astrocytes does not influence the basal membrane properties of medium spiny neurons in the NAc but significantly dampens their excitability and LTP in response to morphine. The observed decreased sEPSCs amplitude (no change in frequency) implies a contribution of astrocytic GR-dependent signaling to the regulation of synaptic transmission via postsynaptic glutamatergic terminals. Previous study provided evidence that lactate induces expression of several plasticity-related genes in cultured cortical neurons by amplifying NMDA receptor-mediated currents and increasing intracellular calcium concentrations [55]. Furthermore, inhibition of lactate release from astrocytes results in reduction of excitability of several populations of neurons [56] and inhibition of astrocytic glycogen phosphorylation reduces LTP in the hippocampus [57]. It is therefore plausible that synaptic transmission is locally influenced by dysfunctional GR-dependent lactate release from astrocytes, which we have observed in our in vitro experiments. In this study, astroglial GR knockdown resulted in enhanced sensitivity to morphine reward, with a parallel reduction of neuronal excitability and LTP in the NAc. Consistently with these results, previous reports show that rewarding stimuli (e.g., sucrose) lead to a major decrease in the activity of NAc neurons [58, 59]. From these various findings, we propose that GR-dependent lactate release from astrocytes modulates morphine-induced changes in synaptic plasticity in the NAc which, in turn, underlies the behavioral response to opioids (Fig. 5e). However, our results do not completely preclude an involvement of neuronal GR in the observed effects, as, although to a lesser extent than astrocytes, we observed that GR-dependent gene transcription also occurs in neurons. What is more, lactate is released in larger quantities from astrocytes than neurons, but both cell types may produce lactate under various conditions [60]. Taken together, our results demonstrate that the astroglial GR is involved in transcriptional, behavioral, and electrophysiological effects of morphine. However, one limitation of this study is the use of different doses for transcriptional and behavioral experiments, respectively. For the whole-genome profiling study, a high dose of morphine (20 mg/kg) was used in order to detect transcriptional alterations in the heterogeneous brain tissue, whereas doses used for behavioral studies (5 and 10 mg/kg) were chosen based on their rewarding properties observed in the CPP.
Alterations in GR function have been previously hypothesized to underlie addiction, with the majority of research being focused on the role of neuronal GR. Further, different types of GR-expressing cells were suggested to underlie behavioral responses to different classes of drugs of abuse, namely psychostimulants and opioids [12]. Here, we show that selective knockdown of GR from Aldh1l1-positive astrocytes enhances sensitivity to the rewarding properties of morphine, accompanied by altered synaptic plasticity in the NAc; our results indicate that the latter result from GR-mediated regulation of astrocytic metabolism. Specifically, our results point out to GR-dependent regulation of lactate release from astrocytes as a possible mechanism responsible for modulation of opioid-related memory. In summary, this work reveals a key role of astroglial GR-dependent mechanisms in the modulation of neuronal plasticity in the NAc, which may ultimately determine opioid reward sensitivity. These findings identify GR in astrocytes as a promising target for treating opioid addiction.
Funding and disclosure
The authors thank Dr O. Almeida for critical reading of the manuscript. Funding for this study was provided by the Polish National Science Centre Grant number 2013/08/A/NZ3/00848 (for RP) The reagents for FISH studies were partially funded by the Human Frontiers Research Project Grant number RGP0039/2017 (for GW). The authors declare no competing interests.
References
Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S, et al. The glucocorticoid receptor as a potential target to reduce cocaine abuse. J Neurosci. 2003;23:4785–90.
Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103.
Heesch CM, Negus BH, Keffer JH, Snyder RW, Risser RC, Eichhorn EJ. Effects of cocaine on cortisol secretion in humans. Am J Med Sci. 1995;310:61–64.
Walter M, Gerber H, Kuhl HC, Schmid O, Joechle W, Lanz C, et al. Acute effects of intravenous heroin on the hypothalamic-pituitary-adrenal axis response: a controlled trial. J Clin Psychopharmacol. 2013;33:193–8.
Ignar DM, Kuhn CM. Effects of specific mu and kappa opiate tolerance and abstinence on hypothalamo-pituitary-adrenal axis secretion in the rat. J Pharmacol Exp Ther. 1990;255:1287–95.
Piazza PV, Maccari S, Deminière JM, Le Moal M, Mormède P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci USA. 1991;88:2088–92.
Piazza PV, Marinelli M, Jodogne C, Deroche V, Rougé-Pont F, Maccari S, et al. Inhibition of corticosterone synthesis by Metyrapone decreases cocaine-induced locomotion and relapse of cocaine self-administration. Brain Res. 1994;658:259–64.
Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30.
Marinelli M, Piazza PV, Deroche V, Maccari S, Le Moal M, Simon H. Corticosterone circadian secretion differentially facilitates dopamine-mediated psychomotor effect of cocaine and morphine. J Neurosci. 1994;14:2724–31.
McReynolds JR, Peña DF, Blacktop JM, Mantsch JR. Neurobiological mechanisms underlying relapse to cocaine use: contributions of CRF and noradrenergic systems and regulation by glucocorticoids. Stress. 2014;17:22–38.
Hearing M, Graziane N, Dong Y, Thomas MJ. Opioid and psychostimulant plasticity: targeting overlap in nucleus accumbens glutamate signaling. Trends Pharm Sci. 2018;39:276–94.
Barik J, Parnaudeau S, Saint Amaux AL, Guiard BP, Golib Dzib JF, Bocquet O, et al. Glucocorticoid receptors in dopaminoceptive neurons, key for cocaine, are dispensable for molecular and behavioral morphine responses. Biol Psychiatry. 2010;68:231–9.
Parnaudeau S, Dongelmans M-L, Turiault M, Ambroggi F, Delbes A-S, Cansell C, et al. Glucocorticoid receptor gene inactivation in dopamine-innervated areas selectively decreases behavioral responses to amphetamine. Front Behav Neurosci. 2014;8:35.
Piechota M, Korostynski M, Solecki W, Gieryk A, Slezak M, Bilecki W, et al. The dissection of transcriptional modules regulated by various drugs of abuse in the mouse striatum. Genome Biol. 2010;11:R48.
Dong Z, Han H, Wang M, Xu L, Hao W, Cao J. Morphine conditioned place preference depends on glucocorticoid receptors in both hippocampus and nucleus accumbens. Hippocampus. 2006;16:809–13.
Marinelli M, Aouizerate B, Barrot M, Le Moal M, Piazza PV. Dopamine-dependent responses to morphine depend on glucocorticoid receptors. Proc Natl Acad Sci USA. 1998;95:7742–7.
Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, et al. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci. 2009;12:247–9.
Carter BS, Hamilton DE, Thompson RC. Acute and chronic glucocorticoid treatments regulate astrocyte-enriched mRNAs in multiple brain regions in vivo. Front Neurosci. 2013;7:139.
Piechota M, Korostynski M, Golda S, Ficek J, Jantas D, Barbara Z, et al. Transcriptional signatures of steroid hormones in the striatal neurons and astrocytes. BMC Neurosci. 2017;18:37.
Slezak M, Korostynski M, Gieryk A, Golda S, Dzbek J, Piechota M, et al. Astrocytes are a neural target of morphine action via glucocorticoid receptor-dependent signaling. Glia. 2013;61:623–35.
Skupio U, Sikora M, Korostynski M, Wawrzczak-Bargiela A, Piechota M, Ficek J, et al. Behavioral and transcriptional patterns of protracted opioid self-administration in mice. Addict Biol. 2017;22:1802–16.
Beitner-Johnson D, Guitart X, Nestler EJ. Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J Neurochem. 1993;61:1766–73.
Song P, Zhao Z-Q. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–6.
Scofield MD, Li H, Siemsen BM, Healey KL, Tran PK, Woronoff N, et al. Cocaine self-administration and extinction leads to reduced glial fibrillary acidic protein expression and morphometric features of astrocytes in the nucleus accumbens core. Biol Psychiatry. 2016;80:207–15.
Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84.
Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol. 2015;20:316–23.
Boury-Jamot B, Carrard A, Martin JL, Halfon O, Magistretti PJ, Boutrel B. Disrupting astrocyte-neuron lactate transfer persistently reduces conditioned responses to cocaine. Mol Psychiatry. 2016;21:1070–6.
Magistretti PJ, Allaman I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018;19:235–49.
Sada N, Lee S, Katsu T, Otsuki T, Inoue T. Epilepsy treatment. targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science. 2015;347:1362–7.
Allaman I, Pellerin L, Magistretti PJ. Glucocorticoids modulate neurotransmitter-induced glycogen metabolism in cultured cortical astrocytes. J Neurochem. 2004;88:900–8.
Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, et al. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci USA. 2004;101:10380–5.
Carrard A, Elsayed M, Margineanu M, Boury-Jamot B, Fragnière L, Meylan EM, et al. Peripheral administration of lactate produces antidepressant-like effects. Mol Psychiatry. 2018;23:488.
Sikora M, Tokarski K, Bobula B, Zajdel J, Jastrzębska K, Cieślak PE, et al. NMDA receptors on dopaminoceptive neurons are essential for drug-induced conditioned place preference. eNeuro. 2016;3:pii: ENEURO.0084-15.201.
Kosak ST, Skok JA, Medina KL, Riblet R, Beau MML, Fisher AG, et al. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296:158–62.
Clowney EJ, LeGros MA, Mosley CP, Clowney FG, Markenskoff-Papadimitriou EC, Myllys M, et al. Nuclear aggregation of olfactory receptor genes governs their monogenic expression. Cell. 2012;151:724–37.
Attarzadeh-Yazdi G, Karimi S, Azizi P, Yazdi-Ravandi S, Hesam S, Haghparast A. Inhibitory effects of forced swim stress and corticosterone on the acquisition but not expression of morphine-induced conditioned place preference: involvement of glucocorticoid receptor in the basolateral amygdala. Behav Brain Res. 2013;252:339–46.
McNally GP, Akil H. Selective down-regulation of hippocampal glucocorticoid receptors during opiate withdrawal. Mol Brain Res. 2003;118:152–5.
Navarro-Zaragoza J, Hidalgo JM, Laorden ML, Milanés MV. Glucocorticoid receptors participate in the opiate withdrawal-induced stimulation of rats NTS noradrenergic activity and in the somatic signs of morphine withdrawal. Br J Pharmacol. 2012;166:2136–47.
Linthorst AC, Flachskamm C, Barden N, Holsboer F, Reul JM. Glucocorticoid receptor impairment alters CNS responses to a psychological stressor: an in vivo microdialysis study in transgenic mice. Eur J Neurosci. 2000;12:283–91.
Miranda MI, Quirarte GL, Rodriguez-Garcia G, McGaugh JL, Roozendaal B. Glucocorticoids enhance taste aversion memory via actions in the insular cortex and basolateral amygdala. Learn Mem. 2008;15:468–76.
Ikegami M, Uemura T, Kishioka A, Sakimura K, Mishina M. Striatal dopamine D1 receptor is essential for contextual fear conditioning. Sci Rep. 2014;4:srep03976.
Bagot RC, Parise EM, Peña CJ, Zhang H-X, Maze I, Chaudhury D, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7626.
Mächler P, Wyss MT, Elsayed M, Stobart J, Gutierrez R, von Faber-Castell A, et al. In Vivo Evidence for a Lactate Gradient from Astrocytes to Neurons. Cell Metab. 2016;23:94–102.
Carter BS, Meng F, Thompson RC. Glucocorticoid treatment of astrocytes results in temporally dynamic transcriptome regulation and astrocyte-enriched mRNA changes in vitro. Physiol Genom. 2012;44:1188–200.
Haydon PG, Blendy J, Moss SJ, Rob Jackson F. Astrocytic control of synaptic transmission and plasticity: a target for drugs of abuse? Neuropharmacology. 2009;56:83–90. Suppl 1
Koyanagi S, Kusunose N, Taniguchi M, Akamine T, Kanado Y, Ozono Y, et al. Glucocorticoid regulation of ATP release from spinal astrocytes underlies diurnal exacerbation of neuropathic mechanical allodynia. Nat Commun. 2016;7:13102.
Zhang Y, Xue Y, Meng S, Luo Y, Liang J, Li J, et al. Inhibition of lactate transport erases drug memory and prevents drug relapse. Biol Psychiatry. 2016;79:928–39.
Chefer V, Shippenberg T. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology. 2009;34:887–98.
Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–7.
Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216.
Margolis EB, Hjelmstad GO, Fujita W, Fields HL. Direct bidirectional μ-opioid control of midbrain dopamine neurons. J Neurosci. 2014;34:14707–16.
Borgkvist A, Usiello A, Greengard P, Fisone G. Activation of the cAMP/PKA/DARPP-32 signaling pathway is required for morphine psychomotor stimulation but not for morphine reward. Neuropsychopharmacology. 2007;32:1995–2003.
Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–94.
Tertil M, Skupio U, Barut J, Dubovyk V, Wawrzczak-Bargiela A, Soltys Z, et al. Glucocorticoid receptor signaling in astrocytes is required for aversive memory formation. Transl Psychiatry. 2018;8:255.
Yang J, Ruchti E, Petit J-M, Jourdain P, Grenningloh G, Allaman I, et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci USA. 2014;111:12228–33.
Sada N, Lee S, Katsu T, Otsuki T, Inoue T. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science. 2015;347:1362–7.
Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, et al. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell. 2011;144:810–23.
Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol. 2004;91:1840–65.
Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci. 2006;26:217–22.
Walz W, Mukerji S. Lactate release from cultured astrocytes and neurons: a comparison. Glia. 1988;1:366–70.
Author information
Authors and Affiliations
Contributions
US and RP designed the study. US and RP wrote the manuscript. MT designed and generated lentiviral plasmids. WB performed immunohistochemistry and obtained confocal images. MT, JB, US, and SG performed biochemical experiments. US, LK, and LW performed behavioral testing. MK and US analyzed data. US, RP, and KT designed electrophysiological study. KP, BR, and GW performed FISH staining and interpreted FISH results. BB, JES, and MS performed electrophysiological experiments. All authors read and approved the manuscript.
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Skupio, U., Tertil, M., Bilecki, W. et al. Astrocytes determine conditioned response to morphine via glucocorticoid receptor-dependent regulation of lactate release. Neuropsychopharmacol. 45, 404–415 (2020). https://doi.org/10.1038/s41386-019-0450-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-019-0450-4
This article is cited by
-
Major depressive disorder: hypothesis, mechanism, prevention and treatment
Signal Transduction and Targeted Therapy (2024)
-
Astrocytic transcriptional and epigenetic mechanisms of drug addiction
Journal of Neural Transmission (2023)
-
Single nucleus transcriptomic analysis of rat nucleus accumbens reveals cell type-specific patterns of gene expression associated with volitional morphine intake
Translational Psychiatry (2022)
-
Knockdown of Astrocytic Monocarboxylate Transporter 4 in the Motor Cortex Leads to Loss of Dendritic Spines and a Deficit in Motor Learning
Molecular Neurobiology (2022)
-
Astroglial Knockout of Glucocorticoid Receptor Attenuates Morphine Withdrawal Symptoms, but Not Antinociception and Tolerance in Mice
Cellular and Molecular Neurobiology (2022)