Abstract
The fungal microbiota (mycobiota) is an integral part of the microbial community colonizing the body surfaces and is involved in many key aspects of human physiology, while an imbalance of the fungal communities, termed fungal dysbiosis, has been described in pathologies ranging from infections to inflammatory bowel disease. Commensal organisms, such as the fungus Candida albicans, induce antigen-specific immune responses that maintain immune homeostasis. Adaptive immune mechanisms are vital in this process, while deficiencies in adaptive immunity are linked to fungal infections. We start to understand the mechanisms by which a shift in mycobiota composition, in particular in C. albicans abundance, is linked to immunopathological conditions. This review discusses the mechanisms that ensure continuous immunosurveillance of C. albicans during mucosal colonization, how these protective adaptive immune responses can also promote immunopathology, and highlight therapeutic advances against C. albicans-associated disease.
Similar content being viewed by others
Introduction
Fungi are an integral part of the human microbiota interacting with the immune system and affecting human physiology. The human mycobiota is composed of 390 different fungal species belonging to the phyla Ascomycota, Basidiomycota, and Zygomycota1, which are required for microbial community structure, metabolic function, and immune priming2,3,4. Candida species such as Candida albicans are commonly found on the oral, gastrointestinal, and vaginal mucosae. Besides its host-beneficial effects protecting the host from various microbial insults, C. albicans can itself become pathogenic and cause diverse pathologies in immunocompetent and immunocompromised individuals. Candida-mediated disorders range from mild superficial infections such as neonatal thrush to extensive and/or recurrent infections of the esophageal or vaginal tract that can cause significant morbidity5. Translocation of Candida across epithelia and fungal dissemination through the bloodstream can finally result in life-threatening systemic manifestations, which are responsible for several hundred thousand annual deaths worldwide6. In addition, C. albicans is also associated with inflammatory not primarily infectious diseases, such inflammatory bowel disease (IBD)7. Given the continuous presence of C. albicans in barrier tissues and their significant pathogenic potential, tight control of the fungus at the host interface is a prerequisite of homeostasis. The adaptive immune system plays a key role in providing long-lasting defence against uncontrolled fungal growth, acquisition of virulence traits, and invasion of normally sterile tissues.
Evidence for a protective role of T cells against C. albicans is provided by individuals displaying an enhanced susceptibility for mucocutaneous candidiasis due to acquired or inherited defects in the frequency, activation, or function of CD4+ T cells, especially those producing IL-178,9. Although historically, the evidence for antibodies contributing to fungal control was sparse, recent studies highlight the relevance of antibody-mediated immunity in maintaining C. albicans commensalism10,11,12. Key hallmarks of cellular and humoral adaptive immunity are the antigen-specificity, which accounts for the directionality of the response, the requirement for lymphocytes to undergo tightly regulated differentiation and polarization processes, which generate highly specialized and qualitatively distinct responses, and the capacity to form long-lasting memory.
Here, we review the current understanding of T- and B-cell mediated antifungal defence in barrier tissues, discuss how these normally protective immune mechanisms can also promote immunopathology under certain conditions, and highlight outstanding challenges that still impede harnessing our knowledge for preventative & therapeutic advances against Candida-associated (infectious and non-infectious) disorders.
T cell-mediated immunity against C. albicans
C. albicans-responsive CD4+ T cells are primed in all healthy individuals as a consequence of their constant exposure to the fungus13,14. They are characterized predominantly by a Th17 profile producing IL-17A, IL-17F and IL-22, with a minor fraction belonging to the Th1 or Th2 subsets15. While most studies assessing human antifungal T cells focused on circulating T cells due to their easy accessibility in the blood, C. albicans-specific Th17 cells have also been evidenced in the healthy skin16. Similarly, in experimental models of oropharyngeal candidiasis (OPC) and epicutaneous candidiasis, C. albicans-specific T cells with a selective Th17 phenotype are detected in the colonized epithelial tissue within a week of infection17,18. In addition to Th17 cells, innate-like T cells and TCR-negative innate lymphoid cells (ILCs) also contribute to the overall IL-17 production in mice where these IL-17 producers rapidly accumulate in response to the primary exposure of the murine host with the fungus, especially in response to virulent strains of C. albicans, which only transiently colonize mice19,20,21,22. The diverse cellular subsets appear to play redundant roles19, with γδ T cells being particularly prominent/important during cutaneous experimental candidiasis22.
The signals driving polarization of C. albicans-specific Th17 cells including IL-23, IL-6 and IL-1 contribute also to the activation of innate-like T cells and ILCs19,21,23,24. Different dendritic cell subsets have been implicated at the interface between the fungus and IL-17-production in the initiation of the response depending on the IL-17-producing cellular subset and the tissue compartment (skin vs. mucosa)23,25,26. In addition, neurons (in the skin) can sense the fungus and link fungal recognition to the induction of IL-23 by dermal DCs22. Moreover, the cellular damage caused by the fungal peptide toxin candidalysin, which is secreted by high virulent strain of C. albicans, contributes to IL-1 release for rapid IL-17 induction by innate-like T cells21.
The continuous presence of C. albicans during commensalism requires a long-lived response for maintenance of homeostasis over time. As such, C. albicans-reactive Th17 in humans exhibit a memory phenotype13. Within barrier tissues, they express markers characteristic of tissue-resident memory T (TRM) cells16. This has also been reproduced in experimental mice that have been persistently colonized with C. albicans to closely mimic the situation in humans18.
Accumulation of C. albicans-specific Th17 during commensalism depends on cognate antigen presentation and on Card9-dependent signals including IL-2318,27, but overall remains not well understood. The initiation and maintenance of the antifungal T cell response represent separate processes characterized by different signal requirements. Overall, the immunosurveillance response is uncoupled from inflammation, in line with the notion that inflammation is not compatible with homeostasis and consistent with what was also shown for the homeostatic T cell response against commensal bacteria28. The tonic signal controlling long-term maintenance of antifungal T cells during commensalism in colonized tissue remains to be determined. Rapid decline of the antifungal T cell population following antimycotic-mediated removal of the fungus indicates that maintenance of the homeostatic Th17 response depends on the continuous presence of the fungus18. This is reminiscent of the situation of other commensal- and virus-specific tissue-resident CD4+ T cells responses29,30. The dependence on fungal persistence may reflect the requirement for continuous antigen recognition by commensal-specific Th17 cells, as shown to be the case for homeostatic Th1 cells in the skin, which depend on keratinocyte-intrinsic MHC-II31. Alternatively, fungal persistence might induce cytokine signals for homeostatic T cell survival, proliferation and/or renewal or modulate the microenvironment in another way to favour T cell persistence. The observed dependence of the C. albicans-specific T cell response on the continuous presence of the fungus contradicts the paradigm of immunological memory. Therefore, clarification of the relationship between homeostatic T cells providing immunosurveillance of commensals and memory T cells that protect against recurring infections awaits further investigations. Taken together, long-lived Th17 cells residing in the colonized tissue are the most notable ones of the diverse lymphoid cells that contribute to the overall IL-17 response for immunosurveillance of commensalism and homeostasis (Fig. 1A), although their identity and regulation remains to be defined in more detail.
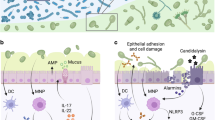
(Left). Homeostatic immunity against C. albicans is maintained by cellular and humoral adaptive immunity. C. albicans drives fungus-specific and tissue-resident Th17 cells. IL-17 and IL-22 contribute to fungal control by promoting antimicrobial and barrier functions of the epithelium, the latter of which is counteracted by overt IFN-γ/STAT1-signalling. Antifungal Th17 immunity can also promote barrier defence against heterologous infections, both locally and systemically, and modulate social behaviour. Mucosal IgA targets fungal virulence determinants to repress C. albicans pathogenicity. C. albicans-specific IgG contributes to systemic antifungal immunity. (Right). Enhanced C. albicans-specific T cell and antibody responses have been observed in patients with gut barrier defects as in IBD. Whether IgA and Th17 cells act in a host-protective manner or whether antifungal T cells adopt features of pathogenic Th17 cells in the inflamed gut remains unclear. During airway inflammation, C. albicans-specific pathogenic Th17 cross-react with A. fumigatus. Enhanced C. albicans-reactive IgG antibodies have been associated with alcoholic and non-alcoholic liver disease, albeit their role in disease pathogenesis remains to be determined. See text for more details. The figure was generated with BioRender.com.
C. albicans-specific Th17 cells act locally in the colonized tissue to keep homeostasis under control and prevent fungal overgrowth and tissue invasion by strengthening the antimicrobial effector functions and the barrier integrity of the epithelium. Reinforcement of the barrier function and protection from intestinal injury in the gut was attributed to IL-22, which is produced by antifungal Th17 cells in addition to IL-17A and IL-17F32. Of note, the T cell response mounted in response to fungal colonization enhances resistance not only against the mycobiota itself but also against bacterial infections as shown in experimental models with C. difficile and C. rodentium32,33.
While an impairment of the IL-17/IL-22 pathway predisposes individuals to mucocutaneous candidiasis, the same fungal infection also manifests in individuals with an intact antifungal Th17 response but a dysregulated balance between type 17 and type 1 immunity. As such, APECED patients suffering from chronic mucocutaneous candidiasis (CMC) exhibit exacerbated type 1 responses while type 17 immune responses are intact34. Aire-deficient mice recapitulating the situation in APECED patients revealed that IFN-γ-driven interferonopathy drives pronounced epithelial barrier defects, which underlie the mucosal fungal susceptibility34. Barrier-protective effects of type 17 immunity can thus be overridden by aberrant IFN-γ/STAT1 responses that promote epithelial cell death and barrier disruption.
Beyond the local host-protective effects, C. albicans-induced Th17 immunity has also been reported to mediate systemic antimicrobial effects35,36 including heterologous protection from systemic S. aureus35. Mechanistically, it was proposed that Th17 primed locally in the gut promote systemic neutrophil activation to enhance protection against an intravenous challenge35. Moreover, the mycobiota can impact physiological processes beyond host protection. As such, fungal gut colonization was recently proposed to promote social behaviour in mice32. The neuromodulatory role of mucosa-associated fungi occurs in an IL-17-dependent manner with neuronal cells acting as direct targets of IL-1732. This expands the previously noted effects of IL-17 on social behaviour37,38,39,40 and provides a mechanistic basis for the reported associations of fungal dysbiosis and neuropsychiatric conditions in humans41,42.
During homeostasis, C. albicans-specific Th17 cells are not associated with inflammatory processes despite their continuous engagement by the commensal fungus and the proinflammatory potential of IL-17. This is reminiscent of homeostatic T cells directed against commensal bacteria in the gut, which display a metabolic profile characteristic of resting memory cells29. Whether immunoregulatory processes contribute to stable homeostasis by actively preventing plasticity of C. albicans-specific homeostatic Th17 cells towards pathogenicity (see below) remains currently unclear with no evidence for a contribution of regulatory T cells or IL-1043. Moreover, the antifungal effects of Th17 cells during fungal commensalism are not accompanied by an accumulation of neutrophils in the colonized tissue, which would be incompatible with homeostasis, even though neutrophil-recruiting chemokines are stimulated by IL-17 under some conditions. Examples of mucocutaneous C. albicans infections characterized by a strong neutrophil response in the infected tissue are those from experimental cutaneous and oropharyngeal infections with highly virulent strains of C. albicans44,45, where the inflammatory response is an acute and direct reaction to fungal virulence factors and release of keratinocyte-derived alarmins such as IL-1α21, and is largely independently of the IL-17 pathway45. In conclusion, the homeostatic role of IL-17 is non-inflammatory and expands beyond microbial control, and it may be mediated by mechanisms beyond strengthening antimicrobial barrier functions of the epithelium (Fig. 1A).
Antibody-mediated immunity against C. albicans
Antibodies play a vital role in homeostasis and protective immunity at mucosal sites, as well as during systemic infections46,47, and can be divided into five isotypes, which operate in distinct places and have distinct effector functions48. Antibody effector functions have classically been studied in the context of pathogenic infections49. However, it is apparent that luminal and serum antibodies bind members of the microbiota50.
The fungal cell wall contains various pattern-associated molecular patterns (PAMPs), such as β-glucans, for innate immune recognition51,52,53,54. However, the C. albicans cell wall and secretory proteins are also a significant source of antigens55. During the course of an immune response, depending on the nature of the eliciting antigen and its entry mode, class-switch recombination (CSR) replaces immunoglobulin constant regions for the isotype that can best protect against commensal or pathogenic microorganism56. As a result, mature B cells express antibodies of the IgA, IgG, or IgE classes that differ in effector functions without altering the specificity for the immunizing antigen57. Intestinal C. albicans colonization induces distal CSR and B cell expansion in germinal centers (GC) to induce high-affinity IgG. CSR and GC-B cell expansion is controlled by CARD9 and CX3CR1+ mononuclear phagocytes (MNPs)58. Antifungal IgG responses are reduced in patients with polymorphisms in the coding region of the CX3CR1 or CARD9 genes58,59, in whom CD has been described60,61,62,63. Furthermore, CARD9-deficient patients manifest fungal-specific infection susceptibility, predominantly in the central nervous system by C. albicans64,65.
A significant portion of C. albicans is recognized by systemic antifungal IgG antibodies in humans and mice, where IgG3 binds the largest fraction of intestinal fungi58. IgG3 Abs are potent mediators of effector functions, including enhanced Ab-mediated cellular cytotoxicity, opsonophagocytosis, complement activation, and neutralization, compared with other IgG subclasses66. Accordingly, mucosal fungal colonization induces distal humoral immunity and systemic protection against invasive candidiasis.
Cross-reactive antibody responses provide protective immunity to related pathogens or antigenic variants in natural epidemiology. In this line, intestinal-induced anti-C. albicans IgG protects against systemic infection with the emerging drug-resistant fungus C. auris58. However, IgG isotypes generated by intestinal C. albicans colonization are not cross-reactive with environmental, food-derived, or skin-resident fungi such as Saccharomyces, Aspergillus, and Malassezia spp58.
IgA is the dominant isotype at mucosal barriers67, and predominantly induced in response to colonization with commensal organism to maintain homeostasis68. Within the mucosa, commensal microorganism are coated by low-affinity and antigen-specific secretory IgA (SIgA)69. Furthermore, IgA serves as the first line of defense in protecting the epithelium, from toxins or potential pathogenic microorganism overgrowth68. While immune exclusion is a dominant IgA effector mechanism68, IgA is able to enchain pathogens, thereby preventing organism separation after replication resulting in clumping70. Accordingly, mucosal C. albicans colonization induces IgA10,11,12 to reduce fungal-associated virulence attributes11. IgA binding to C. albicans prevents adhesion, invasion, and damage of epithelial cells11,71 resulting in reduced inflammation11. In particular, IgA binds to hyphae-associated virulence factors, such as C. albicans adhesins or the toxin candidalysin10,12. Consequently, IgA binding controls commensal homeostasis by eliminating C. albicans-associated virulence traits, while the absence of IgA results in mucosal dysbiosis, C. albicans overgrowth, and pathology10,11,12. Associated pathology is observed in patients with CD, in whom reduced virulence factor targeting SIgA have been described10, and in NDV3 vaccinated mice, which generate IgA against the adhesion Als3 and have reduced pathology during DSS-induced colitis12. Together, by modulating the C. albicans virulence IgA-dependent immunity favours fungal commensalism and homeostasis at mucosal barriers.
Most antibody-secreting cells (ASCs) in mucosal tissues produce IgA72. Oral mucosal C. albicans colonization increases CD19+ CD138− B cell, plasmablast, and plasma cell enrichment in the mucosa11, while intestinal colonization increases the IgA+ frequency and strong IgA CSR in the PP B cell compartment associated with high-affinity antibody responses10. These IgA responses are mediated through innate immune interaction with intestinal CD11c+CD11b+CD103+ dendritic cells and CX3CR1+ MNPs10. Trafficking of IgA ASCs is regulated by a combination of chemokine receptors as well as integrins46. Recent evidence points at a role for gut-educated IgA ASCs in modulating antifungal immune responses outside of mucosal tissues. During homeostasis, meninges, the membranes surrounding the brain and spinal cord, contain gut-derived and commensal-specific IgA ASCs73. Mice lacking IgA or with a selective loss of meningeal IgA plasma cells exhibit reduced protection against C. albicans and is associated with increased fungal invasion of the brain74.
SIgA can cross-react with a diverse fraction of the microbiota by canonical Fab-dependent and non-canonical carbohydrate-dependent binding75. Oral mucosal colonization with C. albicans increases total levels of cross-specific IgAs against the common oral commensal Streptococcus oralis11 suggesting that fungal colonization and cross-reactive IgA responses shape the microbial community.
B cells have diverse antibody-independent functions, such as production of regulatory and pro-inflammatory cytokines, as well as antigen presentation and T cell stimulation76. Human B cells can present fungal antigens to T cells in an HLA-DR–restricted manner, provide critical costimulatory signals through CD80 and CD86, and induce Th17 cell differentiation through an IL-6-dependent mechanism77, while secretion of IL-6 depends on hyphal stimulation and is mediated by MyD8878.
Collectively, mucosal C. albicans colonization induces a variety of antibody responses, which not only restricts fungal overgrowth, but also limit virulence, locally and systematically (Fig. 1A).
Effects of the adaptive antifungal immunity on the intraspecies diversity of C. albicans
C. albicans displays high intraspecies diversity, notably variations in the distribution of heterozygous polymorphisms along the genome79,80,81. Genetically distinct isolates differ in their phenotype such as the degree of filamentation, expression of virulence factors, the induction of epithelial cell damage or the degree of inflammation-induced at the host interface. These phenotypic variations translate in differential outcomes of the interaction with the host as evidenced when probing genetically distinct isolates in model hosts which exclude any inter-individual differences on the host side prior to infection20,79,82,83,84. From studying large sets of isolates it became clear that the high-virulent strain SC5314, which is broadly used in in vitro and in vivo experimental studies, is a poor colonizer of mucosal surfaces in immunocompetent hosts20,85 and thereby represents rather an outlier than the norm within the species of C. albicans20. Despite striking differences between high- and low-virulent isolates in their efficiency to colonize mucosal surfaces, the adaptive immune response mounted in response to oral colonization is surprisingly comparable with strong T cell and antibody responses induced against widely differing isolates11,20. This is consistent with the observation that all human individuals mount a C. albicans-specific T cell and antibody response, irrespective of the isolate that they are colonized with10,12,13,14. This suggests that C. albicans virulence and immunogenicity are largely uncoupled for T cell priming in the oral mucosa and that antigens, as well as PRR ligands mediating dendritic cell activation for efficient lymphocyte priming, are more strongly conserved between isolates than the expression of virulence factors. PAMP exposure by C. albicans seems to depend on the body site of isolation86. Moreover, because C. albicans actively masks PAMPS in response to its environment to reduce immune recognition87, niche-specific colonization and consequently PAMP exposure may influence immune activation and thereby favour commensalism.
Adaptive immunity can modulate the pathogenicity of C. albicans10,12. Thus, variations in the activity of antibody and/or T cell effector functions over time may generate a spectrum of phenotypes within an individual. If different phenotypes become epigenetically stabilized through DNA methylation88 or genetically fixed over time through (micro)evolutionary processes, this might contribute to the generation of genetic diversity. Indeed, C. albicans also undergoes genomic rearrangement during oral infection of mice89. Growth in the oral mucosa of mice selects for trisomy of chromosome 6, resulting in a commensal-like phenotype90. Importantly, C. albicans within-host diversity was also observed in humans and appears common in the context of commensalism as demonstrated by recent genome sequencing of isolates collected from single healthy individuals, which showed that they differed by numerous single nucleotide polymorphisms and short-range loss-of-heterozygosity (LOH)91. C. albicans genomic and epigenetic variations can facilitate adaptation to environmental changes and improve the persistence of the fungus in various host niches92. When considering inter-individual variations in the host adaptive immune activity, including those arising from to the increasing population of immunocompromised individuals, lineage diversification resulting from the close contact of C. albicans with the human host is an important driver of the genetic diversity observed within the species of.C. albicans79,80,81. Therefore, mutual adaptations of C. albicans and its mammalian host shape the outcome of their interaction both, at an individual’s level as well as at a population scale.
Immunopathological consequences of adaptive immunity against C. albicans
While C. albicans is a common inhabitant of mucosal tissues in healthy individuals, fungal dysbiosis has been associated with diverse human diseases, including inflammatory disorders of colonized tissues such as gut, oral cavity, and skin, as well as pathologies in distant organs, including liver and lung. Whether and how the development and/or progression of pathology is causally linked with the observed shift in mycobiota composition remains often unclear and in many cases, the connection appears to be rather unspecific relying on innate mechanisms. However, several examples have recently emerged, where adaptive immune responses directed against C. albicans have been implicated in disease pathogenesis, comprising both T cell and antibody-mediated scenarios (Fig. 1B).
T cell-mediated immunopathologies
C. albicans (as well as other commensal fungi) are potent inducers of type-17 polarized responses, and IL-17 is implicated in the aetiology of many inflammatory disorders. As such, re-activation of mycobiota-specific Th17 cells can convey host-adverse effects and significantly aggravate tissue inflammation. In an experimental model of psoriasis, epicutaneous association of the murine skin with C. albicans was shown to exacerbates psoriaform skin inflammation in a Th17-dependent manner93, in line with the observed enrichment of Candida-species in lesional skin of psoriasis patients94 and the positive response of defined psoriatic patients to antifungals95. Similar results have been obtained with other skin commensal yeasts and in other models of skin inflammation93,96.
Dysbiosis in Crohn’s disease (CD) patients is characterized by an increase in C. albicans and other Candida species97,98,99,100,101,102. Accordingly, C. albicans-specific Th17 cells are increased in the blood of CD patients14, presumably as a consequence of enhanced microbial translocation in the inflamed and barrier-disrupted gut, and their frequencies correlate with the faecal abundance of C. albicans in intensive care unit patients35. Most recently, the pathogenicity of gut-colonizing C. albicans isolates was identified as a decisive factor for enhanced inflammation in IBD patients103. In light of the harmful rather than protective effects of IL-17 blockade in inflammatory bowel disease (IBD) patients104, the role of C albicans-specific Th17 cells in CD pathogenesis remains controversial. Whether C. albicans-specific T cells acquire pathogenicity in the context of colitis and which factors would drive such functional plasticity remains unclear. Serum amyloid A (SAA) is one factor proposed to promote the development of colitogenic Th17 cells in the gut in a C. albicans-independent context105. Alternatively, C. albicans-specific Th17 may exert host-protective effects in the inflamed gut, presumably via strengthening barrier repair functions106 or by targeting translocated microbes. This notion is supported by the observation that C. albicans can enhance protection from colitis in experimental models, although T cell-dependence of this effect was not elaborated in all cases3,59,107.
C. albicans-specific Th17 cells are not restricted to colonized epithelia but can also be found systemically and in distant organs such as the lung, where C. albicans is not a common resident108. Th17 cells are strongly expanded in the inflamed lung of chronic obstructive pulmonary disease (COPD) and asthma patients, which are frequently sensitized to Aspergillus14. In fact, Th17 cells responding to A. fumigatus also responded to C. albicans14. Thereby, normally protective intestinal Th17 responses mounted against C. albicans are directly linked to lung pathologies caused by airborne fungi, albeit without signs of plasticity towards Th2 as observed in the skin109. While bystander activation via T cell receptor-independent mechanisms cannot be excluded for the dual responsiveness of these cells, cross-reactivity has emerged as a common mechanism for the modulation of immune responses by the microbiota110. Of note, the majority of Th17 cells responsive against widely diverse fungal species are cross-reactive to C. albicans suggesting broad modulation of human anti-fungal Th17 responses by a single fungal species14. Antigen-specificity is a key hallmark of adaptive immunity. Only a few fungal antigens have been identified so far14,15,111. Knowing the antigenic epitopes recognized by antifungal T cells is an important basis for understanding the mechanism that underlies the cross-reactivity of C. albicans-specific T cells. Towards that goal, several protein targets of cross-reactive T cells have been identified in A. fumigatus proteins displaying sequence similarity with their homologues in C. albicans14. Identification of C. albicans epitopes further offers the possibility to track fungal-specific T cells in the endogenous repertoire in an antigen-specific manner by means of MHC tetramers, providing a high-resolution approach to dissect immune responses directed against the mycobiota in health and disease, and it may reveal new targets for diagnostics and therapy.
Antibody-mediated immunopathologies
Antibodies against fungal cell wall components and embedded proteins have been associated with various pathological conditions. Anti-Saccharomyces cerevisiae antibodies (ASCA), which target cell-wall components in Saccharomyces and Candida species were found elevated in the serum (IgG) and intestinal lumen (IgA) of CD patients10,112. High ASCA levels are a consequence of intestinal injury suggesting that ASCA is not an epiphenomenon but is probably due to a cross-reactivity with antigens involved in the immunopathology of CD113. Mannans from other yeasts, such as C. albicans114, can cross-react with ASCA suggesting that other yeast may induce ASCA-associated diseases115. In fact, in a mouse model of dextran sodium sulfate (DSS)-induced colitis C. albicans colonization generates ASCA and promotes inflammation116. In this context, systemic ASCA develops consistently in patients with CD117,118, in whom fungal dysbiosis and overgrowth of Candida species have been described101,119. Furthermore, an increase in Candida abundance and serum ASCA generation was shown in patients with alcohol-associated liver disease120,121. ASCA levels correlated with disease severity and increased mortality122, and were reversed upon 2 weeks of alcohol abstinence120. Similarly, anti-C. albicans antibodies were also elevated in non-alcoholic fatty liver disease patients and correlated with the degree of fibrosis123. Antifungal therapy prevented ethanol-induced liver disease in mice121, although it remained open whether this effect was mediated in an antibody-dependent manner and whether fungal dysbiosis and C. albicans overgrowth increased inflammatory responses independently of ASCA to promote pathological conditions.
In autoimmune diseases, autoantibodies (auto-Abs) often develop before any clinical symptoms can be detected124. While fungal dysbiosis has not been associated with the generation of auto-Abs, antibodies against cytokines and chemokines involved in mucosal anti-fungal immunity were proposed to predispose for C. albicans outgrowth and infection125. High antibodies titers against IL-17A, IL-17F, and IL-22 can be found in patients with CMC and autoimmune polyendocrine syndrome type I126,127. However, some patients with persistent IL-17 auto-Abs lack CMC, and some patients with CMC lack auto-Abs128,129 suggesting the existence of CMC subtypes across a spectrum of impaired type 17 immunity, whereby in patients with an intact IL-17 pathway CMC was shown to develop as a consequence of immunopathology caused by excessive type 1 inflammation34,130.
Vaccines against C. albicans
Vaccines are among the most successful public health interventions131 resting on the principle of immune memory, whereby a secondary challenge induces an enhanced immune response against a previously encountered pathogen. The fact that C. albicans has coevolved with humans for at least 2,000 years132, that this fungus is a lifelong inhabitant of mucosal surfaces133, and every colonized individual harbour a memory response directed against C. albicans10,12,13,14, presents a number of conceptual and technical challenges in the development of an anti-C. albicans vaccine. Furthermore, rather than acquisition, C. albicans infections result from translocation or fungal outgrowth6,134,135 implying that anti-C. albicans vaccines should target specific virulence factors to diminish pathogenic attributes. The use of the N-terminal region of C. albicans agglutinin like sequence 3 protein (Als3) is the most promising and advanced vaccination strategy yet tested. This vaccine, referred to as NDV-3, induces T cell136, IgG, and IgA12,137 responses in mice and humans. NDV-3 vaccinated mice are protected against systemic and mucosal C. albicans infection136,138,139,140, while inducing cross-reactive protective responses against C. auris and Staphylococcus aureus in mice136,141. Furthermore, NDV-3 vaccination prevents C. albicans-associated damage in mice with colitis by inducing fungal adhesin-specific IgA responses12. Importantly, NDV-3 has been shown to be safe and efficacious in a clinical trial against recurrent vulvovaginal candidiasis142. A different study uses the recombinant C. albicans secreted aspartyl proteinase 2 (Sap2) protein143. Sap2 plays an important role in fungal pathogenesis by degrading host proteins at mucosal sites and inactivate complement components144,145,146. Vaccination with Sap2 results in the generation of antigen specific protective antibodies147, which cross-react with different members of the Sap family148. These strategies demonstrate that vaccination against C. albicans virulence factors not only protects against this fungus itself, thus, preventing pathology at different body sites, but also provide protection against other fungi, and even bacteria. As a result, research into the production of universal fungal vaccines have gained traction in the scientific community, which may not only aid in the discovery of novel fungal vaccine candidates from unexpected and distant species but may also aid in repurposing of some of the effective vaccines used against other pathogens.
Concluding statement
Informed by mouse studies and fuelled by new techniques, such as single cell RNA sequencing and spatial omics149,150, human immunological research is advancing disease treatment while also generating insights into basic immunological concepts151. This has led to exciting discoveries regarding the relevance and role of adaptive immunity in immuosurveillance of C. albicans commensalism, but also revealed pathological consequences that these responses can have in predisposed patients. However, most work to decipher human immune responses to C. albicans has been (understandably) limited to blood. Recently, Lionakis and colleagues used oral mucosal biopsies of APECED patients with CMC to show that tissue-specific immunity is not always reflected by immune responses of circulating cells34. Thus, studying tissues directly will aid our understanding of tissue-resident cell types (such as resident memory lymphocytes and tissue-specific stroma) and inform of tissue-specific immune mechanisms driving or preventing pathologies. Accordingly, studying the diverse C. albicans-mediated pathologies requires usage of relevant mouse models. For instance, C. albicans infections and immune responses should be studied in colonized animals rather than naïve and acutely infected mice. In this line, commensal isolates, which are able to colonize fully immunocompetent animals20,84 should be used to closely mimic scenarios seen in patients.
References
Gouba, N. & Drancourt, M. Digestive tract mycobiota: a source of infection. Med. et. maladies infectieuses 45, 9–16 (2015).
Underhill, D. M. & Pearlman, E. Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity 43, 845–858 (2015).
Wheeler, M. L. et al. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe 19, 865–873 (2016).
Iliev, I. D. & Leonardi, I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 17, 635–646 (2017).
Revankar S. G., Sobel J. D. Mucosal Candidiasis. In: Calderone R. A., Clancy C. J. (eds.) Candida and Candidiasis, 2nd edn. ASM Press.
Pappas, P. G., Lionakis, M. S., Arendrup, M. C., Ostrosky-Zeichner, L. & Kullberg, B. J. Invasive candidiasis. Nat. Rev. Dis. Prim. 4, 18026 (2018).
Li, X. V., Leonardi, I. & Iliev, I. D. Gut mycobiota in immunity and inflammatory disease. Immunity 50, 1365–1379 (2019).
Lionakis, M. S. & Levitz, S. M. Host control of fungal infections: lessons from basic studies and human cohorts. Annu. Rev. Immunol. 36, 157–191 (2018).
Sparber, F. & LeibundGut-Landmann, S. Interleukin-17 in antifungal immunity. Pathogens 8, 54 (2019).
Doron, I. et al. Mycobiota-induced IgA antibodies regulate fungal commensalism in the gut and are dysregulated in Crohn’s disease. Nat. Microbiol. 6, 1493–1504 (2021).
Millet, N., Solis, N. V. & Swidergall, M. Mucosal IgA prevents commensal candida albicans dysbiosis in the oral cavity. Front. Immunol. 11, 555363 (2020).
Ost, K. S. et al. Adaptive immunity induces mutualism between commensal eukaryotes. Nature 596, 114–118 (2021).
Acosta-Rodriguez, E. V. et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8, 639–646 (2007).
Bacher, P. et al. Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against Candida albicans. Cell 176, 1340–1355 e1315 (2019).
Becattini, S. et al. T cell immunity. Functional heterogeneity of human memory CD4(+) T cell clones primed by pathogens or vaccines. Science 347, 400–406 (2015).
Park, C. O. et al. Staged development of long-lived T-cell receptor alphabeta TH17 resident memory T-cell population to Candida albicans after skin infection. J. Allergy Clin. Immunol. 142, 647–662 (2018).
Conti, H. R. et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 206, 299–311 (2009).
Kirchner, F. R. & LeibundGut-Landmann, S. Tissue-resident memory Th17 cells maintain stable fungal commensalism in the oral mucosa. Mucosal Immunol. 14, 455–467 (2021).
Gladiator, A., Wangler, N., Trautwein-Weidner, K. & LeibundGut-Landmann, S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J. Immunol. 190, 521–525 (2013).
Schonherr, F. A. et al. The intraspecies diversity of C. albicans triggers qualitatively and temporally distinct host responses that determine the balance between commensalism and pathogenicity. Mucosal Immunol. 10, 1335–1350 (2017).
Verma, A. H. et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci. Immunol. 2, eaam8834 (2017).
Kashem, S. W. et al. Nociceptive sensory fibers drive Interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 43, 515–526 (2015).
Sparber, F. et al. Langerin+ DCs regulate innate IL-17 production in the oral mucosa during Candida albicans-mediated infection. PLoS Pathog. 14, e1007069 (2018).
Kashem, S. W. et al. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity 42, 356–366 (2015).
Igyarto, B. Z. et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272 (2011).
Trautwein-Weidner, K. et al. Antigen-specific Th17 cells are primed by distinct and complementary dendritic cell subsets in oropharyngeal candidiasis. PLoS Pathog. 11, e1005164 (2015).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Ansaldo, E., Farley, T. K. & Belkaid, Y. Control of immunity by the microbiota. Annu. Rev. Immunol. 39, 449–479 (2021).
Omenetti, S. et al. The intestine harbors functionally distinct homeostatic tissue-resident and inflammatory Th17 cells. Immunity 51, 77–89.e76 (2019).
Swarnalekha, N. et al. T resident helper cells promote humoral responses in the lung. Sci. Immunol. 6, eabb6808 (2021).
Tamoutounour, S. et al. Keratinocyte-intrinsic MHCII expression controls microbiota-induced Th1 cell responses. Proc. Natl. Acad. Sci. USA 116, 23643–23652 (2019).
Leonardi, I. et al. Mucosal fungi promote gut barrier function and social behavior via Type 17 immunity. Cell 185, 831–846 e814 (2022).
Markey, L. et al. Pre-colonization with the commensal fungus Candida albicans reduces murine susceptibility to Clostridium difficile infection. Gut Microbes 9, 497–509 (2018).
Break, T. J. et al. Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. Science 371, eaay5731 (2021).
Shao, T. Y. et al. Commensal Candida albicans Positively Calibrates Systemic Th17 Immunological Responses. Cell Host Microbe 25, 404–417.e406 (2019).
Tso, G. H. W. et al. Experimental evolution of a fungal pathogen into a gut symbiont. Science 362, 589–595 (2018).
Chen, C. et al. IL-17 is a neuromodulator of Caenorhabditis elegans sensory responses. Nature 542, 43–48 (2017).
Reed, M. D. et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 577, 249–253 (2020).
Choi, G. B. et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016).
Alves de Lima, K. et al. Meningeal gammadelta T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol. 21, 1421–1429 (2020).
Strati, F. et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5, 24 (2017).
Zou, R. et al. Dysbiosis of gut fungal microbiota in children with autism spectrum disorders. J. Autism Dev. Disord. 51, 267–275 (2021).
Kirchner, F. R. et al. Persistence of Candida albicans in the oral mucosa induces a curbed inflammatory host response that is independent of immunosuppression. Front Immunol. 10, 330 (2019).
Huppler, A. R. et al. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J. Immunol. 192, 1745–1752 (2014).
Trautwein-Weidner, K., Gladiator, A., Nur, S., Diethelm, P. & LeibundGut-Landmann, S. IL-17-mediated antifungal defense in the oral mucosa is independent of neutrophils. Mucosal Immunol. 8, 221–231 (2015).
Keppler, S. J., Goess, M. C. & Heinze, J. M. The wanderings of gut-derived iga plasma cells: impact on systemic immune responses. Front Immunol. 12, 670290 (2021).
Dimitrov, J. D. & Lacroix-Desmazes, S. Noncanonical functions of antibodies. Trends Immunol. 41, 379–393 (2020).
Janeway, C. Immunobiology 5: the immune system in health and disease. (Garland Pub., New York, 2001).
Rollenske, T. & Macpherson, A. J. Anti-commensal Ig—from enormous diversity to clear function. Mucosal Immunol. 13, 1–2 (2020).
Sterlin, D., Fadlallah, J., Slack, E. & Gorochov, G. The antibody/microbiota interface in health and disease. Mucosal Immunol. 13, 3–11 (2020).
Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 11, 275–288 (2011).
Lionakis, M. S., Iliev, I. D. & Hohl, T. M. Immunity against fungi. JCI Insight 2, 93156 (2017).
Swidergall, M., Solis, N. V., Lionakis, M. S. & Filler, S. G. EphA2 is an epithelial cell pattern recognition receptor for fungal β-glucans. Nat. Microbiol. 3, 53–61 (2018).
Swidergall, M. et al. EphA2 is a neutrophil receptor for Candida albicans that stimulates antifungal activity during oropharyngeal infection. Cell Rep. 28, 423–433.e425 (2019).
López-Ribot, J. L., Casanova, M., Murgui, A. & Martínez, J. P. Antibody response to Candida albicans cell wall antigens. FEMS Immunol. Med. Microbiol. 41, 187–196 (2004).
Roco, J. A. et al. Class-switch recombination occurs infrequently in germinal centers. Immunity 51, 337–350.e337 (2019).
Stavnezer, J., Guikema, J. E. & Schrader, C. E. Mechanism and regulation of class switch recombination. Annu Rev. Immunol. 26, 261–292 (2008).
Doron, I. et al. Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies. Cell 184, 1017–1031.e1014 (2021).
Leonardi, I. et al. CX3CR1(+) mononuclear phagocytes control immunity to intestinal fungi. Science 359, 232–236 (2018).
Luo, P., Yang, Z., Chen, B. & Zhong, X. The multifaceted role of CARD9 in inflammatory bowel disease. J. Cell. Mol. Med. 24, 34–39 (2020).
Yamamoto-Furusho, J. K. et al. Caspase recruitment domain (CARD) family (CARD9, CARD10, CARD11, CARD14 and CARD15) are increased during active inflammation in patients with inflammatory bowel disease. J. Inflamm. 15, 13 (2018).
Brand, S. et al. Increased expression of the chemokine fractalkine in Crohn’s disease and association of the fractalkine receptor T280M polymorphism with a fibrostenosing disease Phenotype. Am. J. Gastroenterol. 101, 99–106 (2006).
Sabate, J. M. et al. The V249I polymorphism of the CX3CR1 gene is associated with fibrostenotic disease behavior in patients with Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 20, 748–755 (2008).
Drummond, R. A. et al. CARD9-dependent neutrophil recruitment protects against fungal invasion of the central nervous system. PLoS Pathog. 11, e1005293 (2015).
Drummond, R. A. et al. CARD9(+) microglia promote antifungal immunity via IL-1β- and CXCL1-mediated neutrophil recruitment. Nat. Immunol. 20, 559–570 (2019).
Damelang, T., Rogerson, S. J., Kent, S. J. & Chung, A. W. Role of IgG3 in infectious diseases. Trends Immunol. 40, 197–211 (2019).
Li, Y., Jin, L. & Chen, T. The effects of secretory IgA in the mucosal immune system. Biomed. Res Int. 2020, 2032057 (2020).
Mantis, N. J., Rol, N. & Corthesy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4, 603–611 (2011).
Mathias, A., Pais, B., Favre, L., Benyacoub, J. & Corthésy, B. Role of secretory IgA in the mucosal sensing of commensal bacteria. Gut microbes 5, 688–695 (2014).
Moor, K. et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature 544, 498–502 (2017).
Wich, M. et al. Functionality of the human antibody response to Candida albicans. Virulence 12, 3137–3148 (2021).
Mora, J. R. & von Andrian, U. H. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 1, 96–109 (2008).
Hepworth, M. R., Greenhalgh, A. D. & Cook, P. C. B cells on the brain: meningeal IgA and a novel gut-brain firewall. Immunol. Cell Biol. 99, 17–20 (2021).
Fitzpatrick, Z. et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature 587, 472–476 (2020).
Pabst, O. & Slack, E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 13, 12–21 (2020).
Fillatreau, S. B cells and their cytokine activities implications in human diseases. Clin. Immunol. 186, 26–31 (2018).
Li, R. et al. Antibody-independent function of human B cells contributes to antifungal T cell responses. J. Immunol. 198, 3245–3254 (2017).
Ferreira-Gomes, M. et al. B cell recognition of candida albicans hyphae via TLR 2 promotes IgG1 and IL-6 secretion for T(H)17 differentiation. Front Immunol. 12, 698849 (2021).
Hirakawa, M. P. et al. Genetic and phenotypic intra-species variation in Candida albicans. Genome Res. 25, 413–425 (2015).
Ropars, J. et al. Gene flow contributes to diversification of the major fungal pathogen Candida albicans. Nat. Commun. 9, 2253 (2018).
Wang, J. M., Bennett, R. J. & Anderson, M. Z. The genome of the human pathogen Candida albicans is shaped by mutation and cryptic sexual recombination. mBio 9, e01205–e01218 (2018).
MacCallum, D. M. et al. Property differences among the four major Candida albicans strain clades. Eukaryot. Cell 8, 373–387 (2009).
Marakalala, M. J. et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog. 9, e1003315 (2013).
McDonough, L. D. et al. Candida albicans Isolates 529L and CHN1 Exhibit Stable Colonization of the Murine Gastrointestinal Tract. mBio 12, e0287821 (2021).
Fan, D. et al. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat. Med. 21, 808–814 (2015).
Gerwien, F. et al. Clinical Candida albicans vaginal isolates and a laboratory strain show divergent behaviors during macrophage interactions. mSphere 5, e00393–00320 (2020).
Ballou, E. R. et al. Lactate signalling regulates fungal beta-glucan masking and immune evasion. Nat. Microbiol. 2, 16238 (2016).
Mishra, P. K., Baum, M. & Carbon, J. DNA methylation regulates phenotype-dependent transcriptional activity in Candida albicans. Proc. Natl. Acad. Sci. USA 108, 11965–11970 (2011).
Forche, A. et al. Rapid phenotypic and genotypic diversification after exposure to the oral host niche in Candida albicans. Genetics 209, 725–741 (2018).
Forche, A. et al. Selection of Candida albicans trisomy during oropharyngeal infection results in a commensal-like phenotype. PLoS Genet. 15, e1008137 (2019).
Sitterle, E. et al. Within-host genomic diversity of Candida albicans in healthy carriers. Sci. Rep. 9, 2563 (2019).
Braunsdorf, C. & LeibundGut-Landmann, S. Modulation of the fungal-host interaction by the intra-species diversity of C. albicans. Pathogens 7, 11 (2018).
Hurabielle, C. et al. Immunity to commensal skin fungi promotes psoriasiform skin inflammation. Proc. Natl. Acad. Sci. USA 117, 16465–16474 (2020).
Ovcina-Kurtovic, N., Kasumagic-Halilovic, E., Helppikangans, H. & Begic, J. Prevalence of Candida species in patients with psoriasis. Acta Dermatovenerol. Croat. 24, 209–213 (2016).
Crutcher, N. et al. Oral nystatin in the treatment of psoriasis. Arch. Dermatol. 120, 435–436 (1984).
Sparber, F. et al. The Skin Commensal Yeast Malassezia triggers a Type 17 response that coordinates anti-fungal immunity and exacerbates skin inflammation. Cell Host Microbe 25, 389–403.e386 (2019).
Chehoud, C. et al. Fungal signature in the gut microbiota of pediatric patients with inflammatory bowel disease. Inflamm. Bowel Dis. 21, 1948–1956 (2015).
Hoarau, G. et al. Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial Crohn’s disease. mBio 7, e01250–01216 (2016).
Li, Q. et al. Dysbiosis of gut fungal microbiota is associated with mucosal inflammation in Crohn’s disease. J. Clin. Gastroenterol. 48, 513–523 (2014).
Liguori, G. et al. Fungal dysbiosis in mucosa-associated microbiota of Crohn’s disease patients. J. Crohns Colitis 10, 296–305 (2016).
Sokol, H. et al. Fungal microbiota dysbiosis in IBD. Gut 66, 1039–1048 (2017).
Standaert-Vitse, A. et al. Candida albicans colonization and ASCA in familial Crohn’s disease. Am. J. Gastroenterol. 104, 1745–1753 (2009).
Li, X. V. et al. Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature 603, 672–678 (2022).
Fauny, M. et al. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann. Rheum. Dis. 79, 1132–1138 (2020).
Lee, J. Y. et al. Serum Amyloid A proteins induce pathogenic Th17 cells and promote inflammatory disease. Cell 180, 79–91.e16 (2020).
Kumar, P. et al. Intestinal Interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity 44, 659–671 (2016).
Jiang, T. T. et al. Commensal fungi recapitulate the protective benefits of intestinal bacteria. Cell Host Microbe 22, 809–816.e804 (2017).
Nguyen, L. D., Viscogliosi, E. & Delhaes, L. The lung mycobiome: an emerging field of the human respiratory microbiome. Front Microbiol 6, 89 (2015).
Harrison, O. J. et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 2019; 363(6422).
Scheffold, A., Bacher, P. & LeibundGut-Landmann, S. T cell immunity to commensal fungi. Curr. Opin. Microbiol. 58, 116–123 (2020).
Bar, E. et al. A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J. Immunol. 188, 5636–5643 (2012).
McKenzie, H., Main, J., Pennington, C. R. & Parratt, D. Antibody to selected strains of Saccharomyces cerevisiae (baker’s and brewer’s yeast) and Candida albicans in Crohn’s disease. Gut 31, 536–538 (1990).
Vermeire, S. et al. Anti-Saccharomyces cerevisiae antibodies (ASCA), phenotypes of IBD, and intestinal permeability: a study in IBD families. Inflamm. Bowel Dis. 7, 8–15 (2001).
Standaert-Vitse, A. et al. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn’s disease. Gastroenterology 130, 1764–1775 (2006).
Schaffer, T. et al. Anti-Saccharomyces cerevisiae mannan antibodies (ASCA) of Crohn’s patients crossreact with mannan from other yeast strains, and murine ASCA IgM can be experimentally induced with Candida albicans. Inflamm. Bowel Dis. 13, 1339–1346 (2007).
Jawhara, S. et al. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through Galectin-3. J. Infect. Dis. 197, 972–980 (2008).
Limon, J. J. et al. Malassezia is associated with Crohn’s disease and exacerbates colitis in mouse models. Cell Host Microbe 25, 377–388.e376 (2019).
Israeli, E. et al. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut 54, 1232–1236 (2005).
Lewis, J. D. et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host Microbe 18, 489–500 (2015).
Hartmann, P. et al. Dynamic changes of the fungal microbiome in alcohol use disorder. Front Physiol. 12, 699253 (2021).
Yang, A. M. et al. Intestinal fungi contribute to development of alcoholic liver disease. J. Clin. Invest. 127, 2829–2841 (2017).
Lang, S. et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology 71, 522–538 (2020).
Demir, M. et al. The fecal mycobiome in non-alcoholic fatty liver disease. J. Hepatol. 76, 788–799 (2021).
Horst, A. K., Kumashie, K. G., Neumann, K., Diehl, L. & Tiegs, G. Antigen presentation, autoantibody production, and therapeutic targets in autoimmune liver disease. Cell Mol. Immunol. 18, 92–111 (2021).
Browne, S. K. & Holland, S. M. Anti-cytokine autoantibodies explain some chronic mucocutaneous candidiasis. Immunol. Cell Biol. 88, 614–615 (2010).
Kisand, K. et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 207, 299–308 (2010).
Puel, A. et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med 207, 291–297 (2010).
Ferre, E. M. et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight 1, e88782 (2016).
Orlova, E. M. et al. Expanding the phenotypic and genotypic landscape of autoimmune polyendocrine Syndrome Type 1. J. Clin. Endocrinol. Metab. 102, 3546–3556 (2017).
Break, T. J. et al. Response to comments on “Aberrant type 1 immunity drives susceptibility to mucosal fungal infections”. Science 373, eabi8835 (2021).
Doherty, M., Buchy, P., Standaert, B., Giaquinto, C. & Prado-Cohrs, D. Vaccine impact: Benefits for human health. Vaccine 34, 6707–6714 (2016).
Bougnoux, M. E. et al. Multilocus sequence typing reveals intrafamilial transmission and microevolutions of Candida albicans isolates from the human digestive tract. J. Clin. Microbiol 44, 1810–1820 (2006).
Tso, G. H. W., Reales-Calderon, J. A. & Pavelka, N. The elusive Anti-Candida Vaccine: lessons from the past and opportunities for the future. Front Immunol. 9, 897 (2018).
Kullberg, B. J. & Arendrup, M. C. Invasive Candidiasis. N. Engl. J. Med 373, 1445–1456 (2015).
Swidergall, M. & Filler, S. G. Oropharyngeal Candidiasis: fungal invasion and epithelial cell responses. PLoS Pathog. 13, e1006056 (2017).
Lin, L. et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 5, e1000703 (2009).
Schmidt, C. S. et al. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 30, 7594–7600 (2012).
Alqarihi, A., Singh, S., Edwards, J. E. Jr., Ibrahim, A. S. & Uppuluri, P. NDV-3A vaccination prevents C. albicans colonization of jugular vein catheters in mice. Sci. Rep. 9, 6194 (2019).
Ibrahim, A. S. et al. NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine 31, 5549–5556 (2013).
Spellberg, B. J. et al. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J. Infect. Dis. 194, 256–260 (2006).
Singh, S. et al. The NDV-3A vaccine protects mice from multidrug resistant Candida auris infection. PLoS Pathog. 15, e1007460 (2019).
Edwards, J. E. Jr. et al. A fungal immunotherapeutic vaccine (NDV-3A) for treatment of recurrent vulvovaginal Candidiasis-A Phase 2 randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 66, 1928–1936 (2018).
Cassone, A., Boccanera, M., Adriani, D., Santoni, G. & De Bernardis, F. Rats clearing a vaginal infection by Candida albicans acquire specific, antibody-mediated resistance to vaginal reinfection. Infect. Immun. 63, 2619–2624 (1995).
Schaller, M. et al. The secreted aspartyl proteinases Sap1 and Sap2 cause tissue damage in an in vitro model of vaginal candidiasis based on reconstituted human vaginal epithelium. Infect. Immun. 71, 3227–3234 (2003).
Gropp, K. et al. The yeast Candida albicans evades human complement attack by secretion of aspartic proteases. Mol. Immunol. 47, 465–475 (2009).
Svoboda, E. et al. Secreted aspartic protease 2 of Candida albicans inactivates factor H and the macrophage factor H-receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18). Immunol. Lett. 168, 13–21 (2015).
De Bernardis, F. et al. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 65, 3399–3405 (1997).
Shukla, M., Chandley, P. & Rohatgi, S. The Role of B-cells and antibodies against Candida vaccine antigens in invasive candidiasis. Vaccines 9, 1159 (2021).
Huang, W., Wang, D. & Yao, Y. F. Understanding the pathogenesis of infectious diseases by single-cell RNA sequencing. Micro. Cell 8, 208–222 (2021).
Butler, D. et al. Shotgun transcriptome, spatial omics, and isothermal profiling of SARS-CoV-2 infection reveals unique host responses, viral diversification, and drug interactions. Nat. Commun. 12, 1660 (2021).
Varade, J., Magadan, S. & Gonzalez-Fernandez, A. Human immunology and immunotherapy: main achievements and challenges. Cell Mol. Immunol. 18, 805–828 (2021).
Acknowledgements
The authors would like to thank members of their labs for discussions. Research in the LeibundGut-lab is funded by Swiss National Science Foundation (grant # CRSII5_173863, #310030_189255) and the University of Zürich. Research in the Swidergall-lab is supported by NIH grant R01DE031382, R00DE026856, an American Association of Immunologists Careers in Immunology Fellowship, and a TLI grant. The figure was generated using BioRender.
Funding
Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the preparation of this review article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Swidergall, M., LeibundGut-Landmann, S. Immunosurveillance of Candida albicans commensalism by the adaptive immune system. Mucosal Immunol 15, 829–836 (2022). https://doi.org/10.1038/s41385-022-00536-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-022-00536-5
This article is cited by
-
The hyphal-specific toxin candidalysin promotes fungal gut commensalism
Nature (2024)
-
Immune responses to human fungal pathogens and therapeutic prospects
Nature Reviews Immunology (2023)
-
Microbial underdogs: exploring the significance of low-abundance commensals in host-microbe interactions
Experimental & Molecular Medicine (2023)
-
The Role of Host and Fungal Factors in the Commensal-to-Pathogen Transition of Candida albicans
Current Clinical Microbiology Reports (2023)
-
IL-23 signaling prevents ferroptosis-driven renal immunopathology during candidiasis
Nature Communications (2022)