Abstract
The principal neurons of the arousal and sleep circuits are comprised by glutamate and GABA neurons, which are distributed within the reticular core of the brain and, through local and distant projections and interactions, regulate cortical activity and behavior across wake-sleep states. These are in turn modulated by the neuromodulatory systems that are comprised by acetylcholine, noradrenaline, dopamine, serotonin, histamine, orexin (hypocretin), and melanin-concentrating hormone (MCH) neurons. Glutamate and GABA neurons are heterogeneous in their profiles of discharge, forming distinct functional cell types by selective or maximal discharge during (1) waking and paradoxical (REM) sleep, (2) during slow wave sleep, (3) during waking, or (4) during paradoxical (REM) sleep. The neuromodulatory systems are each homogeneous in their profile of discharge, the majority discharging maximally during waking and paradoxical sleep or during waking. Only MCH neurons discharge maximally during sleep. They each exert their modulatory influence upon other neurons through excitatory and inhibitory receptors thus effecting a concerted differential change in the functionally different cell groups. Both arousal and sleep circuit neurons are homeostatically regulated as a function of their activity in part through changes in receptors. The major pharmacological agents used for the treatment of wake and sleep disorders act upon GABA and neuromodulatory transmission.
Similar content being viewed by others
Introduction
Arousal circuits comprise multiple diverse, partially redundant systems which promote behavioral arousal that is accompanied by cortical activation. Arousal is stimulated by multiple conditions of the external and/or internal environments, which elicit varying somatic and/or autonomic responses appropriate to those conditions. Waking (W) is a state characterized by behavioral arousal along with cortical activation of differing degrees, which are reflected in polygraphic recordings by muscle tone on the electromyogram (EMG) and fast activity within a gamma range (30–60 Hz and/or above) on the electroencephalogram (EEG) (Fig. 1). Active or attentive waking (aW) is distinguished from quiet waking (qW) by high tonic and phasic muscle tone along with maximal amplitude of gamma activity coincident with high rhythmic theta activity (6–10 Hz) [1]. Sleep which is typified by a behavioral quiescent state is actually comprised of two distinct states: slow wave sleep (SWS) characterized by the distinctive spindle (10–14 Hz) and slow wave (delta 0.5–4 Hz) activity on the EEG and rapid eye movement (REM) or paradoxical sleep (PS) characterized by REM or also more essentially by behavioral sleep with complete postural muscle atonia accompanied paradoxically by cortical activation similar to that during W (Fig. 1). Although sleep was once considered to be a passive deactivation of the central nervous system, it has been found to involve an active process of inhibition of the multiple arousal circuits, which is differential for SWS and PS. The following review is intended to provide an overview of the major arousal circuits and their regulation by sleep-promoting circuits.
Sleep-wake states in the rat shown by EEG and EMG activity across the three major states, Waking (W), Slow Wave Sleep (SWS), and Paradoxical Sleep (PS) and their sub states (active, a, and quiet, q, W) or transitional (t) stages. Colors represent different activities of the EEG and EMG in the different states and the symbols the different neurons which discharge maximally during those states or activities, as represented in Figs. 2 and 3. PF, prefrontal cortex; RS, retrosplenial cortex
Neurons in the reticular core utilizing amino acid neurotransmitters constitute arousal and sleep circuits
The reticular formation and ascending reticular activating system
From the classical work of Moruzzi and Magoun and their colleagues [2], it has been known that the brainstem reticular formation (RF) is critically important for the stimulation and maintenance of W, including behavioral arousal and cortical activation, since large lesions of the RF resulted in coma in animals and humans [3,4,5]. Neurons distributed through the medullary, pontine, and mesencephalic RF (RFMes) give rise to descending reticulo-spinal projections which facilitate movement and postural muscle tone with multiple behaviors during W [6, 7] (Figs. 1 and 2). Also distributed through these regions, but more highly concentrated in the more rostral pontine and RFMes are neurons which give rise to ascending projections into the forebrain which stimulate cortical activation [8,9,10]. Although yet to be studied fully with in situ hybridization or genetic techniques, most of the large projection neurons of the RF are considered to be glutamate (Glu) neurons and can be distinguished from smaller intermingled neurons which contain the synthetic enzyme for GABA (glutamic acid decarboxylase). GABA neurons represent approximately a third of all neurons in the RF [10, 11] and relatively small percentages of the long spinal and forebrain projecting neurons [12, 13]. Single unit recording studies indicated that most neurons in the RFMes with ascending projections discharge in association with cortical activation during both W and PS [8] (Figs. 1 and 2). Other neurons of the pontomesencephalic tegmentum (PMT) which would have been involved in lesions that were associated with coma or diminished arousal would encompass those of the neuromodulatory systems, including acetylcholine (ACh), noradrenaline (NA), and dopamine (DA) neurons along with intermingled Glu and GABA neurons (see below) [14]. Moreover, Glu neurons in the parabrachial nuclei which receive relays from autonomic afferent inputs have also been found to be important in stimulating and maintaining cortical activation [15, 16].
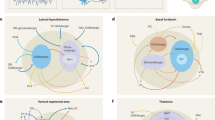
Sleep-wake state neural systems. Sagittal schematic view of the rat brain depicting neurons with their chemical neurotransmitters, pathways, and discharge profiles by which they influence cortical activity or behavior across the sleep/wake cycle. Waking (W) is characterized by fast (gamma, >30 Hz) activity on the cortical EEG (upper left) and high postural muscle tone on the neck EMG (lower right); slow wave sleep (SWS) by slow EEG (delta, <4 Hz) and low tone on the EMG; and paradoxical sleep (PS) by fast EEG and atonia on the EMG (Fig. 1). Neurons that are active during W (red symbols) include cells with ascending projections toward the cortex, which stimulate fast cortical activity (filled symbols), and cells with descending projections toward the spinal cord, which stimulate motor activity with postural muscle tone typical of behavioral W (open symbols). Those with predominantly ascending projections discharge in association with fast EEG activity (gamma+) and decrease or cease firing with delta activity (delta−) to be maximally active during W and PS (W/PS-max active, filled red symbols). (Those with similar average rates of discharge but different modes of discharge across states are red filled with gray.) They include glutamate (Glu), GABA, and acetylcholine (ACh) neurons. Those with more diffuse or descending projections discharge in association with behavioral arousal and EMG activity (EMG+) and decrease or cease firing with muscle atonia to be active during W and quiet during PS (EMG+, W-max active, open red symbols); they include Glu, GABA, noradrenaline (NA), serotonin (Ser), histamine (HA), and orexin (Orx or hypocretin) neurons. Neurons that are active during sleep include cells with ascending projections toward the cortex, which discharge maximally with slow wave activity during SWS (gamma−, delta+, SWS-max active, blue symbols) and those with descending projections toward the hypothalamus, brainstem, or spinal cord, which discharge maximally with muscle atonia during PS (EMG-, PS-max active, aqua symbols). They include GABA (with some glycine, Gly), Glu, and MCH neurons. Among these functionally distinguished cell types, certain have been shown to bear specific receptors to the neuromodulatory chemicals, including ACh inhibitory muscarinic type 2 receptors (AChM2R) on behavioral wake-active neurons, adrenergic excitatory (Aα1R) receptors on behavioral wake-active neurons, adrenergic inhibitory (Aα2R) receptors on slow EEG-active and behavioral sleep-active neurons, serotonergic (5-hydroxytryptamine) inhibitory (5HT1AR) receptors on fast EEG-active neurons and Orx excitatory (Orx1/2R) receptors on behavioral wake-active neurons. 7 g, genu 7th nerve; ac, anterior commissure; ACh, acetylcholine; BF, basal forebrain; CPu, caudate putamen; Cx, cortex; DA, dopamine; DR, dorsal raphe; EEG, electroencephalogram; EMG, electromyogram; GiA, gigantocellular, alpha part RF; Gi RF, gigantocellular RF; GiV, gigantocellular, ventral part RF; Glu, glutamate; Gly, glycine; GP, globus pallidus; HA, histamine; Hi, hippocampus; ic, internal capsule; LC, locus coeruleus nucleus; LDT, laterodorsal tegmental nucleus; PH, posterior hypothalamus; MCH, melanin-concentrating hormone; Mes RF, mesencephalic RF; NA, noradrenaline; opt, optic tract; Orx, orexin; PnC, pontine, caudal part RF; PnO, pontine, oral part RF; POA, preoptic area; PPT, pedunculopontine tegmental nucleus; PS, paradoxical sleep; RF, reticular formation; RT, reticular thalamic nucleus; s, solitary tract; scp, superior cerebellar peduncle; Ser, serotonin; SN, substantia nigra; Sol, solitary tract nucleus; SWS, slow wave sleep; Th, thalamus; VTA, ventral tegmental area; W, wake. (Modified with permission from Jones [199])
Forming the ascending reticular activating system, the RF neurons project together with other neuromodulatory systems into the forebrain along a dorsal pathway to the midline and intralaminar thalamic nuclei of the nonspecific thalamo-cortical projection system, which gives rise in turn to widespread or diffuse projections to the cerebral cortex and has the capacity to stimulate widespread and long-lasting cortical activation [17,18,19]. In addition, there is a ventral extra-thalamic pathway which ascends via the medial forebrain bundle (MFB) through and into the lateral hypothalamus (LH) and basal forebrain (BF) from where other neurons, including ACh containing neurons, project up to the cerebral cortex [9, 20]. Like the neurons of the brainstem RF, those residing along this ventral pathway have long radiating dendrites sitting amongst the MFB passing fibers and thus form a rostral continuation of the reticular core, which is also crucially involved in the relay and maintenance of cortical activation [15, 21, 22].
Neurons of the nonspecific thalamo-cortical projection system, like those of the specific thalamic relay projection system discharge in a relatively tonic manner in association with cortical activation during W and PS, but they also discharge during SWS not at a different average rate but in a different mode, a bursting mode that is correlated with cortical spindles and slow waves [23] (Fig. 2, gray filled red symbols). All thalamo-cortical projection neurons are excitatory neurons utilizing Glu as a neurotransmitter. Their discharge and activity pattern is under the influence of GABA neurons located in what was called the thalamic reticularis or reticular nucleus (TRN) because of the long dendrites of its neurons. The TRN neurons surround the dorsal thalamus and project in a topographically organized manner onto the thalamo-cortical projection neurons (Fig. 2). Like the thalamic relay neurons, the thalamic reticular neurons discharge in a relatively tonic or single spike mode during W and PS and discharge in a bursting mode during SWS [24, 25]. Indeed, these GABA TRN neurons function within a thalamo-cortico-thalamic loop to shape the firing of the relay and nonspecific thalamo-cortical neurons [26]. Within this circuit, the average discharge rate of the neurons is not necessarily different across states, but the mode or pattern of discharge is, such that SWS represents a metabolically restful state for the thalamus and cortex [27], despite the bursting discharge and likely because of the long hyperpolarization between bursts through Up-Down states of the slow oscillation [28]. The most recent studies employing genetic techniques for specific labeling and optogenetic activation of thalamo-cortical neurons have confirmed the importance of the nonspecific thalamic nuclei in stimulating widespread cortical activation of W but also the Up state of the slow oscillation during SWS, when these neurons burst and lead the Up state in the cortex [29].
In contrast to the thalamic nonspecific cortical projection neurons, the neurons within the ventral extra-thalamic pathway and relay do show significant differences in their average rate of discharge across wake-sleep states, such that some can be virtually off during SWS and on during W and PS, suggesting a fundamental role in determining the wake-sleep states. Such a role was first suggested for and attributed to neuromodulatory systems, including the ACh and monoamine neurons [30], as will be reviewed below, but appears increasingly to be essentially mediated by networks of excitatory and inhibitory neurons, as we first learned in the BF cholinergic cell area (Fig. 2). Whereas it was originally thought that the basalo-cortical projection neurons were uniquely ACh neurons, it was learned that roughly equivalent numbers of GABA and Glu neurons contributed to that projection while innervating different target neurons in the cortex [20, 31, 32]. Other GABA and Glu neurons in the BF project caudally to the posterior hypothalamus (PH) and beyond into the PMT [33,34,35]. Using juxtacellular recording and identification of neurons in the BF, it was determined that approximately half of the neurons in the region discharge at a maximal rate in association with fast gamma cortical activity during W and PS and were thus called W/PS-max-active neurons [36] (Figs. 1 and 2). These units were comprised by ACh, GABA, and Glu neurons. However, whereas all ACh neurons were W/PS-max-active neurons (below), the GABA and Glu neurons were heterogeneous and comprised other principal functional cell types, including SWS-max active, W-max active, and PS-max active in addition to state (wsp)-equivalent or state-indifferent neurons (Fig. 3). Each of these different functional cell types displayed significant changes in discharge rate across the three major states and manifested changes in rate between aW and qW and in the transitional stages between W and SWS (tSWS) and between SWS and PS (tPS). Based upon neuroanatomical and electrophysiological evidence, it is assumed that the W/PS-max and SWS-max active neurons give rise to ascending projections to the cortex and can reciprocally influence fast gamma EEG activity such as to respectively promote cortical activation or slow wave activity on the cortex. It is also assumed that W-max-active and PS-max-active neurons give rise to descending projections and can reciprocally influence postural muscle tone recorded on the EMG such as to respectively promote behavioral W and arousal or behavioral sleep. It was moreover hypothesized and realized in a dynamical systems model that the GABA and Glu neurons through projections onto different distant target neurons and local interactions of mutual inhibition between pairs within the EEG and the EMG modules could generate the oscillations in cortical fast activity and muscle tone across wake-sleep states and accordingly generate the three states of W, SWS, and PS [37].
Discharge rates of principal cell types across sleep-wake stages. Normalized average rates of firing shown with normalized average gamma (30–60 Hz) EEG activity and EMG activity across sleep wake stages of active or attentive wake (aW), quiet wake (qW), transition to SWS (tSWS), slow wave sleep (SWS), transition to PS (tPS), and paradoxical sleep (PS). Rates were taken from one exemplary neuron for each cell type from neurons recorded in the basal forebrain. Cells were recorded and filled with Neurobiotin using the juxtacellular technique for subsequent immunohistochemical identification of their neurotransmitter as GABA or (putative or identified) glutamate (Glu). They were classified into one of four principal cell types, (W/PS-max, SWS-max, W-max, and PS-max active) or state (wsp, Wake-SWS-PS)-indifferent, by statistical analysis of their rates in aW, SWS, and PS. (Modified with permission from Hassani et al. [36])
Preoptic area and hypothalamus
The four principal functional cell types of wake-sleep regulatory circuits have been found in other areas of the forebrain, including importantly the preoptic area (POA) (Fig. 2), where W/PS-max and W-max active neurons were recorded in addition to SWS-max and PS-max active neurons in nuclei, such as the ventrolateral preoptic (VLPO) and median/medial preoptic nuclei, which have been considered to be sleep centers [38,39,40,41,42]. From the early studies of von Economo [43] and Nauta [44], the anterior hypothalamus and POA appeared to be critical for sleep, whereas the PH and LH appeared to be the most important for W as the rostral continuation of the ascending reticular activating system. So, it was surprising to learn that both wake- and sleep-active neurons could be located in both the POA and the PH [38, 45, 46]. With the identification of GABA in addition to Glu neurons through these regions, it was thought that perhaps the GABA neurons were the sleep-promoting neurons [47, 48], but these neurons also proved to be heterogeneous in their involvement in sleep versus wake, as evident in c-Fos studies [49]. On the other hand, in the c-Fos and electrophysiological studies in the POA and BF, it appeared that the GABA neurons, which were the most active during sleep, bore adrenergic α2 receptors (Aα2R) (Fig. 2) associated with inhibition in response to NA (see below) [50, 51]. Thus, as was found in the BF, both GABA and Glu neurons are involved in generating each wake-sleep state, their roles depending upon their discharge profiles, the receptors they bear to neuromodulatory inputs along with their specific projections, which can be to local or distant specific target neurons.
Recent studies applying optogenetic tagging together with retrograde tracing showed that specific photoactivation of those GABA neurons in the POA which project to the tuberomammillary nucleus (TMN), where histamine (HA) neurons are located, promoted SWS along with the transition into PS [52]. Optrode recording of these TMN projecting GABA neurons revealed them to be typical of PS-max active neurons, discharging at progressively higher rates in the passage from W to SWS and PS. On the other hand, photoactivation of all GABA neurons in the POA did not evoke SWS but W. Photoactivation of the specific Glu neurons in the POA which project to the TMN elicited W. These results clearly demonstrate that functionally different GABA and Glu neurons lie intermingled in regions previously considered as sleep centers and can promote different wake-sleep states by their specific activities and projections.
Also in the PH and LH, which was considered a W center, recording studies identified sleep-active GABA neurons that were predominantly PS-max active neurons and were found intermingled with other GABA and non-GABA (including Glu) W/PS-max active and W-max active neurons [53]. Yet, optogenetic and chemogenetic activation of all GABA neurons in the LH did not enhance SWS or PS, but instead promoted W through what was discovered to be disinhibition via a projection from some LH GABA neurons onto GABA neurons in the TRN [54] and others onto GABA neurons in the VLPO [55]. Thus, as in the BF and POA, GABA together with Glu neurons having different activity profiles and projections lie intermingled in the LH and can by their specific activities and projections along with receptors promote different wake-sleep states. Although attempts at identifying these functionally different subtypes by colocalization of different calcium-binding proteins (such as parvalbumin) or peptides (such as somatostatin or galanin) in GABA neurons have been made, these markers have also not allowed the distinction of wake-active versus sleep-active GABA neurons [56,57,58]. In summary, the ostensible predominant influence of any region likely depends upon relative densities of the different GABA and Glu functional cell types.
Pharmacological evidence for the importance of Glu and GABA neurotransmitters
Clearly through the brainstem RF, thalamus, and reticular core of the forebrain, Glu and GABA neurons are integrally involved in the regulation of cortical activity and behaviors of arousal and sleep. It is thus not surprising that the major anesthetics, which produce slowing of EEG activity in association with loss of consciousness, reflexes and postural muscle tone, act upon Glu and GABA receptors in the brain [59]. Many anesthetics antagonize Glu NMDA receptors which would be located upon neurons at every level of the RF and ascending reticular activating system, including the thalamus, hypothalamus, and BF as well as the cortex. Most anesthetics act by potentiating or directly activating (chloride) inhibitory currents in GABAA receptors, which would also be present in all neurons of the arousal circuits as well as the cortex. Used in the treatment for insomnia, most sedative-hypnotic drugs also act upon GABAA receptors [59,60,61,62]. Depending upon the precise receptor sub-type upon which the drug acts, different drugs, such as the benzodiazepine-receptor agonists and direct GABAA receptor agonists, can act most prominently upon neurons within particular sites, such as the thalamus or cortex, to effect particular changes, such as enhanced spindle-like activity or slow waves in the EEG normally associated with SWS. However, since these GABAA receptor acting drugs would nonetheless affect multiple target neurons with different roles in wake-sleep states, these drugs cannot and do not induce natural sleep. For this reason, hypnotic agents have been and are being developed for the treatment of insomnia that act more specifically as antagonists of the neuromodulatory arousal systems that are selective in their roles in arousal and the W state.
Neurons utilizing neuromodulators regulate arousal and sleep circuits
Since early pharmacological studies followed by histochemical and then specific lesion studies pioneered by Jouvet, the important role of ACh and monoamines as neuromodulators in wake-sleep states was revealed in the last century [30]. Indeed, in these original studies, it was believed that the neuromodulatory systems could generate the sleep-wake states.
Acetylcholine neurons
ACh was first proposed to be the major neurotransmitter/modulator of the ascending reticular activating system [63] with projections from the brainstem ascending into the thalamus and ventrally up to the BF where other ACh fibers ascended into the cortex. Although the ascending activating system is now better understood to be comprised predominantly of Glu and GABA neurons (above), the ACh neurons are still considered to play a very important role in cortical activation, as has been the subject of many studies over the past 50 years [64, 65]. At the level of the cortex, ACh was shown to elicit fast cortical activity and prevent slow wave activity through ACh muscarinic receptors [66]. Blocking AChM receptors with atropine blocked fast cortical activity which occurs during W and PS without preventing movement or behavioral arousal [67]. ACh release from the cortex occurred with cortical activation during W and PS and was minimal during SWS [68]. Using juxtacellular recording and labeling for immunohistochemical identification of the recorded neurons, BF ACh neurons were found to discharge at maximal rates in association with fast gamma and theta EEG activity during W and PS and to be virtually silent during SWS [69] (Fig. 4). The virtual silence of ACh neurons with SWS is likely a prerequisite for slow wave activity on the cortex. The BF ACh neurons are thus W/PS-max active neurons, as are also a major proportion of intermingled Glu and GABA neurons, which however, unlike the ACh neurons, are functionally heterogeneous (above) [36] (Figs. 2 and 3). Using optogenetic tagging techniques for identifying the neurons being recorded, the activity of BF ACh neurons across wake-sleep states has recently been confirmed [57], and specific optogenetic or chemogenetic activation of the ACh neurons found to suppress slow waves and stimulate cortical fast activity with W and/or albeit more rarely PS [70,71,72]. Chemogenetic activation of all GABA or parvalbumin GABA BF neurons also elicited wake with fast gamma cortical activity [72, 73]. The effect of stimulating all GABA (or parvalbumin) or all Glu neurons in any region likely reflects the role of the predominant functional cell type (above), which for the BF cell population is W/PS-max-active cells, however such effects do not allow revelation of the roles of other minor GABA (or parvalbumin) and Glu functional cell types. On the other hand, pharmacological activation of the BF cell population has manifested the concerted actions of the different GABA, Glu along with the ACh functional cell types, since they respond differentially to agonists of the monoamine (MA) arousal systems [74, 75]. Thus whereas BF ACh neurons are depolarized and excited by NA through Aα1Rs, other nonACh BF neurons, including identified sleep-active GABA neurons [51], are hyperpolarized and inhibited by NA through Aα2Rs (Fig. 2). Microinjection of NA into the BF evoked prolonged cortical activation with increased fast gamma EEG activity and W [76]. Thus, the BF ACh neurons play a primary role in stimulating cortical activation during W and PS but do so in concert with other intermingled GABA and Glu neurons which comprise different functional cell types that respond differentially to neuromodulators of the arousal circuits.
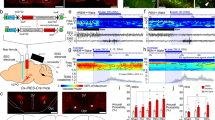
Discharge of an acetylcholine (ACh) basal forebrain (BF) neuron across sleep-wake states. A Record of a neuron labeled by juxtacellular technique with Neurobiotin (Nb) and identified by immunohistochemistry for choline acetyltransferase (ChAT) as cholinergic in the magnocellular preoptic nucleus (MCPO) of the rat. As evident in 10 s traces (above), the unit fired during aW, virtually ceased firing during SWS, resumed firing during tPS, and discharged maximally during PS. As evident in expanded 0.5 s traces (below), the unit discharged in rhythmic bursts of spikes with theta EEG activity that was present intermittently during periods of aW, toward the end of tPS and continuously during PS. B Average discharge rate (Hz) across the sleep-wake states and transitions (t) of the same cell. Avg, average; aW, active wake; EEG, electroencephalogram; EMG, electromyogram; PF, prefrontal cortex; PS, paradoxical sleep; qW, quiet wake; RS, retrosplenial cortex; SWS, slow wave sleep; tPS, transition to PS; tSWS transition to SWS. Bar for horizontal scale: 1 s. Bar for vertical scales: 1 mV for EEG/EMG and 1.5 mV for Unit. (Reprinted with permission from Lee et al. [69])
Like the BF ACh neurons, the PMT ACh neurons also discharge with cortical activation during W and PS with muscle atonia as W/PS-max active neurons [77] (Figs. 1 and 2). Recent studies applying optogenetic or chemogenetic techniques have confirmed these findings of the ACh neuronal discharge while also demonstrating that their stimulation elicits cortical activation including high gamma and theta EEG activity along with W and in some cases PS while diminishing slow wave activity and SWS [78,79,80]. Located in the laterodorsal tegmental (LDT), SubLDT, and pedunculopontine tegmental nuclei, the PMT ACh neurons were originally considered to be components of the RF (above). In this regard, they fit with the neurons of the RFMes, discharging in association with cortical activation, as components of the ascending reticular activating system. The ACh neurons also project rostrally into the intralaminar and midline nonspecific thalamo-cortical projection system which they excite through nicotinic and AChM1 receptors [81]. As in the BF, the ACh neurons are intermingled with equivalent numbers of Glu and GABA neurons in the PMT, which were also found to comprise different functional cell types, mostly W/PS-max, like the ACh neurons, but also PS-max among the Glu and GABA neurons and W-max among the Glu neurons [77]. Whereas chemogenetic stimulation of the ACh PMT neurons evoked cortical activation without enhancing the amount of W, that of Glu neurons evoked prolonged cortical activation together with behavioral arousal and W [79].
Primarily from pharmacological studies, ACh has long been known to play an important role in generating PS [30, 82]. Microinjections of the ACh receptor agonists (carbachol or bethanochol) into the pontine RF elicited PS with muscle atonia which was dependent upon AChM2Rs [83]. Since no PMT ACh neurons were found to discharge specifically during PS, whereas all discharged during both W and PS [77], it was concluded that the PMT ACh neurons may exert an inhibitory action through AChM2Rs upon those RF neurons which normally stimulate muscle tone and movement during W. Indeed, large presumed Glu neurons are distributed through the pontomesencephalic RF which bear AChM2Rs [11] (Fig. 2). From early pharmacological studies, it was known that the cholinesterase inhibitor, physostigmine, could elicit PS, but only following depletion of the monoamines with reserpine [84]. It was conceived that in absence of input upon these Glu neurons from other neuromodulators of the arousal circuits, including importantly NA and orexin (Orx) (below), muscle atonia associated with cortical activation would occur under the influence of ACh neurons during PS.
Although cytotoxic lesions of the PMT cholinergic cell area resulted in a loss of PS with muscle atonia that was correlated with the proportion of ACh neurons destroyed, these lesions included nonACh neurons of the PMT in the cat [85]. As in the BF cholinergic cell area, the Glu and GABA neurons in the PMT cholinergic cell area comprise neurons which discharge maximally during PS, as PS-max active neurons in rats [77]. Some of these likely correspond to PS-active neurons described previously in the cat [86] and more recently in mice [87] that were purported to be most concentrated in the SubCoeruleus and SubLDT region. In studies employing c-Fos, PS-active neurons in this region (also called Sublaterodorsal, SLD) were identified predominantly as Glu and as projecting caudally to the medullary RF where GABA/glycine (Gly) neurons have been identified, which project to the spinal cord to inhibit motor neurons [88,89,90]. GABA and Glycine have both been shown to be involved in the direct inhibition of motor neurons during PS [91, 92]. The region of the medullary RF where GABA/Gly spinally projecting neurons are located [12] corresponds to that which Magoun and Rhines had originally identified by electrical stimulation as having the capacity to effect motor inhibition [93]. Recent optogenetic studies of GABA neurons in the medullary RF suggested that these neurons could generate PS [94] and would act in part by inhibiting other W-active GABA neurons in the midbrain ventrolateral periaqueductal gray (vlPAG) that prevent PS [95]. Yet again here in the medulla and midbrain, GABA (or Gly) inhibitory neurons may play different roles depending upon their activity profile [96], local and distant projections and receptors to the neuromodulators. From c-Fos studies, it is apparent that there are GABA PS-active neurons in some regions and GABA W-active neurons in other regions or overlapping in the same region of the brainstem [97,98,99,100]. These different GABA cell types could be respectively involved in promoting PS with muscle atonia or conversely W with muscle tone and behavioral arousal (Figs. 2 and 3). Indeed, whereas there is evidence that presumed Glu neurons in the SubLDT (or SLD) which promote muscle atonia are excited by ACh through AChM1Rs [101], GABA neurons in the pontine and mesencephalic RF (PnO and RFMes, along with adjacent vlPAG), which are active during W and can prevent muscle atonia with PS, appear to be inhibited by ACh through AChM2Rs [11, 100] (below).
From pharmacological studies in humans and animals, it is known that agents which enhance cholinergic transmission can enhance REM or PS and with that, exacerbate narcolepsy with cataplexy [102]. Many drugs used in the treatment of narcolepsy and cataplexy have anticholinergic actions (below). Anticholinergic agents also comprise certain anesthetics which by blocking nicotinic or ACHM1Rs or by decreasing ACh release are associated with loss of cortical activation and consciousness [103].
Noradrenaline and dopamine neurons
Pharmacological evidence had indicated in the last century that the catecholamines in the brain would play an important role in arousal, like those in the sympathetic nervous system do in the body [30]. Following mapping of the catecholamine neurons in the brain, the role of NA and DA neurons was examined by lesions in rats [104] and cats [30, 105]. Whereas lesions of ascending NA projections were associated with decreases in cortical activation with W, lesions of DA neurons in the ventral mesencephalon did not diminish cortical activation but resulted in akinesia or deficits in behavioral arousal [105].
In neuroanatomical studies, it was revealed that the NA locus coeruleus (LC) neurons were different from other neurons of the brainstem RF because they give rise to highly diffuse projections that reach multiple targets through the brainstem, spinal cord, thalamus, hypothalamus, BF, and the entire cortical mantle [9]. Moreover, like adrenergic neurons of the sympathetic nervous system, the NA LC neurons send highly collateralized fibers bearing varicosities from which NA can be released in a nonsynaptic or paracrine fashion onto target neurons throughout these regions [106]. The NA neurons thus appeared to represent in their structure a central sympathetic system or the central substrate for an arousal system that could simultaneously stimulate cortical activation and behavioral arousal. And indeed, in single unit recording studies in the rat in which the NA neurons form a compact homogeneous nucleus in the LC, they were discovered to discharge during W and in association with behavioral arousal, to diminish firing during SWS and become silent during PS [107] (Fig. 2). Their discharge preceded the transition from PS to W with muscle tone, suggesting that they would play a pivotal role in that transition. It was originally believed that such an arousal circuit would be essential for maintaining W [30], yet following extensive lesions of NA neurons and depletion of NA in the cortex, W was not even significantly decreased to great surprise [108]. Previous effects of lesions of the NA ascending fibers in the midbrain necessitated a reinterpretation which recognized the importance of other presumably Glu neurons in the RFMes. This result was only the first to indicate that NA LC neurons are not essential for W, but also allowed the conclusion that they nonetheless play a very potent role in stimulating W with cortical activation and behavioral arousal. This role was prominently demonstrated in recent optogenetic studies in which activation of the NA LC neurons during SWS led to immediate cortical activation and behavioral arousal along with locomotion in mice [109]. The neuromodulatory role of the NA neurons would entail enlistment of other neurons of the neuromodulatory arousal system (see below) but also Glu and GABA neurons through the RF, thalamus, and reticular core including the BF. Indeed in the BF (above), the ACh neurons along with other nonACh neurons are depolarized and excited by NA through Aα1Rs. Conversely, NA inhibits some nonACh BF and POA neurons, including GABA sleep-active neurons which are hyperpolarized and inhibited through Aα2Rs [49,50,51, 75]. NA neurons can thereby stimulate arousal and regulate wake-sleep states by exciting other neurons of the activating and behavioral arousal circuits and inhibiting neurons of the sleep circuits.
It has been well known that amphetamine, which acts by release of catecholamines, elicits a very strong and prolonged arousal including cortical activation and behavioral arousal with stereotypic motor behaviors. It was thus even more surprising to learn that NA LC lesions in the cat also did not alter quantitatively or qualitatively, the prolonged W produced by amphetamine [108]. These results demonstrated the important role of DA neurons of the substantia nigra (SN) and ventral tegmental area (VTA) in promoting arousal and W. Yet, recordings of presumed DA neurons in the mesencephalon indicated that in contrast to the NA LC neurons, the DA neurons did not change their average rate of discharge across the states of W, SWS, and PS [110]. On the other hand, c-Fos studies indicated that the DA SN and VTA neurons were more active during W and PS than during SWS [111]. Indeed, it became clear that DA neurons change their mode of firing across states from a burst like discharge during W, to a single spike mode during SWS and a burst like discharge during PS that was similar to that occurring during appetitive W behaviors [112]. The DA neurons are thus very different from LC neurons, perhaps because they do not appear to directly increase muscle tone and movement by their predominantly ascending projections into the forebrain thalamus, reticular core, striatal structures, and cortex [113]. On the other hand, they may be active during W and PS, as many neurons are (Fig. 2) in association with central activation of sensory-motor circuits which are peripherally inhibited during PS and dreaming. Recent studies employing fiber photometry with Ca++ indicators expressed in DA VTA neurons confirmed that DA neurons discharge across the wake/sleep cycle but show pronounced population cell activity during W with behavioral arousal and PS [114]. Optogenetic activation of DA VTA neurons in these studies evoked W with behavioral arousal and prevented sleep along with sleep preparatory behaviors. Conversely, optogenetic inhibition promoted sleep preparatory behaviors and enhanced sleep. Indeed, these results confirm a critical role of DA neurons in arousal and W, as would be expected from pharmacological studies.
The primary drugs utilized in the treatment of narcolepsy comprise amphetamine-like drugs or more recently modafinil [115], which was also shown to enhance DA release while additionally acting through other adrenergic receptors [116]. NA and DA must accordingly be viewed as important contingents of the arousal circuits. Though neither may be necessary, both are important potentiators of cortical activation and behavioral arousal of active and attentive W (Fig. 2).
Serotonin neurons
From the results of extensive pharmacological and lesion studies, Jouvet concluded years ago that serotonin raphe neurons played a determinant role in SWS [30]. Depletion of serotonin by drugs or lesions of the raphe nuclei resulted in almost complete insomnia. Yet, single unit recording studies found that the serotonin neurons discharged during W and decreased firing during SWS to become silent during PS, like NA LC neurons [117]. From recording studies examining the role of serotonin neurons of the different raphe nuclei, which are distributed along the midline through the medulla, pons, and midbrain with differential spinal and forebrain projections (including the dorsal raphe, DR, nucleus, Fig. 2), it became clear that serotonin neurons are W-active neurons that may nonetheless play different roles from the NA LC neurons in W [118]. The raphe neurons were thus not found to respond to sensory stimulation or discharge with peripheral sympathetic activation, as NA neurons do, but were found to discharge with movement, particularly movements driven by rhythmic pattern generators which may be associated with dampening of sensory inputs. Through its inhibitory action upon BF ACh neurons, opposite the excitatory action of NA [119], serotonin can reduce cortical gamma activity [76], which would also be associated with diminished sensory responsiveness. Nonetheless, the role of serotonin in arousal and sleep remains incompletely understood. What is clear is that serotonin also inhibits PMT ACh neurons and can thereby prevent PS with muscle atonia [120]. It is likely for this reason that the antidepressant drugs which block serotonin reuptake can be associated with incomplete loss of muscle tone during REM sleep or REM sleep behavior disorder in humans [121] and can also be used to treat narcolepsy with cataplexy [115].
Histamine neurons
Like NA LC neurons, HA neurons were found to have their cell bodies clustered together in a small nucleus, the TMN near the PH [122] and thus in a region considered to be part of the activating system. Also like NA LC neurons, the HA neurons give rise to diffuse projections into the forebrain, including up to the cerebral cortex. Lesions or pharmacological inactivation of the PH and TMN produced a very marked hypersomnia, which could be attributed in part to the suppression or loss of HA neurons [123]. Recording of identified HA TMN neurons revealed them to discharge specifically during W and particularly during attentive W in mice [124]. They differed from NA LC neurons, also recorded in mice [125], in that their discharge did not precede the transition from SWS or PS to W and only responded to sensory stimuli if they elicited attention. A role for HA neurons in attentive W was also apparent in mice in which the gene for histidine decarboxylase was knocked out [126]. Although these mice did not show decreases in the amount of W, they did show deficits in theta EEG activity during W along with lack of behavioral attention to salient stimuli.
A role for HA neurons in cortical activation and attentive W has been known for years in view of the effects of anti-HA medications [61, 62]. Yet, despite H1 receptor antagonist actions of these drugs, which are responsible for the increased slow wave activity, most of the anti-HA drugs, like many of the drugs prescribed for insomnia, also act on other arousal systems, including ACh, NA, DA, and serotonin transmission.
This pharmacological principle converges with the findings from lesion studies (above) that although each neuromodulatory system contributes to arousal, no one system has proven to be essential for the maintenance of W, as evident through lesion studies (above) [85, 105, 108, 127,128,129].
Orexin neurons
Having learned that no one arousal system appeared to be necessary for the maintenance of W, it was very surprising when it was discovered that mice lacking the gene for the peptide Orx or (hypocretin, Hcrt) [130] and dogs lacking the gene for the receptors to Orx (Hcrt or Orx 2R) [131]) manifested the inability to maintain W with muscle tone or what characterizes the disorder of narcolepsy with cataplexy. It was subsequently discovered that patients with this disease show a loss of the Orx neurons associated with diminished levels of Orx in cerebrospinal fluid [132, 133]. Intracerebroventricular administration of Orx can overcome narcolepsy with cataplexy and stimulate prolonged W in Orx KO mice [134]. Neurons containing Orx are located in the PH, tuberal hypothalamus and LH and give rise to diffuse projections throughout the brain including up to the cerebral cortex and down into the spinal cord [135, 136]. They innervate and excite through exclusively excitatory receptors (Orx1 and 2R) all the other neurons of the arousal systems, including those of the RF [11], midline and intralaminar thalamic nuclei [137], BF and PMT ACh neurons [138, 139], NA LC neurons [140], DA VTA neurons [141], serotonin DR neurons [142], and HA TMN neurons [143]. They also release Glu from a certain proportion of their varicosities [144, 145]. Utilizing juxtacellular recording and labeling with immunohistochemical identification, the Orx neurons in the LH were found to discharge selectively during W and particularly during aW in association with muscle tone and movement [146] (Figs. 1, 2, and 5). They were essentially silent during SWS and PS but increased their discharge prior to transitions to W from these states, seemingly driving or participating in the transition to arousal. In recent studies employing optogenetics, specific photoactivation of the Orx neurons shortened transitions to W from SWS and PS [147]. In examining the important effectors of this response, it was learned that the NA LC neurons were critically involved [148]. The Orx neurons are also excited by the NA neurons, since NA depolarizes the Orx neurons through Aα1Rs (Fig. 2) [149]. The Orx neurons thus appear to represent a central hub within the neuromodulatory arousal circuits [150, 151]. Orx receptors are found on the most large presumed Glu neurons, but also on some GABA neurons in the pontine and RFMes [11]. In fact, it would appear that the Orx input would enforce W through excitation of some GABA neurons which are W active and involved in preventing PS with muscle atonia [94, 95, 100, 152] (below).
Discharge of an orexin (Orx) lateral hypothalamus (LH) neuron across sleep-wake states. A Record of a neuron labeled by juxtacellular technique with Neurobiotin (Nb) and identified by immunohistochemistry for Orx in the rat. As evident in 10 s traces (above), the unit fired during W and was virtually silent during SWS, tPS, and PS. As evident in an expanded trace (of ~4 s, below), the unit discharged during active W (aW) and increased firing phasically in association with increases in muscle tone seen on the EMG. B Average discharge rate (Hz) across the sleep-wake states and transitions (t) of the same cell. Avg, average; aW, active wake; EEG, electroencephalogram; EMG, electromyogram; PF, prefrontal cortex; PS, paradoxical sleep; qW, quiet wake; RS, retrosplenial cortex; SWS, slow wave sleep; tPS, transition to PS; tSWS transition to SWS. Horizontal scale bar: 1 s. Vertical scale bar: 1 mV for EEG, 0.5 mV for EMG, and 2 mV for unit. (Reprinted with permission from Lee et al. [146])
The critical role of Orx in maintaining arousal with muscle tone which is lost in patients with narcolepsy and cataplexy has yet to be replaced by drugs for treatment of this disorder, since peptides do not cross the blood–brain barrier. On the other hand, antagonists of Orx1 and 2 receptors have been successfully developed and proven in animal studies and trials to be effective for the treatment of insomnia. Indeed, as antagonists of this most important hub in the arousal circuits, the dual receptor antagonists facilitate sleep without any of the side effects associated with the GABAA R agonists [153].
MCH neurons
Neurons containing melanin-concentrating hormone (MCH) are distributed in the hypothalamus in an overlapping manner with the Orx neurons (Fig. 2). They also give rise to relatively diffuse projections through the brain including the cortex [154]. MCH has a predominantly inhibitory influence on synaptic transmission [155], and MCH neurons may also release GABA from a proportion of their terminals [156, 157]. Through c-Fos studies, it first appeared that the MCH neurons were active in a reciprocal manner to the Orx neurons, since c-Fos was expressed during W enforced by sleep deprivation (SD) in the Orx neurons, whereas it was expressed during sleep recovery (SR) in the MCH neurons [158, 159]. Moreover, intraventricular administration of MCH elicited sleep with enhanced PS [158]. With juxtacellular recording and labeling, the MCH neurons in the LH were found to discharge only during sleep, at lower rates during SWS and higher rates during PS and in a profile which was reciprocal to that of the Orx neurons, suggesting that MCH neurons would promote sleep, whereas Orx neurons promote W and arousal [160]. Recently, optogenetic activation of the MCH neurons elicited sleep and particularly PS [157, 161], though increases in SWS were also reported [162]. That MCH neurons may be an important modulator in sleep circuits is also reinforced by the finding that MCH neurons are inhibited by NA through Aα2Rs, whereas Orx neurons are normally excited by NA through Aα1Rs [149] (Fig. 2).
Homeostatic regulation of arousal and sleep circuits
Obviously, not all GABA neurons are sleep active and promoting, nor are all Glu neurons wake active and promoting, but instead, depending upon their receptors and target neurons, comprise heterogeneous groups of sleep and wake-active neurons operating within local and distant networks in the brainstem and forebrain (above). On the other hand, all neurons of the arousal circuits are subject to inhibition by local or distant GABA neurons, which as shown for the NA LC neurons [163,164,165], as well as for DA VTA neurons, Orx LH and other Glu neurons [166,167,168,169], can regulate their level of discharge in association with arousal but also cause the reduction or cessation of their discharge during sleep. Sleep and conversely, W have been shown to be homeostatically regulated, such that deprivation of sleep is followed by SR with an increase in sleep and decrease in W, as demonstrated years ago by Borbely et al. [170, 171], in humans and rats. The activity of individual neurons is also homeostatically regulated, such that prolonged increases in activity are followed by decreases in excitability and activity, which are associated with increases in GABAA receptors and consequent increases in inhibitory currents on the cells [172,173,174]. Conversely, cessation of activity is followed by increases in excitability and activity, which are associated with decreases in GABAA receptors and consequent decreases in inhibitory currents on the cells [175]. These principles of homeostatic regulation at the cellular level most likely underlie the homeostatic regulation of sleep and W.
Given that Orx and MCH neurons manifest reciprocal profiles of discharge across the wake/sleep cycle, it was deemed likely that they should undergo differential changes in excitability and thus in GABA receptors following enforced W with SD, if the principle of homeostatic regulation of neurons according to their activities holds true [176]. Indeed, it was found that whereas the wake-active Orx neurons showed increases in both GABAA and GABAB receptors [176, 177], the sleep-active MCH neurons showed decreases in these receptors with SD, the control levels of which were restored in each case following SR [176]. The increases in GABAA receptors on the Orx neurons were associated with increased sensitivity of the Orx neurons to GABAA agonists [177]. These results indicated that homeostatic bidirectional changes in GABAARs occur in arousal and sleep circuits as a function of their state selective activities. This same principle was found to apply to other wake-active cell groups, including BF ACh neurons [178], motor neurons [179], and excitatory cortical neurons [180].
In addition, it was found that homeostatic changes could also occur in receptors to the neuromodulators. In Orx neurons, the excitatory action of NA through Aα1Rs found in control conditions was changed to an inhibitory action of NA through Aα2Rs following SD in brain slices of mice [181, 182]. This change in the action of NA likely reflects mobilization of the Aα2Rs to the membrane in the Orx neurons, which do appear to contain both Aα1 and Aα2 receptors [183], in contrast to the MCH neurons which contain only Aα2Rs [159].
Homeostatic changes in inhibitory receptors have also been documented upon the GABA wake-active neurons in the RFMes [100], which together with those in the vlPAG [184], comprise neurons that prevent the occurrence of PS and muscle atonia (above). In this case, GABAA and AChM2 receptors were found to increase on the membrane of GABA neurons in the RFMes that were active during enforced W with SD, as evident by c-Fos expression (Fig. 6). These increases were restored to control levels with SR, during which PS with muscle atonia was increased.
GABA neurons in the mesencephalic reticular formation (RFMes) under conditions of sleep control (SC) and sleep deprivation (SD). I c-Fos in RFMes GABAergic neurons across groups. Fluorescent microscopic images show staining for Nissl with fluorescent Nissl stain (FNS, green, A1, B1), immunostaining for glutamic acid decarboxylase (GAD, blue, A2, B2, with positive staining indicated by filled arrowheads), and immunostaining for c-Fos (red, A3, B3, with positive staining indicated by filled arrowhead) along with dual staining for Nissl and c-Fos in merged images (green and red, A4, B4, with positive c-Fos staining indicated by filled arrowhead). Note that c-Fos immunostaining is prominent in the nucleus of a GABAergic neuron from an SD mouse (B3 and B4), whereas it is not apparent in images from SC mice (A3 and A4, indicated by open arrowheads). Scale bar: 20 μm. Image thickness: 500 nm in all panels. II GABAARs in RFMes GABAergic neurons across groups. Confocal microscopic images show all neurons stained for Nissl with FNS (green, A1, B1), the GABAergic neurons immunostained for GABA (blue, A2, B2, indicated by filled arrowheads), and for the GABAARs in single (red, A3, B3, indicated by filled arrowheads) and merged images (A4, B4, indicated by filled arrowheads). Note that in an SC mouse, the GABAAR immunofluorescence is minimally visible, whereas in an SD mouse, it is prominent and bright. In all cases, the immunostaining is relatively continuous though with nonuniform intensity along the plasma membrane of the GABA+ neurons. Scale bar: 20 μm. Image thickness: 500 nm in all panels. III AChM2Rs in RFMes GABAergic neurons across groups. Confocal microscopic images show all neurons stained for Nissl with FNS (green, A1, B1), the GABAergic neurons immunostained for GAD (blue, A2, B2, indicated by filled arrowheads), and for the AChM2Rs in single (red, A3, B3, indicated by filled arrowheads) and merged images (A4, B4, indicated by filled arrowheads). Note that in an SC mouse, the AChM2R immunofluorescence is minimally visible along the plasma membrane, whereas in an SD mouse, the AChM2R staining is bright and clearly visible along the full membrane of the GAD+ neuron. Scale bar: 20 μm. Image thickness: 500 nm in all panels. (Copied with permission from Toossi et al. [100])
The homeostatic changes in receptors to the amino acid neurotransmitters and neuromodulatory receptors associated with wake-sleep state alterations have major implications for the manifestation and treatment of sleep disorders. First, sleep deficits or deprivation are associated with increases in sleepiness with EEG slow wave activity and also decreases in muscle tone [185, 186] and in narcoleptic patients are associated with increased propensity to attacks of narcolepsy with cataplexy [102]. These changes could be explained by homeostatic increases in inhibitory receptors and resulting decreases in excitability and activity of the neurons in the arousal circuits that promote cortical activation and behavioral arousal with muscle tone. That changes in GABA receptors would be fundamental to these alterations with SD is supported by the finding that certain GABA receptor agonist anesthetics can replace SR [187]. Moreover, one of the major lines of treatment for narcolepsy with cataplexy is the administration of gamma-hydroxybutyrate, which acts on GABAB receptors, during the night following which narcoleptic attacks are greatly reduced the following day [188], likely associated with reduction of GABA receptors on the neurons of the arousal circuits, including importantly Orx neurons.
Sleep homeostasis is also believed to be driven by adenosine, the nucleoside which forms adenosine triphosphate (ATP), adenosine diphosphate (ADP), and cyclic adenosine monophosphate (camp) and is thus linked to energy metabolism [65, 189, 190]. Possibly released from both neurons and glia in a vesicular (with ATP) or nonvesicular manner dependent upon transport, adenosine increases in the extracellular space as a function of neural activity and following SD [191,192,193]. As learned in the last century, increased extracellular adenosine promotes slow wave activity and SWS [194]. This action is effected through adenosine (A)1 inhibitory receptors which are located on neurons of the arousal circuits, including ACh, NA, HA, and Orx neurons, and which have been shown to increase on these neurons with SD [65]. Adenosine also acts upon A2a excitatory receptors identified upon other presumed sleep-promoting neurons [195,196,197]. Accumulation of extracellular adenosine and increases in adenosine receptors during SD would thus effect increasing inhibition of the arousal circuits and excitation of the sleep circuits such as to enhance sleep drive and promote sleep homeostasis. It is not surprising that caffeine, the most highly utilized stimulant in the world, acts as an antagonist on adenosine receptors and mitigator for the effects of SD [198].
Future research directions
With the new armamentarium of genetically based techniques, it has become possible to examine in fine detail the chemical identity of neurotransmitters, modulators, and comodulators in arousal and sleep circuits, the discharge or activity profile of the chemically and anatomically specified neurons in relation to state and behavior and to activate or inactivate these neurons to determine their role in state and behavior. As mentioned for some of the multiple recent discoveries made with these techniques, a better understanding of the role of Glu and GABA neurons as comprising the basic networks of arousal and sleep circuits has begun to emerge. There is still much to learn about the specific roles of local and distant projections of these neurons and their modulation by the neuromodulatory systems. With this knowledge along with developments in pharmaceuticals, more specific or better tailored drugs will become available for the treatment of arousal and sleep disorders.
Funding and disclosure
The author thanks Lynda Mainville and Hanieh Toossi for their assistance with editing the manuscript and figures. The research presented from the Author’s laboratory was funded by grants from NIH (MH-60119) and CIHR (MOP 13458, 82762, and 130502).
References
Maloney KJ, Cape EG, Gotman J, Jones BE. High-frequency gamma electroencephalogram activity in association with sleep-wake states and spontaneous behaviors in the rat. Neuroscience. 1997;76:541–55.
Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–73.
Lindsley DB, Schreiner LH, Knowles WB, Magoun HW. Behavioral and EEG changes following chronic brain stem lesions. Electroencephalogr Clin Neurophysiol. 1950;2:483–98.
Plum F, Posner JB. The diagnosis of stupor and coma. Philadelphia: Davis; 1980.
Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003;126:1524–36.
Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp Brain Res. 1979;36:1–20.
Siegel JM. Behavioral functions of the reticular formation. Brain Res. 1979;180:69–105.
Steriade M, Oakson G, Ropert N. Firing rates and patterns of midbrain reticular neurons during steady and transitional states of the sleep-waking cycle. Exp Brain Res. 1982;46:37–51.
Jones BE, Yang T-Z. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985;242:56–92.
Jones BE. Reticular formation. Cytoarchitecture, transmitters and projections. In: Paxinos G, ed. The rat nervous system. Sydney: Academic Press Australia; 1995. p. 155–71.
Brischoux F, Mainville L, Jones BE. Muscarinic-2 and orexin-2 receptors on GABAergic and other neurons in the rat mesopontine tegmentum and their potential role in sleep-wake state control. J Comp Neurol. 2008;510:607–30.
Jones BE, Holmes CJ, Rodriguez-Veiga E, Mainville L. GABA-synthesizing neurons in the medulla: their relationship to serotonin-containing and spinally projecting neurons in the rat. J Comp Neurol. 1991;312:1–19.
Ford B, Holmes C, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol. 1995;363:177–96.
Fischer DB, Boes AD, Demertzi A, Evrard HC, Laureys S, Edlow BL, et al. A human brain network derived from coma-causing brainstem lesions. Neurology. 2016;87:2427–34.
Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–56.
Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, et al. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci. 2013;33:7627–40.
Starzl TE, Magoun HW. Organization of the diffuse thalamic projection system. J Neurophysiol. 1951;14:133–46.
Steriade M. Mechanisms underlying cortical activation: neuronal organization and properties of the midbrain reticular core and intralaminar thalamic nuclei. In: Pompeiano O, Ajmone Marsan C, eds. Brain mechanisms and perceptual awareness. New York, NY: Raven Press; 1981. p. 327–77.
Herkenham M. New perspectives on the organization and evolution of nonspecific thalamocortical projections. In: Jones EG, Peters A, eds. Cerebral cortex, vol 5. New York, NY: Plenum; 1986. p. 403–45.
Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other non-cholinergic basal forebrain neurons project together with cholinergic neurons to meso- and iso-cortex in the rat. J Comp Neurol. 1997;383:163–77.
Vanderwolf CH, Stewart DJ. Thalamic control of neocortical activation: a critical re-evaluation. Brain Res Bull. 1988;20:529–38.
Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–26.
Steriade M, Hobson JA. Neuronal activity during the sleep-waking cycle. Prog Neurobiol. 1976;6:155–376.
Barrionuevo G, Benoit O, Tempier P. Evidence for two types of firing pattern during the sleep-waking cycle in the reticular thalamic nucleus of the cat. Exp Neurol. 1981;72:486–501.
Steriade M, Deschenes M. The thalamus as a neuronal oscillator. Brain Res Rev. 1984;8:1–63.
Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–85.
Maquet P, Dive D, Salmon E, Sadzot B, Franco G, Poirrier R, et al. Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and [18F]2-fluoro-2-deoxy-D-glucose method. Brain Res. 1990;513:136–43.
Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–85.
Gent TC, Bandarabadi M, Herrera CG, Adamantidis AR. Thalamic dual control of sleep and wakefulness. Nat Neurosci. 2018;21:974–84.
Jouvet M. The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergeb Physiol. 1972;64:165–307.
Freund TF, Meskenaite V. Gamma-aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Natl Acad Sci USA. 1992;89:738–42.
Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–70.
Gritti I, Mainville L, Jones BE. Projections of GABAergic and cholinergic basal forebrain and GABAergic preoptic-anterior hypothalamic neurons to the posterior lateral hypothalamus of the rat. J Comp Neurol. 1994;339:251–68.
Jones BE, Cuello AC. Afferents to the basal forebrain cholinergic cell area from pontomesencephalic–catecholamine, serotonin, and acetylcholine–neurons. Neuroscience. 1989;31:37–61.
Henny P, Jones BE. Vesicular glutamate (VGluT), GABA (VGAT), and acetylcholine (VAChT) transporters in basal forebrain axon terminals innervating the lateral hypothalamus. J Comp Neurol. 2006;496:453–67.
Hassani OK, Lee MG, Henny P, Jones BE. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci. 2009;29:11828–40.
Jones BE. Principal cell types of sleep-wake regulatory circuits. Curr Opin Neurobiol. 2017;44:101–09.
Koyama Y, Hayaishi O. Firing of neurons in the preoptic/anterior hypothalamic areas in rat: its possible involvement in slow wave sleep and paradoxical sleep. Neurosci Res. 1994;19:31–38.
Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–88.
Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543:665–77.
Sakai K. Sleep-waking discharge profiles of median preoptic and surrounding neurons in mice. Neuroscience. 2011;182:144–61.
Takahashi K, Lin JS, Sakai K. Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience. 2009;161:269–92.
von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71:249–59.
Nauta WJH. Hypothalamic regulation of sleep in rats. Exp study J Neurophysiol. 1946;9:285–316.
Koyama Y, Takahashi K, Kodama T, Kayama Y. State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking. Neuroscience. 2003;119:1209–19.
Tamakawa Y, Karashima A, Koyama Y, Katayama N, Nakao M. A quartet neural system model orchestrating sleep and wakefulness mechanisms. J Neurophysiol. 2006;95:2055–69.
Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–19.
Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–31.
Modirrousta M, Mainville L, Jones BE. GABAergic neurons with alpha2-adrenergic receptors in basal forebrain and preoptic area express c-Fos during sleep. Neuroscience. 2004;129:803–10.
Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, et al. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–95.
Manns ID, Lee MG, Modirrousta M, Hou YP, Jones BE. Alpha 2 adrenergic receptors on GABAergic, putative sleep-promoting basal forebrain neurons. Eur J Neurosci. 2003;18:723–7.
Chung S, Weber F, Zhong P, Tan CL, Nguyen TN, Beier KT, et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature. 2017;545:477–81.
Hassani OK, Henny P, Lee MG, Jones BE. GABAergic neurons intermingled with orexin and MCH neurons in the lateral hypothalamus discharge maximally during sleep. Eur J Neurosci. 2010;32:448–57.
Herrera CG, Cadavieco MC, Jego S, Ponomarenko A, Korotkova T, Adamantidis A. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat Neurosci. 2016;19:290–8.
Venner A, Anaclet C, Broadhurst RY, Saper CB, Fuller PM. A novel population of wake-promoting GABAergic neurons in the ventral lateral hypothalamus. Curr Biol. 2016;26:2137–43.
Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458:11-31.
Xu M, Chung S, Zhang S, Zhong P, Ma C, Chang WC, et al. Basal forebrain circuit for sleep-wake control. Nat Neurosci. 2015;18:1641–7.
Chen KS, Xu M, Zhang Z, Chang WC, Gaj T, Schaffer DV, et al. A hypothalamic switch for REM and non-REM Sleep. Neuron. 2018;97:1168–76 e4.
Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86.
Winsky-Sommerer R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur J Neurosci. 2009;29:1779–94.
Feren S, Schweitzer PK, Walsh JK. Pharmacotherapy for insomnia. Handb Clin Neurol. In: Vinken PJ, Bruyn GW, editors. 2011;99:747–62.
Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197–245.
Shute CCD, Lewis PR. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967;90:497–520.
Jones BE. The organization of central cholinergic systems and their functional importance in sleep-waking states. Prog Brain Res. 1993;98:61–71.
Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–187.
Krnjevic K, Phillis JW. Pharmacological properties of acetylcholine-sensitive cells in the cerebral cortex. J Physiol 1963;166:328–50.
Longo VG. Behavioral and electroencephalographic effects of atropine and related compounds. Pharm Rev. 1966;18:965–96.
Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966;16:1053–64.
Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–69.
Han Y, Shi YF, Xi W, Zhou R, Tan ZB, Wang H, et al. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol. 2014;24:693–8.
Irmak SO, de Lecea L. Basal forebrain cholinergic modulation of sleep transitions. Sleep. 2014;37:1941–51.
Anaclet C, Pedersen NP, Ferrari LL, Venner A, Bass CE, Arrigoni E, et al. Basal forebrain control of wakefulness and cortical rhythms. Nat Commun. 2015;6:8744.
Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, et al. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci USA. 2015;112:3535–40.
Fort P, Khateb A, Pegna A, Muhlethaler M, Jones BE. Noradrenergic modulation of cholinergic nucleus basalis neurons demonstrated by in vitro pharmacological and immunohistochemical evidence in the guinea pig brain. Eur J Neurosci. 1995;7:1502–11.
Fort P, Khateb A, Serafin M, Muhlethaler M, Jones BE. Pharmacological characterization and differentiation of non-cholinergic nucleus basalis neurons in vitro. Neuroreport. 1998;9:1–5.
Cape EG, Jones BE. Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci. 1998;18:2653–66.
Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34:4708–27.
Van Dort CJ, Zachs DP, Kenny JD, Zheng S, Goldblum RR, Gelwan NA, et al. Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc Natl Acad Sci USA. 2015;112:584–9.
Kroeger D, Ferrari LL, Petit G, Mahoney CE, Fuller PM, Arrigoni E, et al. Cholinergic, glutamatergic, and GABAergic neurons of the pedunculopontine tegmental nucleus have distinct effects on sleep/wake behavior in mice. J Neurosci. 2017;37:1352–66.
Cisse Y, Toossi H, Ishibashi M, Mainville L, Leonard CS, Adamantidis A, et al. Discharge and role of acetylcholine pontomesencephalic neurons in cortical activity and sleep-wake states examined by optogenetics and juxtacellular recording in mice. eNeuro. 2018;5:pii: ENEURO.0270-18.2018.
Curro Dossi R, Pare D, Steriade M. Short-lasting nicotinic and long-lasting muscarinic depolarizing responses of thalamocortical neurons to stimulation of mesopontine cholinergic nuclei. J Neurophysiol. 1991;65:393–406.
Jones BE. Paradoxical sleep and its chemical/structural substrates in the brain. Neuroscience. 1991;40:637–56.
Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984;306:39–52.
George R, Haslett W, Jenden D. A cholinergic mechanism in the brainstem reticular formation: induction of paradoxical sleep. Int J Neuropharmacol. 1964;3:541–52.
Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res. 1988;458:285–302.
Sakai K, Sastre J-P, Kanamori N, Jouvet M. State-specific neurons in the ponto-medullary reticular formation with special reference to the postural atonia during paradoxical sleep in the cat. In: Pompeiano O, Ajmone-Marsan C, eds. Brain mechanisms and perceptual awareness. New York, NY: Raven Press; 1981. p. 405–29.
Sakai K. Paradoxical (rapid eye movement) sleep-on neurons in the laterodorsal pontine tegmentum in mice. Neuroscience. 2015;310:455–71.
Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94.
Clement O, Sapin E, Berod A, Fort P, Luppi PH. Evidence that neurons of the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are glutamatergic. Sleep. 2011;34:419–23.
Valencia Garcia S, Luppi PH, Fort P. A particular medullary-spinal inhibitory pathway is recruited for the expression of muscle atonia during REM sleep. J Exp Neurosci. 2018;12:1179069518808744.
Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep. J Neurosci. 1989;9:743–51.
Brooks PL, Peever JH. Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis. J Neurosci. 2012;32:9785–95.
Magoun HW, Rhines R. An inhibitory mechanism in the bulbar reticular formation. J Neurophysiol. 1946;9:165–71.
Weber F, Chung S, Beier KT, Xu M, Luo L, Dan Y. Control of REM sleep by ventral medulla GABAergic neurons. Nature. 2015;526:435–8.
Weber F, Hoang DoJP, Chung S, Beier KT, Bikov M, Saffari Doost M, et al. Regulation of REM and non-REM sleep by periaqueductal GABAergic neurons. Nat Commun. 2018;9:354.
Sakai K. Behavioural state-specific neurons in the mouse medulla involved in sleep-wake switching. Eur J Neurosci. 2018;47:1482–503.
Maloney KJ, Mainville L, Jones BE. Differential c-Fos expression in cholinergic, monoaminergic and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci. 1999;19:3057–72.
Maloney KJ, Mainville L, Jones BE. c-Fos expression in GABAergic, serotonergic and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. J Neurosci. 2000;20:4669–79.
Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur J Neurosci. 2003;18:1627–39.
Toossi H, Del Cid-Pellitero E, Jones BE. Homeostatic changes in GABA and acetylcholine muscarinic receptors on GABAergic neurons in the mesencephalic reticular formation following sleep deprivation. eNeuro. 2017;4:pii: ENEURO.0269-17.2017.
Weng FJ, Williams RH, Hawryluk JM, Lu J, Scammell TE, Saper CB, et al. Carbachol excites sublaterodorsal nucleus neurons projecting to the spinal cord. J Physiol. 2014;592:1601–17.
Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78.
Van Dort CJ, Baghdoyan HA, Lydic R. Neurochemical modulators of sleep and anesthetic states. Int Anesth Clin. 2008;46:75–104.
Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122.
Jones BE, Bobillier P, Pin C, Jouvet M. The effect of lesions of catecholamine-containing neurons upon monoamine content of the brain and EEG and behavioral waking in the cat. Brain Res. 1973;58:157–77.
Beaudet A, Descarries L. The monoamine innervation of rat cerebral cortex: synaptic and nonsynaptic axon terminals. Neuroscience. 1978;3:851–60.
Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–86.
Jones BE, Harper ST, Halaris AE. Effects of locus coeruleus lesions upon cerebral monoamine content, sleep-wakefulness states and the response to amphetamine in the cat. Brain Res. 1977;124:473–96.
Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33.
Miller JD, Farber J, Gatz P, Roffwarg H, German DC. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and waking in the rat. Brain Res. 1983;273:133–41.
Maloney KJ, Mainville L, Jones BE. c-Fos expression in dopaminergic and GABAergic neurons of the ventral mesencephalic tegmentum after paradoxical sleep deprivation and recovery. Eur J Neurosci. 2002;15:774–8.
Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–41.
Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topogr J Comp Neurol. 1978;180:545–80.
Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci. 2016;19:1356–66.
Barateau L, Lopez R, Dauvilliers Y. Treatment options for narcolepsy. CNS Drugs. 2016;30:369–79.
Wisor J. Modafinil as a catecholaminergic agent: empirical evidence and unanswered questions. Front Neurol. 2013;4:139.
McGinty D, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–75.
Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends Neurosci. 1993;16:346–52.
Khateb A, Fort P, Alonso A, Jones BE, Muhlethaler M. Pharmacological and immunohistochemical evidence for serotonergic modulation of cholinergic nucleus basalis neurons. Eur J Neurosci. 1993;5:541–7.
Luebke JI, Greene RW, Semba K, Kamondi A, McCarley RW, Reiner PB. Serotonin hyperpolarizes cholinergic low-threshold burst neurons in the rat laterodorsal tegmental nucleus in vitro. Proc Natl Acad Sci. 1992;89:743–47.
Mahowald MW, Schenck CH, Bornemann MA. Pathophysiologic mechanisms in REM sleep behavior disorder. Curr Neurol Neurosci Rep. 2007;7:167–72.
Panula P, Yang HY, Costa E. Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci USA. 1984;81:2572–6.
Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–40.
Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26:10292–8.
Takahashi K, Kayama Y, Lin JS, Sakai K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience. 2010;169:1115–26.
Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–711.
Denoyer M, Sallanon M, Buda C, Kitahama K, Jouvet M. Neurotoxic lesion of the mesencephalic reticular formation and/or the posterior hypothalamus does not alter waking in the cat. Brain Res. 1991;539:287–303.
Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27:14041–8.
Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci. 2008;28:491–504.
Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51.
Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76.
Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7.
Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74.
Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci USA. 2004;101:4649–54.
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015.
van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19:3171–82.
Bayer L, Eggermann E, Saint-Mleux B, Machard D, Jones BE, Muhlethaler M, et al. Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J Neurosci. 2002;22:7835–9.
Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, et al. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–81.
Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by Hypocretin/Orexin peptides: implications for wakefulness and narcolepsy. J Neurosci. 2002;22:2862–72.
Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–59.
Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci. 2008;28:1545–56.
Kohlmeier KA, Inoue T, Leonard CS. Hypocretin/orexin peptide signaling in the ascending arousal system: elevation of intracellular calcium in the mouse dorsal raphe and laterodorsal tegmentum. J Neurophysiol. 2004;92:221–35.
Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–79.
Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience. 2003;119:1033–44.
Henny P, Brischoux F, Mainville L, Stroh T, Jones BE. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience. 2010;169:1150–7.
Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20.
Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–4.
Carter ME, Brill J, Bonnavion P, Huguenard JR, Huerta R, de Lecea L. Mechanism for hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci USA. 2012;109:E2635–44.
Bayer L, Eggermann E, Serafin M, Grivel J, Machard D, Muhlethaler M, et al. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130:807–11.
Jones BE, Muhlethaler M. Modulation of cortical activity and sleep-wake state by hypocretin/orexin. In: de Lecea L, Sutcliffe JG, eds. The hypocretins: integrators of physiological systems. New York, NY: Springer; 2005. p. 289–301.
Eban-Rothschild A, Appelbaum L, de Lecea L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology. 2018;43:937–52.
Kaur S, Thankachan S, Begum S, Liu M, Blanco-Centurion C, Shiromani PJ. Hypocretin-2 saporin lesions of the ventrolateral periaquaductal gray (vlPAG) increase REM sleep in hypocretin knockout mice. PLoS ONE. 2009;4:e6346.
Kumar A, Chanana P, Choudhary S. Emerging role of orexin antagonists in insomnia therapeutics: an update on SORAs and DORAs. Pharm Rep. 2016;68:231–42.
Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, et al. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–45.
Gao XB, van den Pol AN. Melanin-concentrating hormone depresses L-, N-, and P/Q-type voltage-dependent calcium channels in rat lateral hypothalamic neurons. J Physiol. 2002;542:273–86.
Del Cid-Pellitero E, Jones BE. Immunohistochemical evidence for synaptic release of GABA from melanin-concentrating hormone containing varicosities in the locus coeruleus. Neuroscience. 2012;223:269–76.
Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–43.
Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L, et al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19.
Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–16.
Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci USA. 2009;106:2418–22.
Tsunematsu T, Ueno T, Tabuchi S, Inutsuka A, Tanaka KF, Hasuwa H, et al. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci. 2014;34:6896–909.
Konadhode RR, Pelluru D, Blanco-Centurion C, Zayachkivsky A, Liu M, Uhde T, et al. Optogenetic stimulation of MCH neurons increases sleep. J Neurosci. 2013;33:10257–63.
Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997;78:795–801.
Gervasoni D, Darracq L, Fort P, Souliere F, Chouvet G, Luppi PH. Electrophysiological evidence that noradrenergic neurons of the rat locus coeruleus are tonically inhibited by GABA during sleep. Eur J Neurosci. 1998;10:964–70.
Breton-Provencher V, Sur M. Active control of arousal by a locus coeruleus GABAergic circuit. Nat Neurosci. 2019;22:218–28.
Takata Y, Oishi Y, Zhou XZ, Hasegawa E, Takahashi K, Cherasse Y, et al. Sleep and wakefulness are controlled by ventral medial midbrain/pons GABAergic neurons in mice. J Neurosci. 2018;38:10080–92.
Yu X, Li W, Ma Y, Tossell K, Harris JJ, Harding EC, et al. GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat Neurosci. 2019;22:106–19.
Liu K, Kim J, Kim DW, Zhang YS, Bao H, Denaxa M, et al. Lhx6-positive GABA-releasing neurons of the zona incerta promote sleep. Nature. 2017;548:582–87.
Anaclet C, Ferrari L, Arrigoni E, Bass CE, Saper CB, Lu J, et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci. 2014;17:1217–24.
Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–95.
Borbely AA, Tobler I, Hanagasioglu M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav brain Res. 1984;14:171–82.
Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–7.
Marty S, Wehrle R, Fritschy JM, Sotelo C. Quantitative effects produced by modifications of neuronal activity on the size of GABAA receptor clusters in hippocampal slice cultures. Eur J Neurosci. 2004;20:427–40.
Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–7.
Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–37.
Toossi H, Del Cid-Pellitero E, Jones BE. GABA receptors on orexin and melanin-concentrating hormone neurons are differentially homeostatically regulated following sleep deprivation. eNeuro. 2016;3:pii: ENEURO.0077-16.2016.
Matsuki T, Takasu M, Hirose Y, Murakoshi N, Sinton CM, Motoike T, et al. GABAA receptor-mediated input change on orexin neurons following sleep deprivation in mice. Neuroscience. 2015;284:217–24.
Modirrousta M, Mainville L, Jones BE. Dynamic changes in GABAA receptors on basal forebrain cholinergic neurons following sleep deprivation and recovery. BMC Neurosci. 2007;8:15.
Toossi H, Del Cid-Pellitero E, Jones BE. Homeostatic regulation through GABA and acetylcholine muscarinic receptors of motor trigeminal neurons following sleep deprivation. Brain Struct Funct. 2017;222:3163–78.
Del Cid-Pellitero E, Plavski A, Mainville L, Jones BE. Homeostatic changes in GABA and glutamate receptors on excitatory cortical neurons during sleep deprivation and recovery. Front Syst Neurosci. 2017;11:17.
Grivel J, Cvetkovic V, Bayer L, Machard D, Tobler I, Muhlethaler M, et al. The wake-promoting hypocretin/orexin neurons change their response to noradrenaline after sleep deprivation. J Neurosci. 2005;25:4127–30.
Uschakov A, Grivel J, Cvetkovic-Lopes V, Bayer L, Bernheim L, Jones BE, et al. Sleep-deprivation regulates alpha-2 adrenergic responses of rat hypocretin/orexin neurons. PLoS ONE. 2011;6:e16672.
Yamanaka A, Muraki Y, Ichiki K, Tsujino N, Kilduff TS, Goto K, et al. Orexin neurons are directly and indirectly regulated by catecholamines in a complex manner. J Neurophysiol. 2006;96:284–98.
Sastre JP, Buda C, Kitahama K, Jouvet M. Importance of the ventrolateral region of the periaqueductal gray and adjacent tegmentum in the control of paradoxical sleep as studied by muscimol microinjections in the cat. Neuroscience. 1996;74:415–26.
Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–68.
Werth E, Achermann P, Borbely AA. Selective REM sleep deprivation during daytime. II. Muscle atonia in non-REM sleep. Am J Physiol Regul Integr Comp Physiol. 2002;283:R527–32.
Tung A, Bergmann BM, Herrera S, Cao D, Mendelson WB. Recovery from sleep deprivation occurs during propofol anesthesia. Anesthesiology. 2004;100:1419–26.
Boscolo-Berto R, Viel G, Montagnese S, Raduazzo DI, Ferrara SD, Dauvilliers Y. Narcolepsy and effectiveness of gamma-hydroxybutyrate (GHB): a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2012;16:431–43.
Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55.
Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep Med Rev. 2011;15:123–35.
Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–68.
Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–9.
Greene RW, Bjorness TE, Suzuki A. The adenosine-mediated, neuronal-glial, homeostatic sleep response. Curr Opin Neurobiol. 2017;44:236–42.
Radulovacki M, Miletich RS, Green RD. N6 (L-phenylisopropyl) adenosine (L-PHA) increases slow-wave sleep (S2) and decreases wakefulness in rats. Brain Res. 1982;246:178–80.
Gallopin T, Luppi PH, Cauli B, Urade Y, Rossier J, Hayaishi O, et al. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus. Neuroscience. 2005;134:1377–90.
Oishi Y, Xu Q, Wang L, Zhang BJ, Takahashi K, Takata Y, et al. Slow-wave sleep is controlled by a subset of nucleus accumbens core neurons in mice. Nat Commun. 2017;8:734.
Korkutata M, Saitoh T, Cherasse Y, Ioka S, Duo F, Qin R, et al. Enhancing endogenous adenosine A2A receptor signaling induces slow-wave sleep without affecting body temperature and cardiovascular function. Neuropharmacology. 2019;144:122–32.
Urry E, Landolt HP. Adenosine, caffeine, and performance: from cognitive neuroscience of sleep to sleep pharmacogenetics. Curr Top Behav Neurosci. 2015;25:331–66.
Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharm Sci. 2005;26:578–86.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jones, B.E. Arousal and sleep circuits. Neuropsychopharmacol. 45, 6–20 (2020). https://doi.org/10.1038/s41386-019-0444-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-019-0444-2
This article is cited by
-
Neuroligin-2 shapes individual slow waves during slow-wave sleep and the response to sleep deprivation in mice
Molecular Autism (2024)
-
Proteostasis failure exacerbates neuronal circuit dysfunction and sleep impairments in Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
GABA induced by sleep deprivation promotes the proliferation and migration of colon tumors through miR-223-3p endogenous pathway and exosome pathway
Journal of Experimental & Clinical Cancer Research (2023)
-
Probing pathways by which rhynchophylline modifies sleep using spatial transcriptomics
Biology Direct (2023)
-
Basal forebrain cholinergic signalling: development, connectivity and roles in cognition
Nature Reviews Neuroscience (2023)