Abstract
Cognitive control represents an essential neuropsychological characteristic that allows for the rapid adaption of a changing environment by constant re-allocation of cognitive resources. This finely tuned mechanism is impaired in psychiatric disorders such as schizophrenia and contributes to cognitive deficits. Neuroimaging has highlighted the contribution of the anterior cingulate cortex (ACC) and prefrontal regions (PFC) on cognitive control and demonstrated the impact of genetic variation, as well as genetic liability for schizophrenia. In this study, we aimed to examine the influence of the functional single-nucleotide polymorphism (SNP) rs6265 of a plasticity-related neurotrophic factor gene, BDNF (Val66Met), on cognitive control. Strong evidence implicates BDNF Val66Met in neural plasticity in humans. Furthermore, several studies suggest that although the variant is not convincingly associated with schizophrenia risk, it seems to be a modifier of the clinical presentation and course of the disease. In order to clarify the underlying mechanisms using functional magnetic resonance imaging (fMRI), we studied the effects of this SNP on ACC and PFC activation, and the connectivity between these regions in a discovery sample of 85 healthy individuals and sought to replicate this effect in an independent sample of 253 individuals. Additionally, we tested the identified imaging phenotype in relation to schizophrenia familial risk in a sample of 58 unaffected first-degree relatives of schizophrenia patients. We found a significant increase in interregional connectivity between ACC and PFC in the risk-associated BDNF 66Met allele carriers. Furthermore, we replicated this effect in an independent sample and demonstrated its independence of structural confounds, as well as task specificity. A similar coupling increase was detectable in individuals with increased familial risk for schizophrenia. Our results show that a key neural circuit for cognitive control is influenced by a plasticity-related genetic variant, which may render this circuit particular susceptible to genetic and environmental risk factors for schizophrenia.
Similar content being viewed by others
Introduction
Successful goal-directed behavior critically depends on the ability to adapt to an ever-changing environment, requiring a flexible yet resilient control mechanism [1]. Although recent research reports the involvement of several neural networks in cognitive control [2], a meta-analysis of functional neuroimaging studies highlights the importance of two interacting brain regions, namely the anterior cingulate cortex (ACC), and the dorsolateral and medial shares of the prefrontal cortex (PFC) [3]. Theoretical and empirical accounts suggest a contribution of both regions to different components of cognitive control. Although the ACC is preferentially involved in conflict detection and allocation of cognitive resources for conflict resolution [4, 5], the PFC is associated with top–down regulatory control and response selection [6, 7].
Primate studies point to a structurally linked network in which ACC acts as a modulator of signal-to-noise ratios in the PFC via its projections to inhibitory interneurons in the lateral and medial parts of Brodmann area 9 (BA9) [8]. Aberrations in cognitive control form a central component of neuropsychological deficits in mental disorders, e.g., in schizophrenia [9]. Task-based functional magnetic resonance imaging (fMRI) work relates cognitive control dysfunction in schizophrenia patients and their healthy siblings to reduced activity in PFC and ACC, as well as to aberrant functional connectivity between both regions [10]. Moreover, altered functional interaction of PFC and ACC during cognitive control has been observed in carriers of a genome-wide significant psychosis risk variant [11]. These data highlight the value of this neuroimaging phenotype for psychiatric research.
A promising locus for genetic research in cognition is the brain-derived neurotrophic factor (BDNF) gene that stands out for its impact on synaptic plasticity, neural development [12], and brain functional connectivity [13]. In BDNF, a functional single-nucleotide polymorphism (SNP) at codon 66 leads to a Valine (Val) to Methionine (Met) substitution (BDNF Val66Met, G to A) causing the 66Met-carriers (A;G and A;A) of this variant (relative to the more frequent “wild type” G;G-carriers) to show disrupted intracellular trafficking of the BDNF-mRNA [14], and diminished activity-dependent secretion of the neurotrophin [15].
The majority of imaging genetics research on BDNF Val66Met has focused on memory function, while few studies have examined the effects of the variant on cognitive control. Using electroencephalography, Soltesz reported a BDNF-dependent modulation of error monitoring and behavioral adaptation during an assay of cognitive control, the flanker task [16]. Here, 66Met allele carriers showed relative decrease in neural activity and synchrony, pointing to an effect of the variant on the neuronal networks subserving error and conflict monitoring.
A wealth of studies has implicated BDNF Val66Met as a genetic risk factor and/or modifier of heritable mental disorders such as bipolar disorder or schizophrenia [17,18,19,20]. Specifically, although the role of the Met allele as a risk-conferring factor for schizophrenia has recently been questioned by a genome-wide association (GWA) data [21, 22], mounting evidence suggests that BDNF Val66Met plays a role as a modifier of the genetic risk architecture [19, 23, 24] and clinical presentation of schizophrenia [25,26,27], including age of onset [24], severity of cognitive deficits [28,29,30,31], and treatment response [32, 33].
In this study, we aimed to investigate the effects of the BDNF Val66Met polymorphism on cognitive control using fMRI and a neuroimaging implementation of the flanker task [1]. Our work pursued specific aims: first, as ACC has been proposed as a critical modulator of signal-to-noise ratios in the PFC [8], we tested for potential effects of BDNF genotype on the activation and functional connectivity of both regions in a discovery sample of 85 healthy individuals; second, we sought to replicate the same task and comparable neuroimaging procedures in an independent sample of 253 healthy individuals; third, to address whether the identified neuroimaging phenotype may relate to schizophrenia familial risk, we examined 58 healthy first-degree relatives of schizophrenia patients for the presence of matching neural alterations. We conducted two follow-up analyses to probe the specificity of the identified connectivity phenotype for active task performance and its potential relationship to structural confounds.
Materials and methods
Participants
For details on inclusion criteria, see the supplementary “Methods” section.
Discovery sample
We studied 85 right-handed healthy volunteers from the general population of the city of Mannheim, Germany (see Table 1 for demographic details).
Replication sample
For replication purposes of potential genotype effects detected in the discovery sample, we analyzed an independent sample of 253 right-handed healthy volunteers scanned as part of a multicenter imaging genetics study at three sites in Germany: Bonn, Berlin, and Mannheim (see Table 1 for demographic details).
Relatives sample
To probe the potential relationship of the identified neuroimaging phenotype to schizophrenia risk, we further compared a sample of 58 unaffected first-degree relatives of schizophrenia patients with a matched control sample (180 healthy subjects derived from our replication sample). First-degree relatives were scanned as part of the above-mentioned multicenter imaging genetics study at three sites in Germany: Bonn, Berlin, and Mannheim (see Table 1 for demographic details).
Genotyping
Genomic DNA was extracted from whole blood according to standard procedures. For details on SNP genotyping and genotype distributions in the study samples, see the supplementary “Methods” section.
Flanker task
Brain function was studied using a well-established cognitive control task that challenges response inhibition and error monitoring and provokes activations in a network of brain regions including dorsal ACC and lateral and medial PFC (mPFC) [1, 10, 11]. In this event-related task, sets of five symbols are displayed consisting of a central target arrow (pointing to the left or the right side), which is flanked by pairs of non-target symbols on each side. In the “congruent” condition, the central arrow is flanked by pairs of arrows pointing in the same direction as the central arrow. In the “incongruent” condition, the flanking arrows are oriented in the opposite direction of the central arrow. In the “neutral” condition, the central arrow is flanked by pairs of boxes. In all three conditions, the subjects are asked to indicate the direction of the central arrow as quickly and accurately as possible by pressing the left or right button of an MRI-compatible response device using their index and middle finger, respectively. In a fourth condition (“no go”), the central arrow is flanked by pairs of the letter “X”, which instruct the individuals to withhold their responses [1]. Each stimulus was presented for 800 milliseconds (ms) with a variable interstimulus interval of 2200–5200 ms. During this interstimulus interval, a fixation crosshair was shown. Task duration was 10.1 min or 302 whole-brain scans. Task performance was assessed in terms of response time (RT, in ms) and accuracy (in percent correct) for each condition.
Resting-state task
Prior to the 5 min (or 150 whole-brain scans) resting-state task, we instructed participants to close their eyes, relax, and refrain from any specific mental activity. After the end of each scan, investigators verified with the participants that they had not fallen asleep during data acquisition.
MRI data acquisition and fMRI data preprocessing
For details on MRI data acquisition and fMRI data processing, see the supplementary “Methods” section.
Activation analysis
We performed a statistical analysis on individual subject level using a general linear model (GLM) as implemented in SPM8. The four task conditions were modeled as separate regressors of interest, and the six realignment parameters from the preprocessing step were added as regressors of no interest to the model. During model estimation, a high-pass filter of 128 s (SPMs built-in DCT filter) and an autoregressive model of the first order were applied. In order to investigate brain activity related to response inhibition and interference monitoring, first-level models were defined, and differential contrasts were calculated for the contrasts, “no-go vs. neutral” and “incongruent vs. congruent”, respectively. First-level contrast images were subjected to second-level statistical inference by means of: (1) one sample t-tests for within-group analysis of the contrast images to explore the effects of task, and (2) univariate analysis of variance (ANOVA) models for between-group analyses (Val/Val homozygotes vs. Met allele carriers, relatives vs. controls) with group as a factor, whereas age and sex were covariates of no interest. We additionally covaried for site for all the analyses involving multi-site data (replication sample, relatives study).
Functional connectivity analysis
Functional connectivity analyses were performed as previously described and involved a so-called “seeded functional connectivity” approach, a measure of correlated activity between two regions across the full task, for which we have demonstrated superior reliability in earlier work [34]. Consistent with the well-established role of ACC in interference processing [1], the seed region was defined based on the group level activation maximum for the “incongruent vs. congruent” contrast in the dorsal ACC in the discovery analysis (Montreal Neurological Institute (MNI) coordinates: x = 9, y = 32, z = 30). For functional connectivity measures, the entire time series was used. Following our previously published procedures [34], individual seed time series were extracted from 6 mm spheres and first-level models were defined with the subject-specific time series as regressor of interest and the following regressors of no interest: (1) the six movement parameters from the realignment step, (2) the first eigenvariates derived from Cerebrospinal fluid (CSF) and white matter masks, and (3) the regressors encoding the experimental conditions in order to remove mean task-related variance. During model estimation, high-pass filtering and autoregressive modeling was performed as detailed for the activation analysis. Among the resulting beta images, the beta image pertaining to the seed regressor reflects the strength of association of voxel time series with the ACC time course, a well-established measure of functional connectivity [35, 36]. Between-group ANOVA analyses followed the procedures described for the activation analyses.
Statistical inference
Our approach for statistical inference followed a two-step procedure. In our first (discovery) analysis, significance was defined at p < 0.05 family-wise error (FWE) corrected for multiple comparisons across the whole brain. Based on the results of the discovery analysis, we continued to define our regional hypothesis for the subsequent analyses (replication study, relatives study, resting-state connectivity analysis), and specifically tested for ACC functional connectivity effects mapping to the mPFC in a region of interest (ROI) approach. For this, we selected the overlap between the bilateral -Talairach Daemon (TD) based medial frontal area [37], and the BA9 ROI to define a bilateral anatomical mask of medial BA9 containing 673 voxels for subsequent correction of results(p < 0.05 FWE corrected for the ROI).
Resting-state connectivity analysis
We also examined ACC–mPFC connectivity during resting state in the individuals of the replication sample to investigate whether the identified connectivity difference between BDNF genotype groups is specific to cognitive control, or alternatively, represents a task-independent alteration in functional coupling. Resting-state data were available from the vast majority of participants of this sample (Val/Val-carriers n = 164, Met-carriers n = 86). Data processing and analysis steps were kept constant for task-based and resting-state functional connectivity procedures with the exception of data filter choice and target region definition: data were processed using a 0.008–0.1 Hz bandpass filter, and ROIs were derived from the functional connectivity seed in ACC and the functional connectivity peak voxel within mPFC in the flanker task. Thus, we extracted a time series from a 6 mm sphere centered around the respective voxel (MNI ACC: x = 9, y = 32, z = 30; MNI mPFC: x = 18, y = 41, z = 18). The extracted time series were corrected for CSF, White matter (WM), and the six-rigid body alignment parameters as described above. Groups were compared using a univariate ANOVA model with age, sex, and site as covariates of no interest.
Structural analysis
We further analyzed the structural data of the replication sample with voxel-based morphometry (VBM) to test whether the differences during cognitive control in the identified ACC–mPFC functional connectivity phenotype may be influenced by pre-existing structural differences of the examined regions. High-resolution structural MR images were available for the vast majority of participants in this sample (Val-carriers: n = 163, Met-carriers: n = 87). Data processing followed our previously published procedures [38] including the use of the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm8/) with default specifications. Briefly, the structural images were tissue segmented, spatially normalized to the MNI space with a diffeomorphic image registration algorithm (DARTEL), corrected for image intensity non-uniformity and global brain gray matter volume, and smoothed with a 10 mm Full Width at Half Maximum (FWHM) Gaussian kernel. We then extracted mean gray matter volumes for the ACC and PFC as defined above for resting-state analysis and tested for genotype-associated group differences by entering these values as dependent variable in a repeated measurement ANOVA model that included age, sex, and site as covariates of no interest.
Results
Demographics and task performance
Details on all demographic and task performance variables including p-values for group comparisons are provided in Table 1. Notably, the groups compared in this work did not significantly differ in any of these variables, except for a higher number of females in the 66Met allele carrier group of the discovery sample. Notably, however, sex was included as a covariate of no interest in all of our analyses.
Discovery sample
Flanker activation analysis
The analysis of brain activation in the contrasts “incongruent > congruent” and “no go > neutral” revealed robust activations of brain areas known to be involved in conflict monitoring and inhibitory control, respectively [1]. See Supplemental Table 1 and 2 for details. We detected no significant effects of BDNF genotype on brain activation for either contrast in the discovery sample. Consistent with our step-wise analysis strategy, the activation data of the replication and relatives samples were thus not further analyzed.
Flanker connectivity analysis
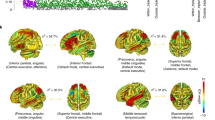
The results of our connectivity analyses are illustrated in Fig. 1.
Effects of BDNF genotype and schizophrenia familial risk on ACC–mPFC functional connectivity. Effects of BDNF Val66Met genotype on ACC–PFC functional connectivity in discovery (a) (t = 5.02; pFWE = 0.022, whole-brain corrected) and replication sample (b) (t = 3.72; pFWE = 0.022, region of interest corrected). c ACC–PFC functional connectivity in a sample of healthy first-degree relatives compared with healthy controls without a history of mental illness (t = 3.39; pFWE = 0.05, region of interest corrected). Depictions at p = 0.005 uncorrected
Seeded functional connectivity analysis demonstrated a significant effect of genotype on the functional coupling of the ACC and PFC. Specifically, 66Met-carriers showed a significant increase in the seeded connectivity between ACC and medial BA9 surviving correction for multiple comparisons across the whole brain (x = 12, y = 53, z = 27; t = 5.02; pFWE = 0.022, four voxels surpassing the peak voxel statistical correction level of significance for the whole brain).
Further analyses
Replication study
In a second step, we aimed to reproduce the detected genotype effect in our replication sample. Analogous to the discovery sample, we detected a significant increase in the seeded connectivity between ACC and the medial parts of PFC BA9 in 66Met allele carriers (MNI: x = 18, y = 41, z = 18; t = 3.72; pFWE = 0.022, two voxels surpassing the peak voxel statistical correction level of significance for the ROI). In contrast, the analysis of the resting-state data of the same individuals revealed no significant effects of genotype on ACC–PFC functional connectivity (p = 0.762), which suggests that the coupling abnormality related to 66Met is task dependent. Moreover, there were no significant genotype-dependent differences in the mean gray matter volume of the seed and target regions (See Supplementary Figure 1) of the functional connectivity analysis, arguing against a relevant role of structural confounds.
Relatives study
We continued to probe the potential relationship of the identified phenotype to schizophrenia risk in unaffected first-degree relatives of schizophrenia patients. Consistent with our genotype findings, we detected a significant increase in the seeded connectivity between ACC and the medial parts of PFC BA9 in the healthy first-degree relatives of schizophrenia patients compared with healthy controls without a family history of mental illness (MNI: x = 15, y = 53, z = 27; t = 3.39; pFWE = 0.05, one voxel surpassing the peak voxel statistical correction level of significance for the ROI).
Discussion
Cognitive control mechanisms interface key neural processes ensuring adequate and efficient behavioral responses to goal-relevant information. Prior research has demonstrated that brain functional abnormalities during cognitive control are frequent in heritable mental disorders [9], and that a synergistic brain circuitry involving ACC and PFC is necessary for accomplishing these functions [2]. However, the neurogenetic architecture of this network is still under researched. To address this point, we examined the potential impact of a functional variant in BDNF associated with effects on neurodevelopment, synaptic plasticity, and mental disorders. Focusing on the functional coupling of two putative key hubs of the cognitive control network, we studied the impact of BDNF Val66Met genetic variation and schizophrenia familial risk on brain activation and interregional connectivity with fMRI and a well-established cognitive control task.
Our study provided several interesting outcomes. In a first step, we examined the effects of BDNF variation on PFC and ACC activation during conflict monitoring and response inhibition in a discovery sample, which yielded a negative finding. We simultaneously detected a whole-brain significant effect of the variant on ACC–mPFC interregional connectivity during cognitive control, which manifested as a relative increase in the functional coupling of regions in the risk-associated Met allele carriers. In a second step, we were able to reproduce the significant increase in ACC–mPFC connectivity in the 66Met allele carriers in a larger independent sample. The outcome of the replication analysis increased our confidence that the effect of the variant on the identified coupling phenotype is indeed credible. We further demonstrated the specificity of the genotype effect for active task performance and its independence from brain structural confounds within the same individuals. Given that the fMRI data collected from cognitive performance tasks also carry task-unrelated (i.e., more generic and/or hemostasis-related) information on brain functional states [39], and since the examined variant in BDNF has been repeatedly associated with brain structural effects [40, 41], we found these two additional control analyses to be particularly important.
Interestingly, we did not find any effects of BDNF Val66Met on ACC or PFC activation in response to cognitive control. Similar dissociations of genotype effects on activity vs. connectivity phenotypes have been previously described [42] and discussed as a variant underlying neurobiological mechanism [43]. Notably, although alterations in dorsal ACC activation have been repeatedly related to changes in dopamine transmission [44,45,46], the interpretation of the observed ACC–mPFC coupling effect is still less clear. However, given the established effects of BDNF on the experience-dependent organization of neural circuits, a neural plasticity-related mechanism appears plausible. This notion is supported by a study demonstrating effects of repetitive transcranial magnetic stimulation on PFC connectivity, but not activity, which is in line with a particularly close link of fMRI coupling signatures to brain plasticity mechanisms [34]. On the cellular level, Val66Met influences dendritic growth and synaptic communication via acute and chronic effects on neurotransmitter signaling [47, 48]. Thus, a lack of BDNF in the 66Met allele carriers can result in reduced synaptic efficacy, which in turn may facilitate a (likely compensatory) increase in ACC interregional coupling at the neural system level, as observed in this study. We thus propose that the observed ACC coupling increase in 66Met allele carriers may indicate a plasticity-related compensatory signal related to the diminished neurotrophin secretion and synaptic efficacy in this group [15]. We further propose that this mechanism requires active challenge of the cognitive network and is not a mere epiphenomenon of its basic architecture, such as its structural composition or functional configuration in the absence of external stimulation.
In psychiatric disorders, alterations in the connectivity of brain regions as a consequence of genetic variation are typically interpreted as conferring risk through impaired functional organization of brain circuits responsible for integrated human behaviors [49, 50]. As cognitive control deficits [9, 51], and related alterations in ACC interregional coupling [10] are well-established observations in psychosis, we tested whether an increase in the functional coupling of ACC and mPFC during cognitive control is evident in subjects with familial risk for schizophrenia. Indeed, compared with controls, unaffected first-grade relatives showed a “compensatory network signature” that paralleled the one observed in the 66Met allele carriers. Although both sample stratification principles, i.e., BDNF genotype and kinship, base their rationale on the logic of increased neurogenetic risk, the distribution of BDNF alleles in our relatives sample did not deviate from that of a European ancestry population (see Supplementary Methods). The joined interpretation of these findings is not simple and requires further discussion.
Healthy first-grade relatives of patients with a heritable psychiatric condition carry, per definition, an enriched set of genetic risk variants that increase their liability to develop the disorder. Together with the absence of important confounds such as medication effects, this makes this population particularly suitable for the exploration of genetic risk-associated “intermediate” phenotypes at the neural system level. BDNF 66Met, in turn, is an established genetic risk factor for neural plasticity-related deficits, including those believed to be effective in neurodevelopmental disorders such as schizophrenia. Nevertheless, as BDNF itself does not increase the genetic risk for schizophrenia directly, as per the current state of knowledge [22], a simple explanatory model of our findings (e.g., BDNF genotype increases psychosis risk through deficits in ACC–mPFC coupling) is unlikely true. Interestingly, a recent study found that plasma BDNF levels as a direct measure of BNDF secretion predicted planning abilities across different stages of psychosis with low BDNF levels only in the at-risk state of schizophrenia [52]. Concurrently, neuroimaging risk markers cannot be attributed to the effects of a single genetic variant only, but rather reflect the accumulation and complex interplay of many risk factors from different sources, including gene–environment interactions. As BDNF Val66Met influences experience-dependent plasticity directly, and also modifies schizophrenia neurogenetic risk and symptoms indirectly [53], our data are likely better explained by a model assuming a convergence of the effects of different risk constellations at the neural system level. Our results in healthy participants indicate the investigated neural circuits to be susceptible to plasticity-related effects, which subsequently may render those circuits more vulnerable to the impact of adverse events [54]. For example, in addition to the direct influence of BDNF on neural plasticity and ACC–mPFC network function, indirect effects may exist that facilitate comparable neural deficits through interaction with psychosis risk-enriched gene sets and/or adverse environmental factors. Considering that healthy relatives of patients are enriched for schizophrenia genetic risk, and also share many of the environmental influences of their affected family members [55], we find this concept plausible and worthwhile to investigate in detail in future studies.
In summary, although this similar connectivity pattern in BDNF 66Met allele carriers and unaffected relatives of patients with schizophrenia on connectivity is intriguing, we interpret these results as indicators for plasticity-related effects on the cognitive control circuit. However, a causal relationship between BDNF and schizophrenia or the genetics of schizophrenia itself cannot be inferred from these observations.
Our study has several limitations worth considering. First, although we replicated our genetic association finding in an independent sample, both the absent effects of BDNF Val66Met on ACC activation and the detected association of the 66Met allele with higher ACC–mPFC coupling merit further study in larger cohorts. Second, although our groups were balanced for a broad range of demographic and task performance variables, and we additionally showed the independence of the coupling effect from more generic functional and structural facets of the underlying network, we cannot exclude the possibility that our results were influenced by factors that we did not cover. Third, our study rationale was based on a hypothesis-driven, multi-stage (discovery-replication), and therefore spatially constrained approach. Although we believe that this strategy is a sound and advisable way to optimize sensitivity while minimizing false negatives in imaging genetics, we cannot exclude that relevant connectivity effects outside the deducted target circuitry remained unnoticed. Fourth, despite the broad use [56, 57] and established robustness [34] of correlative connectivity measures in neuroimaging research, the identified coupling differences cannot be interpreted as a proof for a deficit in the causal interaction between ACC and mPFC. Fifth, although our data suggest a link between the phenotype and schizophrenia familial risk, the proposed role of BDNF as an indirect modifier of the neurocognitive risk architecture of psychiatric disorders via its effects on ACC interregional coupling calls for further inquiry [19, 23, 24]. Sixth, we did not collect a direct measure of plasma BDNF levels, which would have provided more detailed information on the relationship between allelic variation in BDNF, BDNF protein levels, and the identified neurocognitive imaging phenotype.
In conclusion, we provide evidence for an ACC–mPFC coupling increase during cognitive control in healthy BDNF 66Met allele carriers that is replicable specific to the active challenge of the cognitive network, independent of structural differences, and detectable in individuals with an increased schizophrenia familial risk. Our findings suggest a mechanism through which common genetic variation can modify the course of schizophrenia. Moreover, our findings encourage further study of the direct and indirect effects of this polymorphism on cognitive control networks in larger cohorts and heritable disorders with established neuropsychological deficits such as schizophrenia and bipolar disorder.
Funding and disclosure
This study was supported by the German Federal Ministry of Education and Research through the Integrated Genome Research Network Molecular Causes of Major Mood Disorders and Schizophrenia (Systematic Investigation of the Molecular Causes of Major Mood Disorders and Schizophrenia; grant 01GS08144 to Drs Nothen, Cichon, and Walter, grant 01GS08147 to Drs Rietschel and Meyer-Lindenberg, and grant 01GS08148 to Dr Heinz), under the auspices of the National Genome Research Network plus (NGFNplus). Further funding was received through the German Research Foundation (Deutsche Forschungsgemeinschaft; Sonderforschungsbereich 636-B7). AM-L acknowledges support from the German Federal Ministry of Education and Research (BMBF, grants 01ZX1314G, 01GS08147, 01GQ1003B, 01GQ1102 subproject B7 and Collaborative Research Center 1158 subproject B09), European Union’s Seventh Framework Programme (FP7, grants 602805, 602450, 115300 and HEALTH-F2-2010-241909, Innovative Medicines Initiative Joint Undertaking (IMI, grant 115008), German Research Foundation (DFG, ME 1591/4-1) and Ministry of Science, Research and the Arts of the State of Baden-Wuerttemberg, Germany (MWK, grant 42-04HV.MED(16)/16/1 and 42-04HV.MED(16)/27/1). AM-L has received consultant fees from Boehringer Ingelheim, Elsevier, Walt Disney Pictures, Brainsway, Lundbeck Int. Neuroscience Foundation, Sumitomo Dainippon Pharma Co., Academic Medical Center of the University of Amsterdam, Synapsis Foundation-Alzheimer Research Switzerland, IBS Center for Synaptic Brain Dysfunction, Blueprint Partnership, University of Cambridge, Dt. Zentrum für Neurodegenerative Erkrankungen, Universität Zürich, L.E.K. Consulting, ICARE Schizophrenia, Science Advances, and has received fees for lectures, interviews and travels from Lundbeck International Foundation, Paul-Martini-Stiftung, Lilly Deutschland, Atheneum, Fama Public Relations, Institut d’investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Jansen-Cilag, Hertie Stiftung, Bodelschwingh-Klinik, Pfizer, Atheneum, Universität Freiburg, Schizophrenia Academy, Hong Kong Society of Biological Psychiatry, Fama Public Relations, Spanish Society of Psychiatry, Reunions I Ciencia S.L., Brain Center Rudolf Magnus UMC Utrecht. HT acknowledges support from the German Federal Ministry of Education and Research (BMBF, grant 01GQ1102) and the German Research Foundation (DFG, TO 539/3-1). MR acknowledges support from German Federal Ministry of Education and Research (BMBF, grants 01ZX1314G und 01ZX1614G). SC acknowledges support by the German Federal Ministry of Education and Research (BMBF) through the Integrated Network IntegraMent (Integrated Understanding of Causes and Mechanisms in Mental Disorders), under the auspices of the e:Med Programme (grant 01ZX1314A/01ZX1614A to SC). The study was also supported by the Swiss National Science Foundation (SNSF grant 310030_156791 to SC). The remaining authors declare no competing interests.
References
Blasi G, Goldberg TE, Weickert T, Das S, Kohn P, Zoltick B, et al. Brain regions underlying response inhibition and interference monitoring and suppression. Eur J Neurosci. 2006;23:1658–64.
Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105.
Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–68.
MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8.
van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001;14:1302–8.
Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–90.
Kim C, Johnson NF, Gold BT. Conflict adaptation in prefrontal cortex: now you see it, now you don’t. Cortex. 2014;50:76–85.
Medalla M, Barbas H. Synapses with inhibitory neurons differentiate anterior cingulate from dorsolateral prefrontal pathways associated with cognitive control. Neuron. 2009;61:609–20.
Glahn DC, Knowles EE, Pearlson GD. Genetics of cognitive control: implications for Nimh’s research domain criteria initiative. Am J Med Genet B Neuropsychiatr Genet. 2016;171B:111–20.
Sambataro F, Mattay VS, Thurin K, Safrin M, Rasetti R, Blasi G, et al. Altered cerebral response during cognitive control: a potential indicator of genetic liability for schizophrenia. Neuropsychopharmacology. 2013;38:846–53.
Thurin K, Rasetti R, Sambataro F, Safrin M, Chen Q, Callicott JH, et al. Effects of ZNF804A on neurophysiologic measures of cognitive control. Mol Psychiatry. 2013;18:852–4.
Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–31.
Wang C, Zhang Y, Liu B, Long H, Yu C, Jiang T. Dosage effects of BDNF Val66Met polymorphism on cortical surface area and functional connectivity. J Neurosci. 2014;34:2645–51.
Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, et al. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25:6156–66.
Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69.
Soltesz F, Suckling J, Lawrence P, Tait R, Ooi C, Bentley G, et al. Identification of BDNF sensitive electrophysiological markers of synaptic activity and their structural correlates in healthy subjects using a genetic approach utilizing the functional BDNF Val66Met polymorphism. PLoS ONE. 2014;9:e95558.
Li M, Chang H, Xiao X. BDNF Val66Met polymorphism and bipolar disorder in European populations: a risk association in case-control, family-based and GWAS studies. Neurosci Biobehav Rev. 2016;68:218–33.
Neves-Pereira M, Cheung JK, Pasdar A, Zhang F, Breen G, Yates P, et al. BDNF gene is a risk factor for schizophrenia in a Scottish population. Mol Psychiatry. 2005;10:208–12.
Notaras MJ, Hill RA, Gogos JA, van den Buuse M. BDNF Val66Met genotype interacts with a history of simulated stress exposure to regulate sensorimotor gating and startle reactivity. Schizophr Bull. 2017;43:665–72.
Penadés R, López-Vílchez I, Catalán R, Arias B, González-Rodríguez A, García-Rizo C, et al. BDNF as a marker of response to cognitive remediation in patients with schizophrenia: a randomized and controlled trial. Schizophr Res. 2018;197:458–464.
Hou L, Bergen SE, Akula N, Song J, Hultman CM, Landen M, et al. Genome-wide association study of 40,000 individuals identifies two novel loci associated with bipolar disorder. Hum Mol Genet. 2016;25:3383–94.
Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Decoster J, van Os J, Kenis G, Henquet C, Peuskens J, De Hert M, et al. Age at onset of psychotic disorder: cannabis, BDNF Val66Met, and sex-specific models of gene-environment interaction. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:363–9.
Mane A, Berge D, Penzol MJ, Parellada M, Bioque M, Lobo A, et al. Cannabis use, COMT, BDNF and age at first-episode psychosis. Psychiatry Res. 2017;250:38–43.
Lu W, Zhang C, Yi Z, Li Z, Wu Z, Fang Y. Association between BDNF Val66Met polymorphism and cognitive performance in antipsychotic-naive patients with schizophrenia. J Mol Neurosci. 2012;47:505–10.
Mezquida G, Penades R, Cabrera B, Savulich G, Lobo A, Gonzalez-Pinto A, et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with negative symptoms severity, but not cognitive function, in first-episode schizophrenia spectrum disorders. Eur Psychiatry. 2016;38:61–9.
Xia H, Zhang G, Du X, Zhang Y, Yin G, Dai J, et al. Suicide attempt, clinical correlates, and BDNF Val66Met polymorphism in chronic patients with schizophrenia. Neuropsychology 2018;32:199.
Aas M, Haukvik UK, Djurovic S, Bergmann O, Athanasiu L, Tesli MS, et al. BDNF val66met modulates the association between childhood trauma, cognitive and brain abnormalities in psychoses. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:181–8.
Kim SW, Lee JY, Kang HJ, Kim SY, Bae KY, Kim JM, et al. Gender-specific associations of the brain-derived neurotrophic factor Val66Met polymorphism with neurocognitive and clinical features in schizophrenia. Clin Psychopharmacol Neurosci. 2016;14:270–8.
Notaras M, Hill R, van den Buuse M. A role for the BDNF gene Val66Met polymorphism in schizophrenia? A comprehensive review. Neurosci Biobehav Rev. 2015;51:15–30.
Zhang XY, Chen DC, Xiu MH, Haile CN, Luo X, Xu K, et al. Cognitive and serum BDNF correlates of BDNF Val66Met gene polymorphism in patients with schizophrenia and normal controls. Hum Genet. 2012;131:1187–95.
Nikolac Perkovic M, Nedic Erjavec G, Zivkovic M, Sagud M, Uzun S, Mihaljevic-Peles A, et al. Association between the brain-derived neurotrophic factor Val66Met polymorphism and therapeutic response to olanzapine in schizophrenia patients. Psychopharmacol (Berl). 2014;231:3757–64.
Zhang JP, Lencz T, Geisler S, DeRosse P, Bromet EJ, Malhotra AK. Genetic variation in BDNF is associated with antipsychotic treatment resistance in patients with schizophrenia. Schizophr Res. 2013;146:285–8.
Bilek E, Schafer A, Ochs E, Esslinger C, Zangl M, Plichta MM, et al. Application of high-frequency repetitive transcranial magnetic stimulation to the DLPFC alters human prefrontal-hippocampal functional interaction. J Neurosci. 2013;33:7050–6.
Esslinger C, Kirsch P, Haddad L, Mier D, Sauer C, Erk S, et al. Cognitive state and connectivity effects of the genome-wide significant psychosis variant in ZNF804A. Neuroimage. 2011;54:2514–23.
Schneider M, Walter H, Moessnang C, Schafer A, Erk S, Mohnke S, et al. Altered DLPFC-hippocampus connectivity during working memory: independent replication and disorder specificity of a putative genetic risk phenotype for schizophrenia. Schizophr Bull. 2017;43:1114–1122.
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131.
Cao H, Bertolino A, Walter H, Schneider M, Schafer A, Taurisano P, et al. Altered functional subnetwork during emotional face processing: a potential intermediate phenotype for schizophrenia. JAMA Psychiatry. 2016a;73:598–605.
Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–51.
Hashimoto T, Fukui K, Takeuchi H, Yokota S, Kikuchi Y, Tomita H, et al. Effects of the BDNF Val66Met polymorphism on gray matter volume in typically developing children and adolescents. Cereb Cortex. 2016;26:1795–803.
Tost H, Alam T, Geramita M, Rebsch C, Kolachana B, Dickinson D, et al. Effects of the BDNF Val66Met polymorphism on white matter microstructure in healthy adults. Neuropsychopharmacology. 2013;38:525–32.
Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605.
Rasetti R, Sambataro F, Chen Q, Callicott JH, Mattay VS, Weinberger DR. Altered cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804A. Arch Gen Psychiatry. 2011;68:1207–17.
Jaspar M, Genon S, Muto V, Meyer C, Manard M, Dideberg V, et al. Modulating effect of COMT genotype on the brain regions underlying proactive control process during inhibition. Cortex. 2014;50:148–61.
Luijten M, Veltman DJ, Hester R, Smits M, Nijs IM, Pepplinkhuizen L, et al. The role of dopamine in inhibitory control in smokers and non-smokers: a pharmacological fMRI study. Eur Neuropsychopharmacol. 2013;23:1247–56.
Vaughn KA, Ramos Nunez AI, Greene MR, Munson BA, Grigorenko EL, Hernandez AE. Individual differences in the bilingual brain: the role of language background and DRD2 genotype in verbal and non-verbal cognitive control. J Neurolinguist. 2016;40:112–27.
Do T, Kerr B, Kuzhikandathil EV. Brain-derived neurotrophic factor regulates the expression of D1 dopamine receptors. J Neurochem. 2007;100:416–28.
Gottmann K, Mittmann T, Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res. 2009;199:203–34.
Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468:194–202.
Braun U, Schaefer A, Betzel RF, Tost H, Meyer-Lindenberg A, Bassett DS. From maps to multi-dimensional network mechanisms of mental disorders. Neuron. 2018;97:14–31.
Daban C, Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Balanza-Martinez V, Salazar-Fraile J, et al. Specificity of cognitive deficits in bipolar disorder versus schizophrenia. A systematic review. Psychother Psychosom. 2006;75:72–84.
Heitz U, Papmeyer M, Studerus E, Egloff L, Ittig S, Andreou C, et al. Plasma and serum brain-derived neurotrophic factor (BDNF) levels and their association with neurocognition in at-risk mental state, first episode psychosis and chronic schizophrenia patients. World J Biol Psychiatry: 2018;1–10.
Notaras M, Hill R, van den Buuse M. The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol Psychiatry. 2015;20:916–30.
Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–54.
van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–82.
Tost H, Bilek E, Meyer-Lindenberg A. Brain connectivity in psychiatric imaging genetics. Neuroimage. 2012;62:2250–60.
Cao H, Dixson L, Meyer-Lindenberg A, Tost H. Functional connectivity measures as schizophrenia intermediate phenotypes: advances, limitations, and future directions. Curr Opin Neurobiol. 2016b;36:7–14.
Acknowledgements
We would like to thank Tracie Ebalu, Sarah Plier, Oliver Grimm, and Leila Haller for their help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Schweiger, J.I., Bilek, E., Schäfer, A. et al. Effects of BDNF Val66Met genotype and schizophrenia familial risk on a neural functional network for cognitive control in humans. Neuropsychopharmacol 44, 590–597 (2019). https://doi.org/10.1038/s41386-018-0248-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-018-0248-9
This article is cited by
-
Schizophrenia in the genetic era: a review from development history, clinical features and genomic research approaches to insights of susceptibility genes
Metabolic Brain Disease (2023)
-
Behavioural and functional evidence revealing the role of RBFOX1 variation in multiple psychiatric disorders and traits
Molecular Psychiatry (2022)