Abstract
Suicide is major public health concern; one million individuals worldwide die by suicide each year of which there are many more attempts. Thus, it is imperative that robust and reliable indicators, or biomarkers, of suicide risk be identified so that individuals at risk can be identified and provided appropriate interventions as quickly as possible. Previous work has revealed a relationship between low levels of circulating cholesterol and suicide risk, implicating cholesterol level as one such potential biomarker, but the factors underlying this relationship remain unknown. In the present study, we applied a combination of bivariate polygenic and coefficient-of-relatedness analysis, followed by mediation analysis, in a large sample of Mexican-American individuals from extended pedigrees [N = 1897; 96 pedigrees (average size = 19.17 individuals, range = 2–189) 60% female; mean age = 42.58 years, range = 18–97 years, sd = 15.75 years] with no exclusion criteria for any given psychiatric disorder. We observed that total esterified cholesterol measured at the time of psychiatric assessment shared a significant genetic overlap with risk for suicide attempt (ρg = −0.64, p = 1.24 × 10−04). We also found that total unesterified cholesterol measured around 20 years prior to assessment varied as a function of genetic proximity to an affected individual (h2 = 0.21, se = 0.10, p = 8.73 × 10−04; βsuicide = −0.70, se = 0.25, p = 8.90 × 10−03). Finally, we found that the relationship between total unesterified cholesterol and suicide risk was significantly mediated by ABCA-1-specific cholesterol efflux capacity (βsuicide-efflux = −0.45, p = 0.039; βefflux-cholexterol = −0.34, p < 0.0001; βindirect = −0.15, p = 0.044). These findings suggest that the relatively well-delineated process of cholesterol metabolism and associated molecular pathways will be informative for understanding the neurobiological underpinnings of risk for suicide attempt.
Similar content being viewed by others
Introduction
Interest in cholesterol as a biomarker for suicide has been growing since the early 1990s when a meta-analysis of randomized primary prevention trials of statins (cholesterol-lowering drugs) indicated that while drug administration resulted in the expected reduction of risk of death by coronary events, it also increased risk of death by suicide [1]. Unsurprisingly, given the major impact of suicide on public health, this initial observation brought about many studies focused on the potential of cholesterol as a biological risk factor, or biomarker, for suicide [2]. Suicide is a major public health concern; one million individuals worldwide die by suicide each year [3], and there are many more attempts. Suicide accounts for 1–2% of global deaths and ranks 17th in terms of leading causes of mortality [4]. The identification of one or more unbiased and robust biomarkers for suicide will enable enhanced risk prediction so that suicidal patients can be identified prior to attempt [5]. While the potential of peripherally measured cholesterol as a biomarker for suicide risk is great, few studies have attempted to address the mechanism underpinning the overlap between cholesterol and suicide, a mechanism that might be exploited to inform risk prediction and, ultimately, prevention. A first step in investigating the mechanistic underpinnings of this relationship is to evaluate (a) the extent to which the two phenotypes (i.e., plasma cholesterol and attempted suicide) are influenced by the same genes (and by extension a shared neurobiology) and (b) the potential role of key processes in cholesterol production pathways.
A biomarker is an objective indicator of the presence of a disease arising from pathogenic biologic processes; this is distinct from a symptom, which is an indicator of illness perceived by the patient [6]. Currently available methods of assessment for suicidality are entirely symptom-based (i.e., asking the person in question whether they feel suicidal). This is an inherently flawed approach because the person may not wish to disclose their suicidal ideation for fear of prevention, or they might impulsively change their mind at a later date [7]. Standard clinical and demographic measures perform only slightly better than chance for suicide risk prediction [8]. Developing objective and reliable indicators of suicide risk, or biomarkers, would help to circumvent this problem. Therefore, in the present study we aimed to evaluate the extent of the genetic overlap between suicide attempt and cholesterol.
There is a sizeable literature focused on the relationship between suicide and low cholesterol. Several syntheses of the literature have been performed via meta-analysis. Heterogeneity between studies focused on cholesterol in individuals exhibiting suicidal behavior has made synthesis of this work challenging; different studies tend to use different methods of assessment resulting in disparate phenotypes (e.g., suicidal tendencies, ideation, attempt, and completion). Nonetheless the most recent meta-analysis demonstrated a robust effect across 65 studies (comprising upward of 500,000 participants), showing that suicidal patients have lower total cholesterol levels, measured in the periphery, than non-suicidal controls and that lower total cholesterol was associated with a 112% higher risk of suicidality [9].
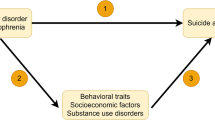
The level of cholesterol in peripheral tissues is determined, at least in part, by reverse cholesterol transport, an active transport pathway that is essential to returning excess cholesterol in peripheral tissues to the liver for excretion. Reverse cholesterol transport is crucial to the maintenance of cholesterol homeostasis in peripheral tissues because these tissues cannot catabolize cholesterol [10]. Briefly (also see Fig. 1), free, or unesterified, cholesterol is effluxed from the peripheral cells and is esterified by the enzyme lecithin:cholesterol acyl-transferase (LCAT) in the form of mature HDL (HDL2) particles. HDL may then be delivered to the liver for excretion via HDL-specific scavenger receptors or exchanged for low-density lipoproteins (LDL or VLDL) [11]. Alterations in one or more steps in this pathway might mediate any existing relationship between cholesterol and suicide risk. For example, cholesterol efflux capacity, reflected by the levels of cholesterol (unesterified or esterified or the ratio of the two) in circulation is inversely associated with risk for cardiovascular events [12]. It is conceivable that the opposite might be true for suicide risk where overactive efflux might be one of multiple factors that adversely affect cholesterol levels resulting in increased risk for suicidal behavior.
Unesterified, or free, cholesterol (COH) from non-hepatic peripheral tissue is transferred, or effluxed, to esterified cholesterol, HDL, via the ABCA1 (ATP-binding cassette transporter) with apoA-I (the principal protein of HDL) as the acceptor. The presence of apoA-I activates LCAT, which esterifies free cholesterol on the surface of apoA-I to generate mature HDL particles. HDL can then transport the cholesterol to the liver for excretion via SR-B1 receptors or it can transfer cholesterol to VLDL or LDL, a process facilitated by CETP (cholesterolester transfer protein). LDL may be taken up by the liver via the LDL receptor (LDLR) while the remainder is taken up by extrahepatic tissues; slowing of LDLR activity results in an increase in plasma LDL levels [10]. Alterations in this process would have implications for the total amount of cholesterol available in blood and may be mechanistically relevant to the link between reduced cholesterol and suicidality, one which might be leveraged for risk prediction and diagnosis and ultimately treatment
We report data from a large sample of extended pedigrees for whom we have a suicide risk assessment as well as cholesterol data from two points in time. The existence of two time points for the cholesterol data enables us to make inferences about the role of confounding environmental factors, for example, diet. While we carefully control for BMI measured at both time points in all our analyses, this does not explicitly rule out the possible influence of diet (given that BMI is an imperfect proxy for adiposity [13]). However, the measurement of cholesterol years prior to psychiatric assessment is conceivably less susceptible to the effects of such environmental factors. Thus, we apply a combination of bivariate polygenic and coefficient-of-relatedness analysis followed by simple mediation analysis. The aims of the study were two-fold: (a) to establish the extent to which cholesterol levels (both unesterified and esterified) measured in plasma shared genetic overlap with risk for suicide attempt; and (b) the extent to which cholesterol efflux capacity of LCAT may have mediated the relationship between cholesterol and suicide attempt. The present study is, to the best of our knowledge, the first to evaluate the extent to which cholesterol is an endophenotype of suicide attempt while at the same time evaluating potentially mediating factors that contribute to the process of reverse cholesterol transport.
Methods
Participants
Data for the present study comprise two separate phases of data collection from the same individuals taken from a large extended-pedigree study comprising a total of 1897 individuals from 96 pedigrees [mean size = 19.17 individuals (sd = 19.06), range = 2–189; 60% female; mean age = 42.58 years, range = 18–97 years, sd = 15.75 years]. The two phases of data collection were a result of two studies conducted in this cohort on overlapping individuals, now collectively termed the San Antonio Family Studies (SAFS). The first is the Genetics of Brain Structure and Function Study (GOBS). GOBS participants were unselected for any given psychiatric disorder and were not excluded based on any current or lifetime psychiatric illness. Psychiatric assessment data were available for the entire sample (N = 1897) and esterified cholesterol (EC) panel data were available for a subset of those individuals (N = 1034; 57% female; mean age = 36.54 years, sd = 14.77 years, range = 18–81 years). The second sample, which overlaps with GOBS (total overlapping N = 604), is the San Antonio Family Heart Study (SAFHS; for recruitment details, see [14]) for whom plasma unesterified, or free, cholesterol (UC), LCAT, and cholesterol efflux were available (65% female; mean age = 34.95 years, sd = 13.10 years, range = 16–81 years).
GOBS data collection occurred between 2006 and 2016. GOBS subjects had previously participated in SAFHS or were blood relatives of individuals who had participated in this family study. SAFHS is an ongoing longitudinal observational study comprising four phases of data collection during a 23-year period. Data used in the present study were collected during the first phase of SAFHS, between the years 1992–1996. Whether recruited as part of GOBS or SAFHS, all participants were randomly selected from the community with the constraints that they were of Mexican-American ancestry, part of a large family, and lived in the San Antonio region. All participants provided written informed consent in compliance with the Institutional Review Board at the University of Texas Health Science Center at San Antonio.
GOBS: psychiatric assessment
During the GOBS assessment participants completed the semi-structured Mini-International Neuropsychiatric Interview (MINI; [15]). Interviews were conducted by masters-level and doctorate-level research staff who had established reliability for conducting suicide risk assessment (κ ≥ 0.85). Subjects who reported possible pathology were discussed in case conference meetings with licensed psychologists and/or psychiatrists. Consensus diagnoses were determined using available medical records, the MINI, and the interviewer’s narrative. Suicidality was assessed in Section C of the MINI. This assessment included item C6, the primary index of suicide for the present study, which asks, “Did you ever make a suicide attempt?”. Our index is therefore a dichotomous trait where a score of 1 indicates that the participant answered yes and 0 indicates that they answered no. The MINI also includes a section on current suicidality (items C1–C5) asking whether in the past month the participant (C1) thought they would be better off dead; (C2) wished that they were dead; (C3) wanted to harm themselves; (C4) thought about suicide; or (C5) had a suicide plan. These items were collapsed to give a categorical index of current suicidality where a score of 1 indicates that any item between C1–C5 was endorsed and a score of 0 indicated that none of these items were endorsed. The presence of any major depressive or anxiety disorder, as well as alcohol and substance use disorders, were also evaluated as part of the MINI assessment.
GOBS EC measurements
GOBS participants completed a fasting venipuncture for the measurement of lipids, completed on the same day as the MINI assessment. Blood was drawn after a 12-h fast. A lipid panel was performed on the plasma samples at an accredited clinical diagnostic laboratory (LabCorp Inc., Burlington, North Carolina). The cholesterol panel included multiple indices of EC, including total EC, LDL and VLDL cholesterol, and high-density cholesterol (HDL), plus triglycerides. Additionally, BMI and current prescriptions for medications linked to metabolic status (lipid, diabetes, and blood pressure medications) were collected.
SAFHS UC, cholesterol efflux, and LCAT measurements
The lipid extraction procedure used in this sample has been described in detail elsewhere [16] and is outlined in the Supplemental Materials. Measures of BMI and prescription status for medications linked to metabolic status were collected at this time point. Measures of plasma-based cholesterol efflux capacity (non-ABCA1 and ABCA-1-specific) and LCAT levels were measured in serum at the same timepoint, details can be found in the Supplemental Materials.
Data analysis
Quantitative genetic analysis
All genetic analyses were performed in SOLAR [17]. The maximum-likelihood variance decomposition approach implemented in SOLAR has been described elsewhere [18] and is outlined in the Supplemental Materials. We first estimated the heritability of all traits. All continuous traits (EC measures, UC, efflux measurements, and LCAT) were normalized using an inverse Gaussian transformation. Continuous traits were residualized for age, age2, sex, and their interactions plus BMI from the relevant time point and medication status (for blood cholesterol, diabetes, and blood pressure). For the dichotomous traits (suicide attempt and current suicidal ideation) age, age2, sex, and their interactions were included as covariates in the univariate polygenic model.
Bivariate polygenic analysis was applied to the residualized EC measures and suicide attempt in order to examine the extent to which the trait pairs were influenced by shared genetic effects. Age, age2, sex, and their interactions were included as covariates for suicide attempt. Parameter estimates from the bivariate analyses were used to calculate endophenotype ranking values (ERVs) for each suicide/cholesterol pairing. We ran the bivariate polygenic models with and without potentially confounding traits as covariates including: psychiatric medication status (including antidepressants, anxiolytics, mood stabilizers, and antipsychotics), any major depressive disorder, any anxiety disorder, and lifetime substance and alcohol dependence disorders.
ERV calculation
The ERV is simply an effect size bounded between 0 and 1, and reflects the degree of genetic overlap between two traits and can be calculated using the parameter estimates from bivariate polygenic analyses (described above). It is useful for prioritizing phenotypes in terms of their shared genetic overlap with a disease of interest. The ERV statistic has been described in detail elsewhere [18], also see Supplemental Materials. In the present study we ranked the EC phenotypes by their genetic overlap with lifetime suicide attempt.
Mean-based ERV (mERV) calculation
The mERV is an extension of the ERV to be used when the disease of interest is not sufficiently common among the sampled individuals. In the larger GOBS sample there was a sufficient number of suicide attempt cases for h2 to be estimated, but in the subset of individuals who also participated in SAFHS (who also had UC, LCAT, and efflux data) there were not; thus, we applied a mERV analysis. For details on the derivation of the mERV, see Glahn et al. [19, 20] and the Supplemental Materials. In the present study, the mERV was applied to a single trait pairing, suicide attempt, and UC.
Mediation analysis: mediating role of efflux and LCAT on the relationship between UC and suicide risk
We performed mediation analysis in MPlus [21] to determine whether the association between genetic liability for suicide attempt (indexed by the coefficient of relationship scalar, see above) and UC was mediated by cholesterol efflux or LCAT. This analysis was applied to the residuals from the univariate genetic analysis (where the effects of age, sex, and where appropriate, BMI and medication status were covaried). Family structure was taken into account using the cluster command. For each potential mediator, we constructed three models. The first posited a direct relationship between the independent variable genetic liability for suicide attempt and the dependent variable UC. In the second model, the relationship between genetic liability for suicide attempt and UC and was partially mediated by efflux or LCAT. In the third model, the relationship was fully mediated (Table 1).
Results
Heritability of risk for lifetime suicide attempt and current suicidal ideation
One hundred and fifty seven individuals had attempted suicide at the time of assessment and 135 individuals endorsed some level of current suicidal ideation (at least one item between C1 and C5 was endorsed). Of the 157 individuals who had attempted suicide, 45 were currently showing some level of current suicidal ideation and less than half (N = 54) met criteria for any major depressive disorder (including current, past, lifetime, or recurrent; Table 1). Individuals who had attempted suicide were distributed across 51 of the 96 pedigrees. Both suicide attempt (h2 = 0.53, se = 0.01, p = 1.90 × 10−06) and current suicidal ideation were significantly heritable (h2 = 0.47, se = 0.14, p = 4.56 × 10−05).
ERV ranking of EC phenotypes by genetic overlap with lifetime suicide attempt
The ERV results are presented in Table 2. The top-ranked cholesterol phenotype for genetic overlap with lifetime suicide attempt was total EC, which was the sum of LDL, VLDL, and HDL cholesterol (ERV = 0.29). The corresponding genetic correlation was statistically significant (ρg = −0.63, p = 1.67 × 10−04). The second-ranked cholesterol phenotype was LDL cholesterol (ERV = 0.23) and the corresponding genetic correlation was statistically significant (ρg = −0.44, p = 7.22 × 10−03) after applying Bonferroni correction for the five tests (α = 0.01). Neither total cholesterol (ρg = −0.32, p = 0.08) nor LDL cholesterol (ρg = −0.32, p = 0.08) exhibited significant genetic correlation with current suicidality. Only the HDL cholesterol phenotype exhibited near zero standardized genetic covariance (ERV = 0.04) with suicide attempt.
Given that there is substantial overlap between those individuals who have attempted suicide and those who have been diagnosed with a major depressive disorder, it is possible that depression confounds the interpretation of our findings. Despite the fact that the inclusion of any major depressive disorder as a covariate could conceivably remove variance in which we are interested (since it can be difficult to disentangle suicide from depressive symptomatology), we re-ran the models including diagnosis of any major depressive disorder as a covariate. Given the link between suicidality and addiction, we also included lifetime alcohol (N = 403, of which 65 had attempted suicide), and substance dependence disorders (N = 187, of which 41 had attempted suicide) as covariates in this secondary analysis. When these covariates were included in the bivariate polygenic models, the genetic correlation between suicide attempt and total EC (ρg = −0.72, se = 0.29, p = 8.98 × 10−03) remained significant, while the genetic correlation between LDL cholesterol and suicide (ρg = −0.40, se = 0.19, p = 0.03) attained a nominal level of significance, but did not withstand multiple testing correction. Failure to appropriately account for medication usage can impact genetic analysis [22]. In the present study, we residualized the cholesterol phenotypes for medications linked to metabolic status including statins; however, the results are extremely similar if these medications are not included in the analysis (Table S1). It is possible that psychiatric medications might also have metabolic effects. Therefore, we re-ran the bivariate polygenic model of suicide attempt risk and EC while including dummy coded antidepressant (186 individuals prescribed antidepressants, 31 had attempted suicide), anxiolytic (68 individuals, 16 had attempted suicide), mood stabilizer (14 individuals, 4 had attempted suicide), or antipsychotic medications (18 individuals, 8 had attempted suicide); the genetic correlation was similar and remained significant (ρg = −0.69, se = 0.23, p = 2.21 × 10−03).
Relationship between UC and lifetime suicide attempt risk
In the subsample of individuals with UC measurements, 51 had attempted suicide; this subsample was underpowered to detect heritability of risk for lifetime suicide attempt relative to the complete sample (h2 = 0.29, se = 0.14, p = 0.07). However, there were 354 individuals who were related to a case (1st degree = 120, 2nd degree = 88, 3rd degree = 72, 4th degree = 58, 5th degree = 13, 6th degree = 3) plus 199 individuals who were unrelated (total N = 553). Therefore, we opted to conduct a mERV [19, 20] or coefficient-of-relatedness approach for this section of the analysis. UC, collected ~20 years prior to psychiatric assessment, was significantly heritable (h2 = 0.21, se = 0.10, p = 8.56 × 10−04); and its level varied as a function of genetic proximity to an individual who had attempted suicide at the time of psychiatric assessment (βsuicide = −0.70, se = 0.25, p = 8.90 × 10−03; Fig. 2b). This result for UC and risk for suicide attempt is in line with the result for EC and risk for suicide attempt, where EC was collected at the time of psychiatric assessment. The parallel in these findings suggests that the relationship between EC and risk for suicide attempt is less likely to have arisen as a consequence of an environmental confound, such as diet.
The same analysis run with a UC phenotype where medications relating to metabolic status including statins were not included as covariates yielded very similar results (βsuicide = −0.63, se = 0.23, p = 7.09 × 10−03). The inclusion of any major depressive, any anxiety, and lifetime alcohol or substance dependence disorders as covariates did not alter this result (βsuicide = −0.64, se = 0.23, p = 6.69 × 10−03; βMDD = −0.04, se = 0.09, p = 0.63; βanxiety = 0.08, se = 0.12, p = 0.49; βalcohol = −0.08, se = 0.11, p = 0.48; βsubstance = −0.11, se = 0.18, p = 0.55).
Do cholesterol efflux or LCAT mediate the relationship between UC and suicide attempt?
Both ABCA-1-specific efflux capacity (βABCA−1 = 0.30, se = 0.05, p = 4.05 × 10−10) and LCAT (βLCAT = −0.10, se = 0.05, p = 0.04) were significant covariates of UC; non-ABCA1 efflux capacity (βnon-ABCA1 = −0.01, p = 0.84) was not a significant covariate. Consequently, we ran mediation analysis using ABCA-1-specific efflux capacity and LCAT. Figure 3 summarizes the results of the mediation analysis. A direct model (Fig. 3a) indicated a strong effect of genetic risk for suicide attempt (indexed by the mERV scalar) on UC. For efflux, a model with partial mediation (Fig. 3b) indicated a significant indirect effect of suicide risk on UC via efflux as well as a significant direct effect of suicide risk on UC. A model with full mediation supported an indirect effect of suicide risk on UC via efflux (Fig. 3c). These results suggest strong partial, if not full, mediation of the relationship between suicide risk and UC by ABCA-1-specific efflux capacity. For LCAT, a model with both partial mediation (Fig. 3d) and full mediation (Fig. 3e) indicated minimal relationship between suicide risk and LCAT level and a non-significant indirect effect of suicide risk on UC via LCAT. These results suggest that LCAT does not mediate the relationship between suicide attempt risk and UC.
Discussion
The results of the present study suggest that there is shared genetic influence between total EC and UC and risk for suicide attempt. Total EC, measured at the time of suicide assessment, was genetically correlated with suicide attempts share a substantial and significant genetic correlation. Total UC, measured around 20 years prior to suicide assessment, varied as a function of genetic proximity to an individual who had attempted suicide in their lifetime. The latter relationship was significantly mediated by cholesterol efflux capacity. These findings are important because they suggest that the relatively well-delineated process of cholesterol metabolism and the associated molecular pathways might be informative for understanding the neurobiological underpinnings of risk for suicide attempt.
The cholesterol data in the present study comprised two different types of cholesterol (EC and UC), derived using two different methods, and collected at two different points in time. Both exhibited genetic overlap with risk for suicide attempt. The reverse cholesterol transport pathway oversees the return of excess cholesterol in peripheral tissues to the liver for excretion (Fig. 1). This process is of great interest in atherosclerosis (weakening and narrowing of arteries caused by fatty buildup in the arterial wall) research, as it is thought to offer protection from the progression of atherosclerotic vascular disease [23]. In the present study, total UC varied as a function of genetic proximity to an individual who had attempted suicide in their lifetime and cholesterol efflux capacity (but not LCAT) mediated the relationship between total UC and suicide attempt risk. Affected individuals—that is, those individuals who endorsed having attempted suicide in their lifetime—were not included in this analysis. Figure 3c shows total efflux capacity across groups of genetic proximity including affected individuals. Intriguingly, unaffected individuals more closely related to an individual who had attempted suicide exhibited lower cholesterol efflux capacity, while suicide attempters exhibited higher efflux capacity than even unrelated unaffected individuals. A tentative conclusion from these findings is that increased efflux capacity is a risk factor for suicide attempt while lowered efflux may be protective to those genetically predisposed to suicidal behavior. At the very least, this generates a testable hypothesis for future work focused on key players in reverse cholesterol transport in the etiology of suicide attempt.
Several feasible mechanisms for neurobiological links between suicide and cholesterol have been proposed in the literature, including the cholesterol-serotonin hypothesis. This hypothesis states that low levels of cholesterol adversely affect serotoninergic activity; more specifically, cholesterol reduction is thought to give rise to changes in the structure and function of cell membranes in the central nervous system which directly influence the affinity and structure of serotonin receptors and transporters [24]. This decrease in serotoninergic activity is ostensibly linked to a reduction in the ability to suppress impulsivity [25]. Impulsivity, defined as the initiation of risky behavior without adequate appraisal of possible adverse consequences [26], makes up an essential feature of at least some aggressive [27] and suicidal behaviors [28]. In particular, it has been argued that low cholesterol appears to be particularly strongly associated with violent rather than non-violent suicide [29, 30]. This further underlines the potential interplay of cholesterol and impulsivity, perhaps within the context of serotoninergic activity. Future work might focus on assessing cholesterol in concert with indicators of serotoninergic activity, the relationship those biologic measures have with indices of impulsivity, brain regions thought to underlie impulsive behavior (e.g., response inhibition and the inferior frontal gyrus [31]), the ways in which brain activity relates to suicidal behavior.
The present study focused on the presence or absence of a lifetime non-fatal suicide attempt. However, suicidal behavior is on a spectrum where ideation can range from fleeting thoughts of not caring if one lives or dies to highly persistent and obtrusive thoughts about killing oneself with a well thought out and lethal method with the spectrum ending at suicide completion [32]. Low cholesterol has been consistently associated with increased risk for suicide [33,34,35,36,37,38,39,40,41]. Numerous genome-wide association studies of suicidal behavior have been carried out, and the results have been largely inconsistent [42]. However, one such study identified a region on 2p25 that influences risk for attempted suicide and contains the ACP1 gene [43], an association that has since been replicated [44]. Interestingly, polymorphisms in ACP1 have been found to modulate both protection and predisposition to dyslpipidemia (elevation of circulating cholesterol levels) that is associated with obesity [45]. The role of this gene in relation to cholesterol metabolism and transport, particularly within the context of the spectrum of suicidal behavior, could be the focus of future work.
Because the data in the present manuscript are observational, this study is best described as correlational, and as such it does not allow us to make causal inferences about the impact of lowered cholesterol on risk for suicide. Future work of a longitudinal design would allow this relationship to be better examined and for directional inferences to be made. Work is ongoing to improve the ways in which suicidality is assessed using sophisticated and elegant adaptive procedures that capture real-time fluctuations suicidal ideation [46]. Future work should focus on repeated assessments of suicidality in tandem with repeated assessment of cholesterol in an effort to ascertain the extent to which one varies as a function of the other. Taking together this information, including both deep phenotypes and blood-based biomarkers, it might be possible to build testable machine learning-based risk algorithms [8]. To this end, researchers in the field are using powerful longitudinal datasets in order to identify blood biomarkers, combined with clinical information gathered electronically, suicidality across psychiatric diagnoses [47, 48]. The results of the present manuscript suggest that blood-based measures related to the process of reverse cholesterol transport might be incorporated into such efforts.
In the present study, we carefully considered the effect of numerous potential confounds on the relationship between risk for suicide attempt and cholesterol. More specifically, we controlled the effects of sex and age, of BMI measured at the two time points of cholesterol measurement, as well as medications linked to metabolic status, and medications linked to psychiatric disorders, as well as the presence of a diagnosis of major depression, anxiety, and substance or alcohol dependence. However, it was unfortunately not possible to explicitly assess the role of dietary factors on the relationship between cholesterol and risk for suicide attempt. While there has been little research on the link between diet and/or exercise and suicide risk [49], it is conceivable that they may be correlated. Though it should be noted that most methods of dietary assessment are, for the most part, not validated and subject to issues of measurement error and thus reliability [50, 51], this includes food frequency questionnaires, food diaries, and 24 h dietary recall, where the latter (and likely the former) are biased by subject underreporting [52]. While we cannot rule out the influence of diet on the present results, three observations suggest that this influence was small. First, we controlled for BMI—in lieu of information pertaining to diet. However, BMI is an imperfect proxy for adiposity; it cannot differentiate between muscle and fat [13]. Second, the measurement of UC 20 years prior to psychiatric assessment, a measure that is less susceptible to confounding by diet, also showed significant correlation with risk for suicide attempt. Third, the present study used an extended pedigree design. It is a widely held view among genetics researchers that one of the benefits of extended pedigrees relative to smaller families is that there is reduced confounding of genetic and shared environmental effects because family members are usually distributed across multiple households [53]. Diet is an example of a shared environmental factor that might be considered strongly tied to household. In the present study, some of the pedigrees were very large: the largest family comprises 189 individuals. Thus, according to the conventional wisdom of genetics research, genetic correlations between cholesterol and suicide risk should have been only modestly influenced by shared environmental effects, including diet.
The extended pedigrees in the present study are of Mexican-American ancestry and it is possible that the genetic overlap that we have observed between risk for suicide attempt and UC and EC are population specific. Previous research suggests that Mexican-Americans born in the United States are at higher risk for suicide-related outcomes than Mexican-Americans born in Mexico, and also higher in Mexican-Americans born in the United States compared to non-Hispanic whites [54,55,56,57]. Moreover, it has been shown that Mexican-American individuals have a high prevalence of high cholesterol, which is coupled with substantially lower rates of screening, awareness, and treatment compared to non-Hispanic whites [58]. It should be noted that in the present study that lowered, not increased, cholesterol levels are associated with risk for suicide attempt. Nonetheless, subsequent work should focus on replicating these results in other ethnicities.
A potential limitation of the present study, and indeed the majority of work in this area, is that it has focused on cholesterol levels measured in the periphery [16]. This is somewhat unavoidable given the inaccessibility of the human brain for direct measurement of cholesterol. Nonetheless, the relevance of these findings to the brain is of interest to the field of psychiatry whose focus is brain-based disorders. Future work might attempt to index metabolites of brain cholesterol metabolism that are measurable in blood [59].
In summary, the present study is, to the best of our knowledge, the first to demonstrate shared genetic influences on cholesterol and risk for suicide attempt, and the potentially mediating influence of cholesterol efflux on the interplay between the two. It highlights circulating cholesterol as a potential biomarker of suicide attempt risk and underscores the utility of cholesterol and cholesterol metabolism pathways as potentially rewarding avenues of research for suicide risk. Going further, the present study generates specific tentative but testable hypotheses regarding the role of cholesterol efflux and associated molecular pathways in suicide risk prevention.
References
Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. Br Med J. 1990;301:309–14.
Cantarelli Mda G, Tramontina AC, Leite MC, Goncalves CA. Potential neurochemical links between cholesterol and suicidal behavior. Psychiatry Res. 2014;220:745–51.
Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, Lee S. Suicide and suicidal behavior. Epidemiol Rev. 2008;30:133–54.
World Health Organization. SuicideData. 2017. http://www.who.int/mental_health/prevention/suicide/suicideprevent/en/. Accessed 18 Jan 2018.
Pandey GN, Dwivedi Y. Peripheral biomarkers for suicide. In: Dwivedi Y, editor. The neurobiological basis of suicide. Boca Raton, FL: CRC Press/Taylor Francis; 2012.
Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5:463–6.
Niculescu AB, Levey DF, Phalen PL, Le-Niculescu H, Dainton HD, Jain N, et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry. 2015;20:1266–85.
Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Huang X, et al. Risk factors for suicidal thoughts and behaviors: a meta-analysis of 50 years of research. Psychol Bull. 2017;143:187–232.
Wu S, Ding Y, Wu F, Xie G, Hou J, Mao P. Serum lipid levels and suicidality: a meta-analysis of 65 epidemiological studies. J Psychiatry Neurosci. 2016;41:56–69.
Feingold KR, Grunfeld C. Introduction to lipids and lipoproteins. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM et al., editors. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000.
Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–32.
Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–93.
Cornier MA, Despres JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, et al. Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011;124:1996–2019.
Mitchell BD, Kammerer CM, Blangero J, Mahaney MC, Rainwater DL, Dyke B, et al. Genetic and environmental contributions to cardiovascular risk factors in Mexican Americans. The San Antonio Family Heart Study. Circulation. 1996;94:2159–70.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33.
Knowles EEM, Huynh K, Meikle PJ, Göring HHH, Olvera RL, Mathias SR, et al. The lipidome in major depressive disorder: shared genetic influence for ether-phosphatidylcholines, a plasma-based phenotype related to inflammation, and disease risk. Eur Psychiatry. 2017;43:44–50.
Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–211.
Glahn DC, Curran JE, Winkler AM, Carless MA, Kent JW Jr, et al. High dimensional endophenotype ranking in the search for major depression risk genes. Biol Psychiatry. 2012;71:6–14.
Glahn DC, Williams JT, McKay DR, Knowles EE, Sprooten E, Mathias SR, et al. Discovering schizophrenia endophenotypes in randomly ascertained pedigrees. Biol Psychiatry. 2015;77:75–83.
Knowles EEM, Meikle PJ, Huynh K, Göring HHH, Olvera RL, Mathias SR, et al. Serum phosphatidylinositol as a biomarker for bipolar disorder liability. Bipolar Disord. 2017;19:107–15.
Muthén LK, Muthén BO. Mplus user’s guide. 6th ed. Los Angeles, CA: Muthén & Muthén; 2011.
Rice TK, Sung YJ, Shi G, Gu CC, Rao D. Genome-wide association analysis of Framingham Heart Study data for the Genetics Analysis Workshop 16: effects due to medication use. BMC Proc. 2009;3:S52.
Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50:S189–94.
Engelberg H. Low serum cholesterol and suicide. Lancet. 1992;339:727–9.
Paaver M, Nordquist N, Parik J, Harro M, Oreland L, Harro J. Platelet MAO activity and the 5-HTT gene promoter polymorphism are associated with impulsivity and cognitive style in visual information processing. Psychopharmacology. 2007;194:545–54.
Barratt E. Impulsiveness and aggression. Chicago: University of Chicago; 1994. pp. 61–79.
Barratt ES, Stanford MS, Dowdy L, Liebman MJ, Kent TA. Impulsive and premeditated aggression: a factor analysis of self-reported acts. Psychiatry Res. 1999;86:163–73.
Corruble E, Damy C, Guelfi JD. Impulsivity: a relevant dimension in depression regarding suicide attempts? J Affect Disord. 1999;53:211–5.
Lester D. Serum cholesterol levels and suicide: a meta-analysis. Suicide Life Threat Behav. 2002;32:333–46.
Alvarez JC, Cremniter D, Lesieur P, Gregoire A, Gilton A, Macquin-Mavier I, et al. Low blood cholesterol and low platelet serotonin levels in violent suicide attempters. Biol Psychiatry. 1999;45:1066–9.
Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatry. 2007;20:255–61.
Sudol K, Mann JJ. Biomarkers of suicide attempt behavior: towards a biological model of risk. Curr Psychiatry Rep. 2017;19:31.
Sarchiapone M, Camardese G, Roy A, Della Casa S, Satta MA, Gonzalez B, et al. Cholesterol and serotonin indices in depressed and suicidal patients. J Affect Disord. 2001;62:217–9.
Atmaca M, Kuloglu M, Tezcan E, Ustundag B, Gecici O, Firidin B. Serum leptin and cholesterol values in suicide attempters. Neuropsychobiology. 2002;45:124–7.
Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Serum leptin and cholesterol values in violent and non-violent suicide attempters. Psychiatry Res. 2008;158:87–91.
Sullivan PF, Joyce PR, Bulik CM, Mulder RT, Oakley-Browne M. Total cholesterol and suicidality in depression. Biol Psychiatry. 1994;36:472–7.
Golier JA, Marzuk PM, Leon AC, Weiner C, Tardiff K. Low serum cholesterol level and attempted suicide. Am J Psychiatry. 1995;152:419–23.
Kunugi H, Takei N, Aoki H, Nanko S. Low serum cholesterol in suicide attempters. Biol Psychiatry. 1997;41:196–200.
Seneviratne SL, Warnasooriya WM, Gunatilake SB, Fonseka MM, Gunawardena MK, de Silva HJ. Serum cholesterol concentrations in parasuicide. Ceylon Med J. 1999;44:11–3.
Partonen T, Haukka J, Virtamo J, Taylor PR, Lonnqvist J. Association of low serum total cholesterol with major depression and suicide. Br J Psychiatry. 1999;175:259–62.
Kunugi H, Takei N, Aoki H, Nanko S. Low serum cholesterol in suicide attempters. Biol Psychiatry. 1997;41:196–200.
Turecki G. The molecular bases of the suicidal brain. Nat Rev Neurosci. 2014;15:802–16.
Willour VL, Seifuddin F, Mahon PB, Jancic D, Pirooznia M, Steele J, et al. A genome-wide association study of attempted suicide. Mol Psychiatry. 2012;17:433–44.
Pawlak J, Dmitrzak-Weglarz M, Wilkosc M, Szczepankiewicz A, Leszczynska-Rodziewicz A, Zaremba D, et al. Suicide behavior as a quantitative trait and its genetic background. J Affect Disord. 2016;206:241–50.
Stanford SM, Bottini M, Bottini N. The role of LMPTP in the metabolic syndrome. In: Bence KK, editor. Protein tyrosine phosphatase control of metabolism. New York, NY, Heidelberg, Dordrecht, London: Springer; 2013. pp. 203–20.
Gibbons RD, Kupfer D, Frank E, Moore T, Beiser DG, Boudreaux ED. Development of a computerized adaptive test suicide scale—the CAT-SS. J Clin Psychiatry. 2017;78:1376–82.
Niculescu AB, Le-Niculescu H, Levey DF, Phalen PL, Dainton HL, Roseberry K, et al. Precision medicine for suicidality: from universality to subtypes and personalization. Mol Psychiatry. 2017;22:1250–73.
Niculescu AB, Levey DF, Phalen PL, Le-Niculescu H, Dainton HD, Jain N, et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry. 2015;20:1266–85.
Perera S, Eisen RB, Bhatt M, Dennis BB, Bawor M, El-Sheikh W, et al. Exploring metabolic factors and health behaviors in relation to suicide attempts: a case-control study. J Affect Disord. 2018;229:386–95.
Tucker KL. Assessment of usual dietary intake in population studies of gene-diet interaction. Nutr Metab Cardiovasc Dis. 2007;17:74–81.
Prentice RL. Dietary assessment and the reliability of nutritional epidemiology research reports. J Natl Cancer Inst. 2010;102:583–5.
Briefel RR, Sempos CT, McDowell MA, Chien S, Alaimo K. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr. 1997;65:1203S–9S.
Docherty AR, Kremen WS, Panizzon MS, Prom-Wormley EC, Franz CE, Lyons MJ, et al. Comparison of twin and extended pedigree designs for obtaining heritability estimates. Behav Genet. 2015;45:461–6.
Sorenson SB, Golding JM. Prevalence of suicide attempts in a Mexican-American population: prevention implications of immigration and cultural issues. Suicide Life Threat Behav. 1988;18:322–33.
Olvera RL. Suicidal ideation in Hispanic and mixed-ancestry adolescents. Suicide Life Threat Behav. 2001;31:416–27.
Fortuna LR, Perez DJ, Canino G, Sribney W, Alegria M. Prevalence and correlates of lifetime suicidal ideation and suicide attempts among Latino subgroups in the United States. J Clin Psychiatry. 2007;68:572–81.
Borges G, Breslau J, Su M, Miller M, Medina-Mora ME, Aguilar-Gaxiola S. Immigration and suicidal behavior among Mexicans and Mexican Americans. Am J Public Health. 2009;99:728–33.
Rodriguez CJ, Cai J, Swett K, Gonzalez HM, Talavera GA, Wruck LM et al. High cholesterol awareness, treatment, and control among Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. J Am Heart Assoc. 2015;4. https://doi.org/10.1161/JAHA.115.001867.
Hughes TM, Rosano C, Evans RW, Kuller LH. Brain cholesterol metabolism, oxysterols, and dementia. J Alzheimers Dis. 2013;33:891–911.
Funding
Grant sponsor: National Institute of Mental Health; Grant numbers: MH078143, MH078111, MH083824; Grant sponsor: SOLAR NIMH; Grant number: MH059490.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Knowles, E.E.M., Curran, J.E., Meikle, P.J. et al. Disentangling the genetic overlap between cholesterol and suicide risk. Neuropsychopharmacol 43, 2556–2563 (2018). https://doi.org/10.1038/s41386-018-0162-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-018-0162-1
This article is cited by
-
The Relationship Between CYP46A1 Polymorphism and Suicide Risk: A Preliminary Investigation
NeuroMolecular Medicine (2024)