Abstract
Psychosocial stress contributes to the development of psychiatric disorders. Repeated social defeat (RSD) is a murine stressor that causes a release of inflammatory monocytes into circulation. Moreover, RSD-induced anxiety-like behavior is dependent on the recruitment of these monocytes to the brain. Activation of the endocannabinoid (ECB) system may modulate both neuroendocrine and inflammatory responses mediated by stress. Therefore, we hypothesized that a cannabinoid receptor agonist would attenuate RSD-induced inflammation, anxiety, and stress sensitization. To test this hypothesis, mice received an injection of the synthetic cannabinoid1/2 receptor agonist, WIN55,212-2 (WIN; 1 mg/kg, intraperitoneally) daily for six consecutive days, 30 min before each exposure to RSD. Anxiety-like behavior, immune activation, neuroinflammation, and microglial reactivity were determined 14 h after RSD. RSD-induced anxiety-like behavior in the open field and in the EPM was reversed by WIN55,212-2. Moreover, WIN55,212-2 reduced the accumulation of inflammatory monocytes in circulation and brain after RSD and attenuated RSD-induced interleukin-1β (IL-1β) messenger RNA (mRNA) expression in microglia/macrophages. Increased ex vivo reactivity of microglia/monocytes to lipopolysaccharides (LPS) after RSD was also attenuated by WIN55,212-2. Next, fear expression, extinction, and recall were evaluated 24 and 48 h, respectively, after contextual fear conditioning, which took place 7 days after RSD. Here, RSD caused prolonged fear expression and impaired fear extinction recall, which was associated with increased IL-1β mRNA in the brain. Moreover, these stress-induced effects were reversed by WIN55,212-2. In conclusion, activation of cannabinoid receptors limited the immune and neuroinflammatory responses to RSD and reversed the short-term and long-term behavioral deficits associated with RSD.
Similar content being viewed by others
Introduction
The neurobiology of neuropsychiatric illness is a complex process and the contributing problems are multifactorial [1]. While inflammation is not involved in all cases of anxiety and depression, recent clinical studies show that leukocytes (CD16−/CD14+ cells) can be detected in these conditions and contribute to the increased inflammatory status of the individual [2, 3]. For example, the increased presence of a pro-inflammatory monocyte signature in circulation was associated with chronic stress/anxiety symptoms [3]. Also, post-traumatic stress disorder (PTSD) and major depressive disorder symptom severity were positively correlated with increased pro-inflammatory cytokine levels and nuclear factor-κB (NF-κB) signaling in monocytes [4,5,6]. Moreover, many of the pro-inflammatory effects of chronic stress are attributed to enhanced monocytopoiesis in the bone marrow, resulting in the selective accumulation of the “inflammatory” Ly6Chi monocytes in circulation [7, 8], and their trafficking to the brain [9]. For instance, postmortem studies of depressed suicide victims revealed microglial activation and macrophage accumulation within the anterior cingulate cortex [10]. Thus, these clinical data provide key evidence linking psychosocial stress, inflammatory monocytes, and neuropsychiatric disorders.
The immune and behavioral responses to stress observed in humans can be modeled in mice using the repeated social defeat (RSD) paradigm [9]. The monocyte profile detected in RSD [11] parallel clinical findings of inflammatory/glucocorticoid-resistant monocytes [4,5,6]. RSD prolongs anxiety-like behavior in mice that depends on microglia activation, and the recruitment of inflammatory/glucocorticoid-resistant monocytes to the brain [12]. Indeed, a recent study confirmed that the development of anxiety after RSD was caused by microglial recruitment of interleukin-1β (IL-1β)-producing monocytes to the neurovasculature of stress response-related brain centers [13].
RSD-exposed mice have exaggerated immunological and behavioral responses following subsequent exposure to a secondary challenge [12, 14]. For instance, exposure to an acute stressor 24 days after RSD re-established monocyte trafficking and anxiety-like behavior [12]. This stress sensitization is also observed in individuals with PTSD. These patients show prolonged, hyperactive response to mild stressors or memories associated with the trauma even in the absence of threat [15]. Impaired processing of conditioned fear memories, resulting in exaggerated fear responses resistant to extinction, has been proposed as a primary cause of persistent fearful memories in PTSD [16]. Not surprisingly, increased plasma levels of several inflammatory markers, such as IL-1β, IL-6, and monocyte chemoattractant protein-1, and a high incidence of inflammatory conditions are observed in PTSD [17].
Activation of the endocannabinoid (ECB) system is a potential intervention for the treatment of anxiety and stress-related disorders [18]. The ECB system is involved in the extinction of aversive memories and alleviation of fear response [19]. Notably, altered ECB system was observed in the brain areas related to fear conditioning in PTSD [20]. Cannabinoid receptors, mainly CB1, are expressed in neurons and are implicated in the stress response and behavioral adaptation to stress [21]. Cannabinoid receptors CB1 and CB2 are also expressed by innate immune cells, including microglia and macrophages, and have anti-inflammatory effects [22]. Overall, cannabinoid receptors may regulate the hypothalamic pituitary adrenal (HPA) axis, lower inflammation, and facilitate fear extinction [19]. Notably, medical marijuana is currently approved to treat PTSD symptoms in several US States [23]. However, this treatment approach has its detractors and several side effects [24]. Therefore, a better understanding of the physiology and pharmacology of the ECB system may reduce side effects and broaden the potential therapeutic targets in neuropsychiatric disorders.
Because activation of CB1 and/or CB2 receptors can modulate both neuroendocrine and inflammatory responses mediated by stress, we aimed to determine if a non-selective agonist of these receptors (WIN) would attenuate RSD-induced inflammation, anxiety, and stress sensitization. This tetrahydrocannabinol-like drug has previously been shown to mitigate anxiety, fear conditioning, and facilitate fear extinction in rodents [19, 25]. Also, WIN prevented stress effects and attenuated inflammation [26]. In the present work, we show novel data indicating that this cannabinoid limits the behavioral, immune, and neuroinflammatory response to RSD.
Methods and materials
Mice
Male C57BL/6-resident mice were housed in cohorts of three per cage, while CD-1 aggressors were singly housed. All procedures were in accordance with the NIH Guidelines and were approved by the OSU Institutional Laboratory Animal Care and Use Committee.
Repeated social defeat
Mice were subjected to RSD as reported [12] and described in the Supplementary Methods. In brief, an aggressive intruder male CD-1 mouse was introduced into cages of established (three) male cohorts for two hours (1700 to 1900) per night for six consecutive nights. During each cycle, submissive behaviors were observed to ensure that the resident mice showed subordinate behavior.
Cannabinoid receptor agonist administration
Mice were administered vehicle or the cannabinoid1/2 receptor agonist WIN55,212-2 (WIN; 1 mg/kg; 0.1 mg/ml intraperitoneally (i.p.); Sigma-Aldrich [27]) 30 min prior to each cycle of RSD.
Anxiety-like behavior
Behavior was determined using the open field and elevated plus maze (EPM) paradigms as reported [13, 28] and described in Supplementary Methods. For the open field test, mice were placed in the corner of the test apparatus and activity was recorded and analyzed using an automated system (VersaMax). For the EPM, mice were placed in the center of the apparatus and activity was recorded and analyzed using an automated system (Ethovision). For each test, time spent moving, the number of entries (open and closed arms or center of the open field), and time spent in (open and closed arms or center of the open field) were determined.
Contextual conditioned fear
Behavior was determined to start 7 days after the cessation of RSD, as reported and described in the Supplementary Methods. In brief, on day 7 mice received three electrical footshocks (0.75 mA, 2 s each) [29] in the conditioning box. Acquisition of the conditioned fear extinction was evaluated 24 h after conditioning, and on day 8, during a 20 min period. The ability to extinguish the conditioned response (i.e., Retention of Fear Extinction) was evaluated after additional 24 h, and on day 9, during a 5 min period. During these sessions, each animal was placed in the same conditioning box, but with no footshock presentation, and the conditioned response was evaluated.
Corticosterone ELISA
Blood samples were collected from submandibular bleeds immediately after RSD and corticosterone concentrations were determined using the Corticosterone EIA kit (Enzo Inc.) following the manufacturer’s instructions.
Isolation of cells from bone marrow and blood
Fourteen hours after the last exposure to RSD, whole blood was collected with EDTA-lined syringes by cardiac puncture and red blood cells were lysed. Bone marrow was collected from the femur and flushed out with ice-cold Hank's balanced salt solution. Samples were washed, filtered, and then cells counts were determined with Particle Count and Size Analyzer (Beckman Coulter, Brea, CA, USA).
Isolation of CD11b+ cells from the brain
CD11b+ cells were isolated from whole-brain homogenates as previously reported [30] and described in the Supplementary Methods. In brief, brains were mechanically dissociated and CD11b+ cells were enriched using Percoll separation gradients.
Flow cytometry
Cells from the bone marrow, blood, and brain were collected, blocked, and then labeled with the appropriate antibodies. Cell surface antigen expression was determined using a Becton-Dickinson FACSCalibur cytometer. Data were analyzed using the FlowJo software and positive labeling for each antibody was determined based on isotype-labeled controls.
RNA isolation and real-time PCR
RNA was isolated from Percoll-enriched CD11b+ cells and whole-brain samples using the PrepEase RNA Isolation kit (Affymetrix, Santa Clara, CA, USA). Real-time quantitative PCR was performed using the Applied Biosystems Assay-on-Demand Gene Expression protocol following the manufacturer’s instructions. mRNA expression was determined on an ABI PRISM 7300 sequence detection system, was analyzed using the ΔΔCT method, and results are expressed as fold difference from glyceraldehyde 3-phosphate dehydrogenase.
Ex vivo stimulation of enriched CNS CD11b+ cells
As reported [31], CD11b cells were isolated via Percoll gradient, counted, plated, and then stimulated with vehicle or with LPS. Supernatants were collected 18 h later and concentration of IL-6 was determined by enzyme-linked immunosorbent assay (ELISA).
IL-6 ELISA
IL-6 levels in the plasma and ex vivo culture supernatants were determined using the BD OptEIA Mouse IL-6 ELISA (BD Biosciences).
Immunohistochemistry for Iba-1
Ionized calcium binding adaptor molecule-1 (Iba-1) labeling of microglia was performed as reported [32] and described in the Supplementary Methods. Briefly, paraformaldehyde-fixed brains sections were blocked and labeled with Iba-1 antibody (Wako, VA, USA; cat# 019-19741). Iba-1 labeling was analyzed via digital image analysis on the ImageJ software.
Statistical analysis
Data were analyzed by IBM® SPSS® Statistics software (version 21). Flow cytometry, ELISA, quantitative PCR, and EPM data were analyzed by two-way analysis of variance (ANOVA). Data from the ex vivo experiment with CD11b+ cells were analyzed by one-way ANOVA. Conditioned fear extinction data were analyzed by repeated-measures analysis. Retention of fear extinction data was analyzed by two-way ANOVA. Each group in the ER was also compared to the same group in the last min of the extinction phase (T20) by Student’s t test to show the recurrence or not of the conditioned behavior. Post hoc comparisons were performed by Student–Newman Keuls.
Results
The cannabinoid1/2 receptor agonist, WIN55, 212-2, attenuated RSD-induced threat appraisal and anxiety-like behavior
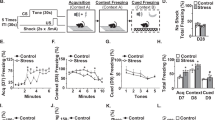
We first aimed to determine if an agonist of the cannabinoid CB1/CB2 receptors (WIN) attenuated RSD-induced threat appraisal and anxiety. Here, mice subjected to RSD were concomitantly administered WIN (Fig.1a). Increases in corticosterone, plasma IL-6, and spleen weight are indexes of increased threat appraisal with RSD [9, 33]. Corticosterone is a key hormone released by activation of the HPA axis [34] and IL-6 is a cytokine that is profoundly increased in circulation after exposure to RSD in mice [9, 12]. As expected, both corticosterone (Fig. 1b, p < 0.05) and IL-6 (Fig. 1c, p < 0.05) were increased in circulation after RSD (main effect of stress, p < 0.05). There was also a main effect of the intervention on corticosterone and IL-6 levels (main effect of intervention, p < 0.05), but there was not a significant stress × intervention interaction (p > 0.05). Spleen weight was also increased by RSD (Fig. 1d, p < 0.05) and this induction was attenuated by WIN (Fig. 1d, interaction, p < 0.02). Next, anxiety-like behavior in the open field and EPM was assessed 14 h after RSD. On the open field paradigm, RSD-exposed mice took longer to enter the center (Fig. 1e, p < 0.05), had fewer entries into the center (Fig. 1f, p < 0.001), and spent less time in the center (Fig. 1g, p < 0.01). Moreover, intervention with WIN reduced the effects of RSD on time to enter the center (interaction: p = 0.08), on the number of center entries (interaction: p < 0.005), and on duration spent in the center (interaction: p < 0.05). Post hoc analysis confirmed the Vehicle-RSD group was different than all other groups in the entries into the center and time to enter the center (p < 0.05). For the EPM, the total number of entries into the arms was unaffected by RSD (Fig.1h). Mice exposed to RSD have reduced entries into the open arm of the maze compared to all other groups (Fig. 1i, stress, p = 0.07; interaction p < 0.01) and this effect was reversed by WIN (Fig. 1j). Mice exposed to RSD also spent less time in the open arms compared to controls and this behavior was reversed by WIN (Fig. 1j, interaction, p < 0.05). Thus, WIN intervention attenuated the increased threat appraisal and anxiety-like behavior in mice promoted by RSD.
The cannabinoid1/2 receptor agonist, WIN55, 212-2, attenuated RSD-induced HPA activation and anxiety-like behavior. a Male C57BL/6 mice were subjected to six repeated cycles of social defeat (Stress) or left undisturbed as controls. Concomitant with each cycle of RSD, WIN55,212-2 (WIN, 1 mg/kg) was administered i.p. 30 min daily prior to each stress session. b Corticosterone levels (n = 7) were determined in the plasma immediately after the third cycle of RSD (stress: F1,27 = 6.5, p < 0.05; intervention: F1,27 = 7.0, p < 0.05). c IL-6 levels (n = 7) were determined in the plasma 14 h after RSD (stress F1,26 = 4.6, p < 0.05; intervention F1,26 = 5.1, p < 0.05). d Spleen weight (n = 11–14/group) was determined 14 h after RSD (stress: F1,44 = 59.1, p < 0.0001; intervention: F1,44 = 11.7, p < 0.005; stress × intervention: F1,44 = 6.2, p < 0.05). Next, anxiety-like behavior was evaluated 14 h after the last cycle on the open field test and the elevated plus maze. For the open field test (n = 6-–/group), e time to enter the center (stress: F1,26 = 16.3, p < 0.0001; stress × intervention: F1,26 = 3.2, p = 0.09), f number of center entries (stress: F1,26 = 20, p < 0.001; stress × intervention: F1,26 = 10.4, p < 0.005), and g duration in the center (stress: F1,28: 8.5, p < 0.01; stress × intervention: F1,28 = 4.9, p < 0.05) were determined. For the elevated plus maze (n = 18–13/group), h the number of closed arm entries (p > 0.05), i percentage of entries into open arms (stress: F1,40 = 3.4, p = 0.07; stress × intervention: F1,40 = 8.6, p < 0.01), and j percentage of time in open arms (stress × intervention: F1,40 = 7.1, p < 0.05) were determined. Bars represent the mean ± SEM. Bars or graphs with different letters (a, b, or c) are significantly different from each other (post hoc analysis, p < 0.05)
RSD-induced myelopoiesis and increase in circulating monocytes were attenuated by WIN
Immune changes with RSD are also related to increased threat appraisal activation. For instance, myelopoiesis and release of Ly6Chi monocytes in circulation are immune alterations that are promoted by RSD [8, 9]. Here, these immune parameters were determined with RSD (stress) and WIN intervention. As expected [8], stress reduced erythrocytes and lymphocytes (Fig. 2a, b, p < 0.0001) and increased monocytes (Fig. 2a, b, p < 0.0001) and granulocytes (Fig. 2a, b, p < 0.001) in the bone marrow. This RSD-induced reduction in erythrocyte and increase in granulocyte production were prevented by WIN (Fig. 2a, b, interaction: p < 0.05, for each), and the decrease in lymphocytes and increase in monocyte production tended to be blocked by WIN (Fig. 2a, b, interaction: p = 0.1 and p = 0.06, respectively). RSD also increased granulocytes (Fig. 2c, d, p < 0.005) and Ly6Chi monocytes (Fig. 2c, e, p < 0.005) in circulation and these effects were attenuated by WIN (Fig. 2d, interaction: p < 0.05; Fig. 2e; interaction: p = 0.1). Post hoc analysis confirmed the Vehicle-RSD group had the highest percentage of granulocytes and monocytes in the bone marrow and blood and lower percentage of erythrocytes and lymphocytes in the bone marrow, compared to all other groups (p < 0.05, for each).
RSD-induced myelopoiesis and increase in circulating monocytes were attenuated by WIN. Male C57BL/6 mice were subjected to six repeated cycles of social defeat (Stress) or left undisturbed as controls. Concomitant with each cycle of RSD, WIN55,212-2 (1 mg/kg) was administered daily 30 min prior to each stress session. Bone marrow and blood samples were collected and immune cell profiles were determined (n = 5). a Representative bivariate dot plots of CD31 and Ly6C labeling in the bone marrow showing: G granulocytes, M monocytes, L lymphocytes, E erythrocytes. b Percentage of erythrocytes (stress: F1,20 = 41.9, p < 0.0001; intervention: F1,20 = 4.0, p = 0.06; stress × intervention: F1,20 = 9.1, p < 0.01), lymphocytes (stress: F1,20 = 23.1, p < 0.0001; intervention: F1,20 = 7.9, p < 0.05; stress × intervention, F1,20 = 3.7, p = 0.07), monocytes (stress F1,20 = 19.9, p < 0.0001; intervention: F1,20 = 3.3, p = 0.09; stress × intervention: F1,20 = 3.3, p = 0.08) and granulocytes (stress: F1,20 = 43.3, p < 0.0001; intervention: F1,20 = 6.6, p < 0.05; stress × intervention: F1,20 = 8.0, p < 0.05). c Representative bivariate dot plots of CD115 and Ly6C labeling in the plasma shown for: G granulocytes, M monocytes. d Granulocytes (n = 7) in the blood (stress: F1,27 = 9.5, p < 0.01; stress × intervention: F1,27 = 5.1, p < 0.05) and e Ly6Chi monocytes (n = 8) in the blood (stress: F1,29 = 9.8; p < 0.005; intervention: F1,29 = 6.2; p < 0.05; stress x intervention: F1,29 = 2.4, p = 0.1). Bars represent the mean ± SEM. Bars or graphs with different letters (a, b, or c) are significantly different from each other (post hoc analysis, p < 0.05)
RSD-induced monocyte accumulation, and IL-1β and FAAH mRNA expression in the brain were attenuated by WIN
Next, several parameters of neuroinflammation were assessed after RSD and WIN intervention. As expected [12], proportional area of Iba-1 labeling, an indicator of morphological restructuring of microglia, was increased in prelimbic cortex after RSD (Fig. 3a, b, p < 0.05). This morphological restructuring of microglia was unaffected by WIN. Nonetheless, WIN reduced the accumulation of CD45hi monocyte/macrophages in the brain after RSD (Fig. 3c, d, interaction: p < 0.005). Post hoc analyses confirmed that the RSD-induced increase in monocyte accumulation was attenuated by WIN (Fig. 3c, d, p < 0.05). In a separate experiment, enriched brain CD11b+ cells (microglia and monocytes) were collected. These cells expressed higher mRNA levels of IL-1β after RSD (Fig. 3e, stress, p < 0.05) and this increase tended to be attenuated by WIN (Fig. 3e, stress × intervention, p = 0.08). Post hoc analysis confirmed that the enriched CD11b cells from Vehicle-RSD group had higher IL-1 β mRNA levels compared to all other groups (p < 0.05). Several ECB system-associated markers were also determined in enriched CD11b+ cells. There was no effect of stress or intervention on CB1/2 receptors expression. The mRNA levels of the fatty acid amide hydrolase (FAAH) enzyme, involved in anandamide metabolism, were increased by RSD (Fig. 3e, p < 0.05). This effect tended to be attenuated by WIN (interaction, p = 0.06). Post hoc analysis confirmed that the enriched CD11b cells from Vehicle-RSD group had higher FAAH mRNA levels compared to Vehicle-Control mice, which was attenuated by WIN (p < 0.05). Collectively, WIN intervention reduced RSD-induced monocyte accumulation in the brain and monocyte/microglial expression of IL-1β and FAAH.
RSD-induced monocyte accumulation and IL-1β mRNA expression in the brain was attenuated by WIN. Male C57BL/6 mice were subjected to six repeated cycles of social defeat (Stress) or left undisturbed as controls. WIN55,212-2 (WIN, 1 mg/kg) was administered i.p. 30 min daily prior to each stress session. At 0.5 days after the last cycle of RSD, mice were CO2 asphyxiated, and perfused with PBS followed by 4% paraformaldehyde. a Representative images of Iba-1 labeling in the prelimbic region of PFC. The arrows show the Iba-1+ cell depicted in the inset (20×). b Proportional area of Iba-1 (n = 6) in the prelimbic PFC (stress: F1,24 = 4.5, p < 0.05). In a separate experiment, mice (either treated with vehicle or WIN) were exposed to six cycles of stress, brain samples were collected 0.5 days after, and enriched CD11b+ cells were isolated. c Representative bivariate dot plots of CD11b-labeled and CD45-labeled cells. d Percentage of CD11b+/CD45hi brain macrophages (n = 8–10/group) (stress, F1,30 = 4.4, p < 0.05; stress × intervention: F1,30 = 12.0, p < 0.005). e A separate experiment was performed in an identical manner, and mRNA expression (n = 5–7/group) of IL-1β (stress: F1,19 = 7.6, p < 0.05, stress × intervention, F1,19 = 3.3, p = 0.08), and endocannabinoid-related molecules, CB1, CB2, FAAH (stress: F1,21 = 5.8, p < 0.05; stress × intervention: F1,21 = 3.8, p = 0.06) and MAGL (stress × intervention: Interaction F1,21 = 3.34, p = 0.08), were evaluated in enriched CD11b+ cells in the brain. Bars represent the mean ± SEM. Bars or graphs with different letters (a, b, or c) are significantly different from each other (post hoc analysis, p < 0.05)
WIN prevented RSD-induced ex vivo myeloid cell reactivity to LPS
We have reported that RSD results in sensitization of brain myeloid cells in which these cells are more reactive to ex vivo LPS stimulation [31]. Here ex vivo cultures of enriched CD11b cells were established after RSD and WIN. As expected, LPS increased secreted IL-6 protein (Fig. 4, p < 0.0001). Moreover, LPS stimulation caused the highest level of IL-6 secretion in the Stress-Vehicle group compared to all other groups (Fig. 4, p < 0.05), including the WIN-Stress group (p < 0.05). Thus, stress-induced immune sensitization of brain myeloid cells was attenuated by WIN.
WIN prevented RSD-induced myeloid cell reactivity to LPS. Male C57BL/6 mice were subjected to six repeated cycles of social defeat (Stress) or left undisturbed as controls. Concomitant with each cycle of RSD, WIN55,212-2 (WIN, 1 mg/kg) was administered i.p. 30 min prior to each stress day. Brain samples were collected 0.5 days after RSD, and Percoll-enriched CD11b+ cells were treated with LPS (400 ng/ml) ex vivo. IL-6 protein was determined in the supernatant (F5,61 = 6.9, p < 0.0001); n = 8–15/group. Bars represent the mean ± SEM. Bars or graphs with different letters (a, b, or c) are significantly different from each other (post hoc analysis, p < 0.05)
WIN reversed the RSD-induced impairment in fear extinction
Because RSD caused sensitization to subsequent immune challenge (Fig. 4), we next examined the sensitivity of RSD and WIN exposed mice to a fear conditioning protocol where fear extinction learning (FEL) and retention of fear extinction (Extinction retention) were determined 8 and 9 days after exposure to RSD (Fig. 5a). Notably, both control and RSD-exposed mice acquired the extinction memory, but RSD mice had a higher conditioned fear response. For example, RSD-exposed mice had higher conditioned fear responses over the 20-min session of FEL (Fig.5b, stress × time, p < 0.05). This higher response was reversed by WIN (Fig. 5b, stress × intervention, p < 0.01). Next, Fear Recall was determined 24 h later (+24 h timepoint in Fig. 5b). Notably, WIN controls were not different from Vehicle-controls in the fear extinction session, but had higher fear during the Extinction retention session compared to Vehicle-Control group (Fig. 5b, p < 0.005). Extinction retention was affected by RSD and by WIN (interaction: p < 0.001). Post hoc analysis confirmed that Vehicle-Stress mice had higher fear during the Extinction retention session compared to all other groups (p < 0.05).
WIN reversed RSD-induced impairment in fear extinction. a Male C57BL/6 mice were subjected to six repeated cycles of social defeat (Stress) or left undisturbed as controls. WIN55,212-2 (WIN, 1 mg/Kg) was administered i.p. 30 min prior to each stress day. Starting at 7 days after exposue to RSD (7 days), mice underwent contextual fear conditioning paradigm. b On 8 days post RSD, conditioned response was determined (fear extinction learning) over a 20-mine time course (time: F19,19 = 10.4, p < 0.001; time × stress: F19,19 = 2.4, p < 0.05; stress × intervention, F1,37 = 8.7, p < 0.01, F3,37 = 4.8, p < 0.01). Retention of the fear extinction (Retention) was also determined 9 days post RSD, which was also 24 h (+24 h in the graph) after fear extinction learning (stress × intervention: F1,37 = 15.2, p < 0.001). Following Retention, brain samples were collected and IL-1β mRNA expression was determined in the c prefrontal cortex (PFC; stress: F1,25 = 4.98, p < 0.05; intervention: F1,25 = 4.33, p < 0.05; stress × intervention: F1,25 = 3.38, p = 0.08) and d hippocampus (HPC; stress: F1,35 = 5.9, p < 0.05; stress × intervention: F1,35 = 11.42, p < 0.005). Bars represent the mean ± SEM. Bars or graphs with different letters (a, b, or c) are significantly different from each other (post hoc analysis, p < 0.05)
In the same mice, mRNA was collected from the prefrontal cortex (PFC) and hippocampus (HPC) after the Extinction retention test. This time point corresponds to 9 days post-RSD. IL-1β mRNA expression was higher in the PFC (Fig. 5c, p < 0.05) of the RSD mice and this effect tended to be dependent on WIN (interaction: p = 0.08). Similar results were detected in the HPC following FEL, where the RSD-associated increase in IL-1β (Fig. 5d, p < 0.05) was attenuated by WIN (interaction: p < 0.005). Post hoc analysis confirmed that Vehicle-Stress mice had the highest IL-1β mRNA expression in the PFC and HPC compared to all other groups (p < 0.05, for both regions). Taken together, RSD causes stress sensitization that is associated with heightened fear response and impaired fear extinction retention and increased IL-1β levels in the PFC and HPC, and these effects were attenuated by WIN.
Discussion
Psychosocial stress contributes to the development of anxiety and the potential recurrence of anxiety. Here we show that WIN, a synthetic CB1/CB2 receptor agonist, attenuates stress-induced anxiety-like behavior. Moreover, WIN reduced the production and release of inflammatory myeloid cells during RSD. The reduction in the release of monocytes into circulation was associated with fewer monocytes in the brain after RSD. Moreover, IL-1β and FAAH mRNA were increased in brain microglia/macrophages after stress and were attenuated by WIN. RSD-associated “sensitization” of microglia/monocytes to LPS was also prevented by WIN. Last, there was a higher fear expression and impaired retention of fear extinction 8 and 9 days, respectively, after RSD. This increase was associated with increased IL-1β expression in the brain. Again, these deficits were prevented by WIN.
A significant finding of this study was that concurrent intervention with WIN resulted in a reversal of the RSD-induced threat appraisal and anxiety-like behavior 14 h after cessation of RSD. Key parameters of increased threat appraisal after RSD were attenuated by WIN. These include splenomegaly and release of inflammatory monocytes into circulation. There was also a reduction of corticostreone and IL-6 with WIN. WIN treatment during RSD was also associated with the attenuation of RSD-induced myelopoiesis, which is dependent on the activation of stress brain circuitry and sympathetic nervous system (SNS) [9]. RSD induces recruitment of peripheral monocytes into the brain where they trigger neuroinflammatory signaling and anxiety-like behavior [12, 13]. In the current study, RSD-induced monocyte recruitment and anxiety in both the open field and EPM were attenuated by WIN. Thus, activation of cannabinoid receptors was effective in reversing key physiological, immunological, and behavioral deficits associated with a chronic stressor in mice.
One relevant point, which was not evaluated in the present study, is if the CB1/2 agonist is affecting central or peripheral signaling induced by RSD. Although this a limitation of this study, we would speculate that WIN works at both levels. For example, stressors increase activation of the SNS and HPA axis. Previous data indicate that WIN decreases noradrenaline release from PFC [35] and hippocampal [36] slices. Moreover, blocking CB1 receptors basally results in increased activation of β-adrenergic receptors and increased anxiety behavior in rodents [37]. Here, the key point is the reduction in the inflammatory monocyte release and reduce accumulation in the brain. We have reported that much of the IL-1β expression in the brain associated with RSD is dependent on the accumulation of monocytes in the brain [13]. Monocyte release during RSD is dependent on SNS and HPA, that could be regulated by WIN via CNS-mediated top-down mechanisms [9, 38]. These effects could also be simultaneously regulated via peripheral actions of WIN. For instance, CB1 receptors modulate noradrenaline release from sympathetic neurons outside the brain [39]. Moreover, WIN may directly interfere with myeloid cells production and trafficking [40, 41] and may prevent the differentiation of myeloid cells to a more adherent phenotype [42]. Thus, WIN can activate CB1 receptors on noradrenergic terminals reducing sympathetic outflow to the bone marrow and can interfere with the traffic of myeloid cells.
Another important finding was that WIN attenuated the neuroinflammatory profile associated with RSD. For instance, there was less monocyte accumulation in the brain after RSD with WIN intervention. Corresponding with this, WIN also reduced the mRNA expression of IL-1β in enriched CD11b cells after RSD. This is important because our recent work shows that IL-1β signaling in the brain after RSD is provided by these accumulating monocytes and this signal is critical for prolonged anxiety [13]. While WIN did not affect microglia morphology in the PFC, this parameter does not necessarily reflect microglial activation [43]. In addition, WIN attenuated the RSD-induced increased reactivity (e.g., IL-6 secretion) to ex vivo LPS stimulation of brain CD11b cells. This finding indicates that WIN lowered the neuroinflammatory profile associated with RSD. Notably, there was increased FAAH mRNA with RSD, which was attenuated by WIN. These data suggest that RSD increases the activity of the enzyme associated with anandamide (an ECB) metabolism, and lower the levels of this anti-inflammatory ECB, ultimately triggering neuroinflammatory signaling. Taken together, WIN intervention attenuated three key components of the neuroinflammatory profile associated with RSD: monocyte accumulation in the brain, IL-1β expression by brain myeloid cells, and ex vivo reactivity of brain myeloid cells to LPS.
Another relevant finding was the impaired fear extinction observed 8–9 days after RSD. Stress-exposed mice showed higher fear expression to conditioned stimuli 8 days later. Moreover, while RSD-exposed mice were able to acquire fear extinction, they presented a higher fear response when tested for fear extinction retention 24 h later. This result is relevant because hyper-consolidation of aversive fearful memories and resistance to extinction are key components of PTSD [16]. Recent evidence suggests that chronic stress in rodents increases fear expression and/or disrupt fear extinction [44, 45]. Other studies using less stressful models of social defeat show enhanced fear expression and more discrete alterations in the extinction process [46, 47]. Here, we also provide evidence that enhanced fear response and impaired fear extinction retention associated with RSD were reversed by WIN. These data support the notion that cannabinoid agonists are useful therapeutic interventions to resolve stress sensitization as with PTSD [18]. Related to this point, the ECB system plays a tonic role in the control of fear-conditioned responses [19]. For example, pharmacological blockade or genetic disruption of these receptors delayed or impaired fear extinction [48]. There is evidence of a dysregulated ECB system in the PFC of PTSD patients when compared to trauma-exposed non-PTSD subjects or healthy controls [20]. Here, expression of the ECB-related gene FAAH in enriched CD11b cells was changed 14 h after RSD, whereas CB1/CB2 receptors were not changed. Nonetheless, RSD may elicit other regional or temporal alterations in ECB-related genes that contribute to enhanced fear expression. Of note, it is suggested that ECBs released during repeated social stress are necessary for later extinction of conditioned fear [47]. Interestingly, there was an enhancement in fear retention in WIN-control mice, similar to a previous report in rats [49]. Taken together, RSD causes stress sensitization that is associated with heightened fear responses and these effects were attenuated by WIN.
Consistent with the hypothesis of long-term stress sensitization after RSD, stress-sensitized mice in the current study had increased IL-1β expression in the PFC and HPC with contextual fear, which was blocked by WIN. Here, IL-1β mRNA expression was significantly higher in the PFC and in the HPC of RSD mice after evaluating retention of fear extinction. Increased IL-1β expression in the brain of RSD mice to the fear conditioning protocol was absent in the mice receiving WIN. This reactivity was triggered by the fear conditioning and was evident more than 7 days after the cessation of social defeat. The induction of IL-1β observed in the present study is relevant because increased IL-1β signaling is involved in the pathophysiology of neuropsychiatric disorders including fear and anxiety [13, 50, 51]. For instance, direct injections of IL-1β into the brain or increased IL-1β following LPS during the consolidation phase of a contextual fear conditioning paradigm impaired fear expression in rats [52]. In our study, nonetheless, IL-1β was associated with higher fear response and impaired fear extinction retention. However, only stressed mice presented higher IL-1β, suggesting that this was a consequence of the previous exposure to RSD, not of the conditioning procedure per se. Likewise, an acute stressor increased IL-1β levels in the dorsal HPC that were associated with delayed stress-induced fear learning and was prevented by central administration of an IL-1 receptor antagonist (IL1RA) [53]. Parallel to this finding, human PTSD patients show increased pro-inflammatory profile. Of note, they present increased levels of cytokines in the periphery, including IL-1β, and reduced anti-inflammatory mediators [17]. Taken together, these results indicate that WIN intervention was effective in reducing stress sensitization that is associated with increased IL-1β reactivity and enhanced fear sensitivity.
An alternative approach would be to use WIN intervention after the RSD protocol was completed. Indeed, a recent report showed that acute administration of WIN after exposing rats to a single prolonged stressor, a PTSD model, attenuated a stress-associated impairment in fear extinction [54]. While we did not complete these post-stress interventions here, it is plausible that this CB1/CB2 agonist strategy would be effective in limiting the stress sensitization after RSD. Thus, post-stress intervention with the CB1/CB2 agonist is a key future direction. Nonetheless, there are many known triggers for anxiety responses, so prophylactic intervention is still relevant and meaningful in preventing stress sensitization and the potential recurrence of anxiety. Indeed, we show evidence that stress sensitization and increased stress reactivity after RSD (induced by fear conditioning or ex vivo LPS challenge) was limited by our concomitant WIN intervention strategy. The data with the CB1/CB2 agonist, however, does not allow the conclusion regarding a possible physiological role of the ECB system in regulating stress responses. Therefore, the use of drugs that facilitate ECB neurotransmission in future experiments, such as a FAAH inhibitor, instead of direct activation of the receptors, will give us more specific information about the involvement of the ECB system in the consequences of RSD.
In conclusion, a non-selective CB1/CB2 receptor agonist, WIN, was effective in limiting the immune and neuroinflammatory response to RSD. Novel data are provided showing that mice exposed to RSD are stress sensitized with heightened fear responses that persist at least a week after stress. These deficits, however, were prevented by pharmacological activation of cannabinoid receptors. Overall, interventions that reduce threat appraisal and neuroinflammatory responses to stress may prevent long-term stress sensitization that is relevant to PTSD.
References
Stone EA, Lin Y, Quartermain D. A final common pathway for depression? Progress toward a general conceptual framework. Neurosci Biobehav Rev. 2008;32:508–24.
Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC, et al. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J Leukoc Biol. 2012;92:959–75.
Miller GE, Murphy ML, Cashman R, Ma R, Ma J, Arevalo JM, et al. Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav Immun. 2014;41:191–9.
Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–3.
Carvalho LA, Bergink V, Sumaski L, Wijkhuijs J, Hoogendijk WJ, Birkenhager TK, et al. Inflammatory activation is associated with a reduced glucocorticoid receptor alpha/beta expression ratio in monocytes of inpatients with melancholic major depressive disorder. Transl Psychiatry. 2014;4:e344.
Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40.
Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–8.
Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110:16574–9.
Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. Beta-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–88.
Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 2014;42:50–59.
Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1799–1805.
Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, et al. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry. 2014;75:970–81.
McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, et al. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. 2017 [Epub ahead of print].
Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–505.
Bonne O, Grillon C, Vythilingam M, Neumeister A, Charney DS. Adaptive and maladaptive psychobiological responses to severe psychological stress: implications for the discovery of novel pharmacotherapy. Neurosci Biobehav Rev. 2004;28:65–94.
Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–82.
Wang Z, Young MR. PTSD, a disorder with an immunological component. Front Immunol. 2016;7:219.
Krystal JH, Davis LL, Neylan TC, Raskind MA, Schnurr PP, Stein MB, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: A Consensus Statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82:e51–e5.
Lisboa SF, Gomes FV, Terzian AL, Aguiar DC, Moreira FA, Resstel LB, et al. The endocannabinoid system and anxiety. Vitam Horm. 2017;103:193–279.
Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry. 2013;18:1034–40.
Viveros MP, Marco EM, Llorente R, Lopez-Gallardo M. Endocannabinoid system and synaptic plasticity: implications for emotional responses. Neural Plast. 2007;2007:52908.
Mecha M, Carrillo-Salinas FJ, Feliu A, Mestre L, Guaza C. Microglia activation states and cannabinoid system: Therapeutic implications. Pharmacol Ther. 2016;166:40–55.
State Medical Marijuana Laws. March 28th, 2016. National Conference of State Legislatures, 2016. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47.
Ganon-Elazar E, Akirav I. Cannabinoids prevent the development of behavioral and endocrine alterations in a rat model of intense stress. Neuropsychopharmacology. 2012;37:456–66.
Crowe MS, Nass SR, Gabella KM, Kinsey SG. The endocannabinoid system modulates stress, emotionality, and inflammation. Brain Behav Immun. 2014;42:1–5
Gomes FV, Casarotto PC, Resstel LB, Guimaraes FS. Facilitation of CB1 receptor-mediated neurotransmission decreases marble burying behavior in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:434–8.
Lisboa SF, Issy AC, Biojone C, Montezuma K, Fattori V, Del-Bel EA, et al. Mice lacking interleukin-18 gene display behavioral changes in animal models of psychiatric disorders: Possible involvement of immunological mechanisms. J Neuroimmunol. 2018;314:58–66.
Lisboa SF, Gomes FV, Silva AL, Uliana DL, Camargo LH, Guimaraes FS, et al. Increased contextual fear conditioning in iNOS knockout mice: additional evidence for the involvement of nitric oxide in stress-related disorders and contribution of the endocannabinoid system. Int J Neuropsychopharmacol. 2015;18 pii: pyv005.
Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–33.
Ramirez K, Shea DT, McKim DB, Reader BF, Sheridan JF. Imipramine attenuates neuroinflammatory signaling and reverses stress-induced social avoidance. Brain Behav Immun. 2015;46:212–20.
Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci. 2014;34:2583–91.
Ramirez K, Sheridan JF. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive- like behaviors. Brain Behav Immun. 2016;57:293–303.
Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603–21.
Richter H, Teixeira FM, Ferreira SG, Kittel A, Kofalvi A, Sperlagh B. Presynaptic alpha(2)-adrenoceptors control the inhibitory action of presynaptic CB(1) cannabinoid receptors on prefrontocortical norepinephrine release in the rat. Neuropharmacology. 2012;63:784–97.
Schlicker E, Timm J, Zentner J, Gothert M. Cannabinoid CB1 receptor-mediated inhibition of noradrenaline release in the human and guinea-pig hippocampus. Naunyn-Schmiedeberg’s Arch Pharmacol. 1997;356:583–9.
Bellocchio L, Soria-Gomez E, Quarta C, Metna-Laurent M, Cardinal P, Binder E, et al. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB(1) receptor blockade. Proc Natl Acad Sci USA. 2013;110:4786–91.
Niraula A, Wang Y, Godbout JP, Sheridan JF. Corticosterone production during repeated social defeat causes monocyte mobilization from the bone marrow, glucocorticoid resistance and neurovascular adhesion molecule expression. J Neurosci. 2018;38:2328–40.
Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118:2023–8.
Hegde VL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur J Immunol. 2010;40:3358–71.
Palazuelos J, Davoust N, Julien B, Hatterer E, Aguado T, Mechoulam R, et al. The CB(2) cannabinoid receptor controls myeloid progenitor trafficking: involvement in the pathogenesis of an animal model of multiple sclerosis. J Biol Chem. 2008;283:13320–9.
Paulsen K, Tauber S, Timm J, Goelz N, Dumrese C, Stolzing A, et al. The cannabinoid receptors agonist WIN55212-2 inhibits macrophageal differentiation and alters expression and phosphorylation of cell cycle control proteins. Cell Commun Signal. 2011;9:33.
Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64:300–16.
Hoffman AN, Lorson NG, Sanabria F, Foster Olive M, Conrad CD. Chronic stress disrupts fear extinction and enhances amygdala and hippocampal Fos expression in an animal model of post-traumatic stress disorder. Neurobiol Learn Mem. 2014;112:139–47.
Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–8.
Laricchiuta D, Centonze D, Petrosini L. Effects of endocannabinoid and endovanilloid systems on aversive memory extinction. Behav Brain Res. 2013;256:101–7.
Dubreucq S, Matias I, Cardinal P, Haring M, Lutz B, Marsicano G, et al. Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology. 2012;37:1885–1900.
Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4.
Lin HC, Mao SC, Chen PS, Gean PW. Chronic cannabinoid administration in vivo compromises extinction of fear memory. Learn Mem. 2008;15:876–84.
Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30:30–45.
Rossi S, Sacchetti L, Napolitano F, De Chiara V, Motta C, Studer V, et al. Interleukin-1beta causes anxiety by interacting with the endocannabinoid system. J Neurosci. 2012;32:13896–905.
Rachal Pugh C, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25:29–41.
Jones ME, Lebonville CL, Barrus D, Lysle DT. The role of brain interleukin-1 in stress-enhanced fear learning. Neuropsychopharmacology. 2015;40:1289–96.
Ganon-Elazar E, Akirav I. Cannabinoids and traumatic stress modulation of contextual fear extinction and GR expression in the amygdala–hippocampal–prefrontal circuit. Psychoneuroendocrinology. 2013;38:1675–87.
Acknowledgements
We thank David Hammond, Daniel Shea, Yufen Wang, and Brooke Benner (Ohio State University) for the excellent technical support. This work was support by FAPESP (2014/212260-0 to SFL, 2012/17626-7 to FSG and SFL) and NIH (R01-MH-093473 and R01-MH-093472 to JFS). All authors report no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lisboa, S.F., Niraula, A., Resstel, L.B. et al. Repeated social defeat-induced neuroinflammation, anxiety-like behavior and resistance to fear extinction were attenuated by the cannabinoid receptor agonist WIN55,212-2. Neuropsychopharmacol 43, 1924–1933 (2018). https://doi.org/10.1038/s41386-018-0064-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-018-0064-2
This article is cited by
-
Enhanced fear memory after social defeat in mice is dependent on interleukin-1 receptor signaling in glutamatergic neurons
Molecular Psychiatry (2024)
-
Are Advanced Oxidation Protein Products (AOPPs) Levels Altered in Neuropsychiatric Disorders? An Integrative Review
Molecular Neurobiology (2024)
-
The neuroimmunology of social-stress-induced sensitization
Nature Immunology (2022)
-
Cannabinoid receptor 1 signalling modulates stress susceptibility and microglial responses to chronic social defeat stress
Translational Psychiatry (2021)
-
Tempering aversive/traumatic memories with cannabinoids: a review of evidence from animal and human studies
Psychopharmacology (2019)