Abstract
A characteristic feature of host responses to helminth infections is the development of profound systemic and tissue-localised Type 2 immune responses that play critical roles in immunity, tissue repair and tolerance of the parasite at tissue sites. These same Type 2 responses are also seen in the tissue-associated immune-pathologies seen in asthma, atopic dermatitis and many forms of allergies. The recent identification of new subtypes of immune cells and cytokine pathways that influence both immune and non-immune cells and tissues creates the opportunity for reviewing helminth parasite–host responses in the context of tissue specific immunity. This review focuses on the new discoveries of the cells and cytokines involved in tissue specific immune responses to helminths and how these contribute to host immunity against helminth infection and allow the host to accommodate the presence of parasites when they cannot be eliminated.
Similar content being viewed by others
Introduction
The Type 2 immune response is the product of a special evolutionary relationship between a host and its parasites
Over millions of years, the selective evolutionary pressures of infectious agents, sex, lifespan and diet has shaped the immune system of many mammalian species1. For the challenges faced by each species the immune inflammatory responses need to be efficient in removing damaging pathogens, but they need to be tightly regulated to avoid immune pathology of vital tissues associated with feeding and reproduction2,3. In this review, we focus on how new knowledge of helminth induced immune responses can be applied to the tissue specific host immune responses that are provoked by the invading parasite and that are relevant to the acquisition of immunity and ongoing immune pathology4. The most common parasites of humans are soil-transmitted helminths (STH) including whipworms, roundworms, and hookworms. Reportedly, one billion people are infected worldwide, mainly in countries with tropical and subtropical climates where people are in regular contact with infective larvae in the environment5. Once they have established themselves in the host, adult STHs reside in the gastrointestinal tract, in intimate contact with the mucosal surfaces of the gut. Infection can often last for many years, with the presence of adult worms being well tolerated by the host immune cells at mucosal sites and there being very little effect on host microbiome, food uptake, reproduction and response to invading pathogens5,6,7. Irrespective of the STH species, a defining characteristic of parasitic infection is that they preferentially stimulate strong systemic and local type 2 immune responses. The potential benefit of these Type 2 responses to host immunity was first revealed in in vitro experiments demonstrating the parasiticidal properties of eosinophils and marking the lineage as one of the key Type 2 effector cell types that confer immunity against the parasite8,9. Further studies using murine models of helminth infections have led to the discovery of other novel immune cells such as type 2 innate lymphoid cells (ILC2s) that are an innate source of the cytokines IL-5 and IL-13 and play a role in expanding and activating eosinophils and inducing mucus secreting goblet cell hyperplasia10,11,12. Furthermore, recent experimental studies of chronic helminth infections have revealed the function of tuft cells in the gastric epithelium13,14, and the importance of T regulatory cells (TREG)15,16,17 and IL-10-producing B regulatory cells (BREG)18 in anti-helminth immunity.
The host immune responses induced by helminth infection
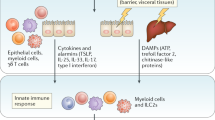
The host Type 2 immune responses stimulated by invading helminths is thought to be regulated by a complex interplay between epithelial cells, eosinophils, basophils, mast cells, ILC2s, T helper type 2 cells (TH2) and effector cytokines such as IL-3, IL-4, IL-5, IL-9, IL-13, and IgE antibodies19. Blood eosinophilia is a hallmark feature of parasitic infections, especially for parasites that migrate through multiple tissues20. Type 2 immune responses are thought to be necessary for parasite killing and clearance, and to prevent detrimental damage to the host3, with mounting evidence that type 2 immune responses are also required for returning damaged tissues to normal physiological function. ILC2 activation is thought to be required in early life for generating anti-inflammatory M2 macrophages and contributing to homeostasis21. Additionally, type 2 immune responses have been shown to be involved in thermogenesis22, and recently IL-13 has been identified as a driving factor for the skin dendritic cell subsets involved in inducing TH2 development in the lymph node23. Investigations of parasite induced responses have also revealed how the release of the so called alarmin cytokines such as IL-25, IL-33, and Thymic Stromal Lymphopoietin (TSLP) from damaged tissues that can act alone or together with other cytokines (e.g. IL-2, IL-9), lipid mediators, hormones, and neurotransmitters to eliminate the parasite and resolve the damage caused by infection19,24,25,26,27,28,29,30,31. The cytokines IL-4, IL-13, IL-3, IL-5, and IL-9 are considered key to helminth induced type 2 immune responses, which together with CD4 T cells, ILC2s, eosinophils, basophils and mast cells form a conserved mechanism that leads to expulsion of helminth species often referred to as the “weep and sweep” response32,33,34.
The potential for gut dwelling parasites to affect host physiology and behavior
The gut is well recognized in human physiology as an immune organ that is key to regulating the health of the whole body. This point is clearly observed with the higher incidence of skin diseases appearing in patients suffering from gut inflammatory conditions such as coeliac disease, Crohn’s disease, and ulcerative colitis35. Also, there is emerging evidence for a gut-brain axis associated with the regulation of host metabolism, neurodevelopmental disorders, neuroinflammation, and stress levels36,37,38,39. Investigations of the molecules secreted by gut dwelling adult parasites have revealed they can bind host cell receptors and mediate functional host responses, including induction of TREG cells, altering DC activity or blocking initiation of type 2 immune responses40,41,42,43,44.
Experimental vs natural models for investigating tissue specific Type 2 immune responses to helminths
Several murine based parasite infection models have been used to explore how the helminth infected host responds immunologically to tissue migrating helminth parasites and how in situations of chronic infection the host is made tolerant, or becomes tolerant, to the presence of parasites in the body. The most commonly used parasites have included Nippostrongylus brasiliensis and Heligmosomoides polygyrus. N. brasiliensis follows the life cycle of skin penetration, tissue migration to the lung, gut colonization and egg reproduction before expulsion by the host45 (Fig. 1). By contrast the strictly enteric H. polygyrus models chronic helminth infection by establishing itself in the small intestine and persisting for several months in the rodent host45,46. Similarly, Strongyloides ratti and Strongyloides venezuelensis, which transit through the lungs before reaching the gut, have been used to model the human parasite Strongyloides stercoralis. Although these parasites do not establish a chronic infection in mouse experimental models, they have given good insights into host-parasite interaction and revealed the cellular and cytokine effector mechanisms involved in parasite tolerance, immunity and eradication47. Other parasites like Trichuris muris have been used to understand T cell activation and tissue changes in the intestinal epithelium at the colonic boundary seen in human whipworm infection48,49, while Schistosoma infection has provided information on the role of dendritic cells in inducing TH2 immunity50,51. Recently, work using the H. polygyrus chronic infection model has enabled the molecular identification and characterization of some of the biologically active molecules secreted by adult worms that bind with high fidelity cytokine receptors and work to regulate the host immune system to enable chronic infection52.
Infective larvae (iL3) survive in warm humid environments. Upon contact with the human host, they penetrate the skin (1), migrate through the circulatory vasculature to the lung (2), where they molt, and are carried up by the trachea by the host muco-ciliary ladder and swallowed. Larvae are retained in the small intestine (3) where they develop to the adult stage, mate, and produce eggs. Eggs are released with the feces into the environment, they hatch, develop through three stages to the infective L3. This review will focus on the immune responses elicited in the skin, lungs and intestine by this and related parasites.
In real world settings, helminth infections have often been associated with impaired immune responses in humans and animals against mycobacteria53, intradermal BCG vaccine54 and tetanus vaccine55. However, in experimental co-infection models, helminths have also been shown to have potential protective role against secondary infection with Influenza A and pneumonia virus of mice (PVM), and protective effect against primary infection with respiratory syncytial virus (RSV) and murid gamma herpes virus 4 (muHV-4)56,57,58,59. Furthermore, in countries where helminth infections are still endemic, a lower incidence of severe atopic diseases has been observed due to the possible induction by the parasitic worm of Type 2 linked regulatory mechanisms60,61,62,63,64. These parasite-induced regulatory mechanisms have been recently associated with decreased severity of COVID-19 in a cohort of African patients65. In support of these observations, a chronic infection model using H. polygyrus showed reduced inflammation in a model of asthma66, experimental autoimmune encephalomyelitis (EAE)67 and contact hypersensitivity68. These findings have supported the proposal that helminth parasites could have a beneficial effect in regulating host inflammatory responses and have led to clinical trials using live parasites to treat conditions like Crohn’s disease, multiple sclerosis, asthma, and celiac disease69. In humans the complexity of the immune system in conjunction with multiple parasitic infections and presence of co-morbidities make the relationship between asthma and helminth infections difficult to untangle. For instance, Ascaris lumbricoides, a roundworm that migrates through the lung, has been identified as a risk factor for asthma and atopic diseases which is opposite to the situation with N. americanus infection, which with a similar life cycle, but which has a negative association with asthma70,71,72,73. Furthermore, helminth-endemic areas have the highest incidence of malaria, HIV and tuberculosis indicating potential helminth induced impairment on the host immune defenses against viruses, bacteria and other parasites74. Certainly, in co-infection models increased mortality following challenge with West Nile virus75 or virus reactivation with γ-herpesvirus76 has been shown. In summary, the mechanisms by which helminth parasites can modulate immune responses at distal tissue and mucosal sites is still largely unidentified, making the field of parasite immunomodulation a productive area for future research endeavour.
Helminth infection induced immune responses in the skin
For many helminths the skin is the first barrier they have to breach to gain entry to the host with several types of skin immune cells reported to confer some protection against invasion and maintenance of barrier integrity at the skin surface77. However, to date natural skin infection studies have proven difficult to model with one of the most commonly used models of helminth infection N. brasiliensis parasites bypassing the skin penetration phase of the parasite with subcutaneous injection. Experiments using percutaneous administration of N. brasiliensis larvae have demonstrated recruitment of neutrophils and eosinophils to areas of parasite penetration with recent elegant studies showing that larvae can sense the presence of inflammatory cells in the skin remaining longer in their protective sheaths before travelling more quickly to the lung once ex-sheathed78. In other studies innate immune cells such as neutrophils and eosinophils have been shown to be required for immobilization and killing of S. ratti larvae in the skin and infected tissues79, while S. stercoralis induces neutrophils extracellular traps (NETs) in vivo that trap and kill the parasite80.
Helminth induced eosinophil responses in the skin
The presence of high numbers of eosinophils in blood and tissues is considered a hallmark of parasite infection and blood eosinophilia is clinically used as a diagnostic marker of parasite infection in humans20. Eosinophils are bone-marrow-derived cells that are recruited to the site of infection by IL-5 and eotaxin and can be specifically identified by the chemical staining of their cytoplasmic granules. Granules are rich in cationic proteins, RNAses and antimicrobial agents and for this reason, eosinophils have been studied as effector cells in human diseases and parasitic infection81. Interestingly the eosinophils that infiltrate the skin after N. brasiliensis injection show evidence of increased degranulation, and being associated with the larvae that remain trapped in the skin and having a clear role in reducing the number of larvae reaching the lungs82. Although a lower number of parasites were observed in the gut of this IL-5/eosinophil transgenic model, and parasites were less fecund, the gut infection was cleared at the same time of wild-type mice83. In a different study, using IL-5-deficient mice, which lack or are unable to mobilise eosinophils, resistance against N. brasiliensis is impaired in both primary and early secondary infection84. This indicates the importance of IL-5 in eosinophil recruitment and the role of IL-5/eosinophils in anti-helminth immunity in the skin. In models of T. spiralis infection which involve infection and long term survival of parasites in host muscle tissues, depletion of eosinophils did not affect the intestinal parasitic burden but did lead to greater numbers of viable larvae being detected in the muscle tissue85. Interestingly the eosinophils in the muscle tissue surrounding the parasite produced IL-10 in the early stage of infection which had the effect of protecting the encapsulated intracellular larvae and supporting chronic infection86. Taken together these studies identified an important role for eosinophils in anti-helminth immunity depending on the parasite and infective tissue stage. However, caution must be observed using experimental models due to the inadequacy of the strategies used to deplete or enhance eosinophils, especially with respect to the inability of the depletion strategies to discriminate between eosinophil subtypes that have functions beyond the observed in vitro killing of parasitic larvae8,87. Subsets of eosinophils have been shown to secrete a wide range of cytokines (e.g. IL-4, Il-6, IL-10, and IL-13)88,89, are involved in liver regeneration90, and in remodeling of the airways in diseases such as asthma81.
Helminth induced basophil responses in the skin
Parasitic infections have been key to understanding the role of basophils in type 2 immune responses (Fig. 2). Basophilia has been described as a feature in several rodent parasite models and in humans their abundance is used as a hallmark feature of helminth infections91. Basophils from patients infected with Ascaris, Strongyloides, or Schistosoma can secrete histamine in response to parasite antigen92. Basophil precursors reside in the bone marrow and respond to IL-3 and TSLP upon helminth infection91. Furthermore, N. brasiliensis models showed that basophils are required to induce protection upon secondary infection by trapping the larvae in the skin, and this is orchestrated by IgE-activated basophils and M2-polarised macrophages93. This study helped to identify basophils as a source of IL-4 during primary infection, placing basophils as a potentially important player in initiating type 2 immune responses, in contrast to the CD4+ T cells that are the major IL-4 producer during re-infection34,93,94. In the absence of basophils, clearance of parasites is impaired in both N. brasiliensis and H. polygyrus infections, due to a lack of basophil-derived IL-4 and IL-13 and reduced expansion of Th2 cells95. In a S. venezuelensis model, which migrates through the skin similarly to N. brasiliensis, basophils had a primary role in trapping larvae in the skin during primary infection, but had no effect during secondary infection96. However, studies using S. ratti indicated that the absence of basophils can be compensated for by other immune cells, such as mast cells. The authors observed no differences in TH2 responses in basophil-depleted mice97. Experiments with different parasite species indicate that the role of basophils is model-dependent and should be evaluated individually. In a pathological context, basophils have been observed in inflamed human and murine skin during atopic dermatitis and early IL-4 production is required for ILC2 recruitment and activation98. However, basophil-derived IL-4 and M-CSF can activate M2 macrophages to induce dermal repair99, therefore basophils have been shown to both promote skin damage as well as skin repair.
Infective L3 penetrate the skin causing damage of the epithelial cells. Alarmins such as IL-33 and TSLP are released from damaged epithelial cells. Both IL-33 and TSLP can activate basophils, which can release IL-4. IL-4 is necessary for 1) the development of TH2 cells and the release of IL-5 involved in eosinophil recruitment, and 2) B cell IgE class switching. Basophil activity is directed against the parasite upon binding of IgE through high affinity receptors and trapping infective larvae. TSLP together with IL-3 induces recruitment of basophils at the site of skin inflammation.
Schistosoma infection induced skin immune responses
When comparing the three main Schistosoma species able to infect humans interesting differences in skin responses are observed leading to quite different host skin penetration times for the parasite. This difference appears to be related to the variation in inflammatory mediators released by S. mansoni and S. haematobium, when compared to the slower migrating S. japonicum100. Investigations of Schistosome larvae, called cercariae, have also helped in understanding the interplay between the optimal immune response that controls the infection, whilst preventing tissue damage and threatening the survival of the host. Cercariae penetrate the skin and secrete excretory/secretory (E/S) products that help maintain the infection as well as regulate immune responses. For instance, E/S products affect the functionality of Langerhans cells and dendritic cells through prostaglandins, IL-4, and IL-13101,102. Percutaneous infection by S. mansoni induced a transient increase in expression of inflammatory cytokines such as TSLP and IL-33, which was associated with increased inflammatory cellular infiltrates in vivo103. While IL-33 plays a key role in orchestrating immune responses in the lung and intestine, TSLP seems to be more important in activating ILC2s in the skin104.
Chronic helminth infection in the gut can influence skin immune responses
Infection with the strictly enteric helminth H. polygyrus has been shown to modulate the skin environment in a number of murine models. Chronic infection with H. polygyrus induces a strong systemic TH2 immunity with the survival of the parasite in the host gut being determined by the expansion of TREG and BREG cells, and induction of a DC subset that expands TREG over TH215,105. Interestingly, despite the fact that H. polygyrus is confined to the lamina propria of the small intestine and the lumen of the small intestine, it or its secreted products appear able to modulate skin inflammation in a mouse model of atopic dermatitis, reducing neutrophil recruitment through reduction of inflammatory chemokines/cytokines68. Additionally, chronic infection with H. polygyrus inhibited the allergen-induced hyperplasia of skin-draining lymph nodes. Similarly, intradermal BCG vaccination in H. polygyrus infected mice is reduced, with lower lymphocyte cells in the draining lymph node106. Interestingly, although these antigen-induced immune responses were inhibited in those mice infected with H. polygyrus alone there was a low level of CD4+ T cell expansion and eosinophil recruitment detected in the skin. Chronic H. polygyrus infection also induced increased expression of skin-homing receptors (e.g. CCR9, CCR4, and CCR10) in CD4+ T cells in the mesenteric LN (mLN), that subsequently migrated to the skin of infected mice and persisted even after deworming107. These gut helminth-induced changes in the composition of the immune cell infiltrate in the skin could potentially affect responses to vaccination or other pathogens and could be a further mechanism used by helminth parasites to protect the host from other invading parasites or limit the number of parasites that the host accommodates to the survival advantage of the host and parasite.
Natural helminth infection versus experimental models of helminth infection
One of the caveats to consider when using experimental parasite infections to model real world human helminth infection is the consideration of the natural routes of host entry and the level of infection that occurs. In endemic areas or the wild, humans and animals are constantly in contact with parasites and re-infection by low numbers of parasites is common. In experimental models, a high dose of parasite is often administered, termed a bolus infection which has been informative in understanding the immune cell types involved in the immune response but not necessarily the role they play in immunity and tolerance. To mimic real-world situations, experimental infections have been performed with low, regular parasite dosing, termed a “trickle infection”. In the case of Schistosoma, a trickle infection of cercariae in the skin induces a different immune response compared to a bolus infection102,108,109. Injection of S. mansoni weekly in the ear pinna in mice, induced recruitment of non-regulatory FoxP3− CD4+ T cells in the skin and the production of IL-10 is required to limit dermal immune responses to other invading parasites, and possibly also to other pathogens109. IL-10 production is essential in maintaining homeostasis. In the process of resolving inflammation, IL-10 can be produced by several T cell subsets and B cells, and it is strongly associated with immunomodulation by helminth parasites64,110. The absence of IL-10 in mice induced an increased inflammatory infiltrate in the skin during cercariae migration, showing the importance of IL-10 in reducing inflammatory responses111. In addition, S. mansoni stimulated the production of prostaglandin E2 (PGE2), possibly from keratinocytes, and consequently induced IL-10 in the skin111. Using trickle infection in models using N. brasiliensis in rats and A. ceylaniucum in hamsters allowed an increase in parasite burden followed by a steady decline, indicating the generation of a partial protective immune responses. The parasites established a longer infection112, but to date, no immunological studies have been performed in trickle N. brasiliensis infection. Trickle infection with T. muris in mice resulted in slower induction of immunity, associated with partial expulsion and a shift from TH1 to TH2 responses113, while trickle infection with S. ratti, induced little difference in the generation of type 2 immune responses affecting the production of IL-4, IL-13, and IgG114.
Helminth infection induced immune responses in the lung
After penetrating the skin, Ancylostoma spp, Strongyloides spp, Ascaris spp, N. americanus, and N. brasiliensis parasites migrate through the lungs before reaching the intestine. During the migratory phase, A. lumbricoides induces persistent airway hyperresponsiveness (AHR) and airway remodeling in mice, that resembles allergic airway disease observed in human asthma115. In human exposures, infection with Ascaris has been linked to lower lung function and increase risk of asthma72,73. Severe S. stercoralis infection in human is associated with acute respiratory distress syndrome (ARSD), acute respiratory failure and pulmonary hemorrhage, however the majority of patients are asymptomatic or present with mild gastrointestinal symtoms116. The migratory phase of N. brasiliensis infection causes extensive damage and hemorrhage in the lung parenchyma with large neutrophilic infiltrate, but tissue repair is promoted once the parasite exits the lungs117, possibly driven by TGF-b-responsive myeloid cells and the production of trefoil factor 2 (TFF2)118,119. Emphysema-like pathology and activated macrophages were observed long term in mice infected with N. brasiliensis120, suggesting long term effect of hookworm infection in the lung environment. However, lung emphysema, hemorrhage and pulmonary pathology have been associated with high dose of infective larvae transiting the lung, and not observed with lower dose of parasite121, possibly lung pathology observed in some infected human reflect high burden of larvae transiting the lungs.
Role of lung neutrophils during helminth infection
Neutrophils are primarily associated with antimicrobial or antifungal responses, and they are not well characterized during parasitic infections. However, significant numbers of neutrophils infiltrate the lungs of Nb-infected mice two days post infection (dpi) in an IL-17-dependent manner122,123. Furthermore, Ancylostoma caninum can secrete a neutrophil inhibitory factor (NIF) protein that can reduce neutrophilic inflammation124. Furthermore, depleting neutrophils increased N. brasiliensis adult worm burden in the gut, suggesting a potential role for neutrophils in defense against N. brasiliensis78,122. Similarly, Chen et al. showed impairment in worm expulsion in the absence of neutrophils. They showed that neutrophils can instruct macrophage polarization and these neutrophils displayed a specific gene expression profile, characterized by markers associated with type 2 immunity e.g. Il13 and Il33125. IL-17A is a key cytokine for neutrophil recruitment with IL-17 responses being associated with pathology in human schistosomiasis126, and an important component of Type 2 immune responses in some cases of asthma127. Recently, Ajendra et al. showed that early production of IL-17A induced down-regulation of IFNγ and this was required for the generation of optimal type 2 immune responses during N. brasiliensis infection. However, IL-17A produced in the later stage of infection negatively regulated type 2 immune response128, showing a novel role for IL-17A in the regulation of type 2 immunity. Vice versa, type 2 immune responses can regulate TH17 cells that express a functional IL-13R, reducing expression of RORγt and production of IL-17 and IL-21129.
Initiation of type 2 immune responses in the lung during helminth infection
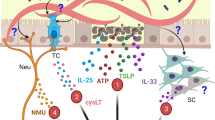
Murine helminth infection models have substantially enhanced our understanding of the agents and factors involved in the initiation of type 2 immunity. When N. brasiliensis and S. venezuelensis migrate through the lung it has been clearly demonstrated that the alarmin IL-33 is released in significant amounts and appears to be important for initiating type 2 immune responses in the lung. IL-33 is normally released by damaged epithelial cells, and it acts on several immune cells that express the receptor for IL-33 (ST2). The IL-33 cytokine acts as an alarmin and it has broad effects on TREG, CD8 + T cells, NK cells as well as ILC2 and TH2130,131. IL-33 has also been shown to have a role in asthma development in early life132, in allergen-induced asthma42,133 and is associated with type-2 cytokines in asthmatic patients134. Furthermore, in a clinical trial of therapeutic anti-IL-33 monoclonal antibody beneficial outcomes were achieved in atopic dermatitis patients with reduced eosinophilia and skin neutrophil recruitment135. IL-33 is also known to rapidly activate ILC2s to secrete IL-5 which is important for eosinophil recruitment, and secrete IL-13 which is involved in mucus production and airway hyperresponsiveness133 (Fig. 3). Neurotransmitters have recently been identified and additional contributors or regulators to Type 2 immune responses. Mucosal sites such as the lungs and the gut are highly innervated, and ILC2s can respond to neuromedin U (NMU), a neuropeptide produced by cholinergic neurons. The parasitic burden during N. brasiliensis infection is increased in Nmur1-/- mice, suggesting that NMU has an important role in the generation of protective type 2 immune responses26,31. In addition, ILC2s can synthesize acetylcholine (ACh), a neurotransmitter that influences the immune system (Fig. 3). The lung migration of N. brasiliensis increased the expression of choline acetyltransferase (ChAT) on lung ILC2s, the enzyme involved in the production of ACh. Pulmonary ChAT+ ILC2s can be induced by IL-25 and IL-33, and ACh production is required for optimal anti-helminth type 2 immune responses30,136. In support of the importance of this novel pathway in the induction of optimal type 2 immune responses, parasitic nematodes can produce acetylcholinesterase44, which could be involved in suppressing the effect of ACh to allow the parasite to establish infection in the host. ACh has a broad effect on several immune cells, such as eosinophils, lung macrophages, and DCs137. DCs treated with ACh induce expression of OX40L, which is involved in the promotion of TH2 responses, and induces production of IL-4, IL-5, and IL-13 by T cells138.
Hookworm larvae can cause extensive damage during the lung migratory phase. The release of IL-33 and IL-25, from damaged epithelial cells, and NMU from neurons drives the activation of ILC2s. Activated ILC2s release IL-5 and ACh, involved in eosinophils recruitment, and IL-13 that acts on goblet cells and increases mucus production. Both the presence of activated eosinophils and increased mucus production help with the killing and removal of parasites.
Furthermore, ACh antagonists have been used to treat asthma and chronic obstructive pulmonary disease (COPD), ameliorating mucus production and airway inflammation30. Neuroimmune interactions are starting to be explored in the context of helminth infections. Neuroendocrine cells are localized in the lungs and intestine. Therefore, by using lung/gut-dwelling parasites as a model, we can gain insight into the role of neurotransmitters and neuropeptides in type 2 immune responses.
Type 2 responses involved in anti-helminth immunity
IL-13 is a key cytokine produced during type 2 immune responses to parasites, it has been shown to be crucial for expulsion of parasites from the gut33,139,140, as well as tissue repair and fibrosis123. TH2 cells induced by parasites have been associated with the production of IL-13, with, ILC2s being an innate source of IL-1311,12,141. IL-13 induces goblet cell hyperplasia and mucus production and has been linked to collagen synthesis and fibrotic deposition during S. mansoni infection or egg challenge142,143, indicating the broad role IL-13 plays in type 2 immunity. In the absence of IL-13, vascular damage and lung injury is exacerbated during early N. brasiliensis infection, suggesting a protective role for IL-13, while it was required for complete eosinophil recruitment the lung144. IL-13 signals through IL-4 receptor alpha (IL-4Rα), sharing this receptor with IL-4. IL-13 and IL-4 both signal through STAT6 and for this reason it has been suggested that the cytokines could have a redundant function145. Both cytokines are rapidly induced after infection, and they are both necessary to induce tissue repair through macrophage activation146. IL-4/IL-13-deficient mice showed impairment in helminth expulsion147, but while IL-4 seems to play an important role in expulsion of H. polygyrus46, IL-13 may play a major role in driving N. brasiliensis clearance33. In addition, in experimental asthma model the role of IL-13 and IL-4 did not overlap as IL-4-independent type 2 immune responses have been reported148,149. Several other factors can influence anti-helminth immunity, such as the surfactant protein A (SP-A), in a mechanism dependent on IL-4Rα. Lack of SP-A was associated with increased worm burden, egg production, and impaired repair processes. SP-A deficient mice had increased lung damage, with a higher number of red blood cells and neutrophils in the bronchoalveolar lavage (BAL) of infected mice, along with reduced expression of resistin-like molecule α (RELMα), YM1, and arginase by macrophages150. Recently, arginase 1 (Arg1) was shown to be highly expressed in alveolar macrophages following N. brasiliensis infection. Interesting, during infection monocytes are rapidly recruited in the inflamed lung environment where they acquired an alveolar macrophage-like phenotype. These macrophages expressed SiglecF, CD11c and Arg1, and they can kill N. brasiliensis larvae in vitro possibly depleting arginine, an essential amino acid for parasite metabolism151.
Effect of helminth induced immune responses on coinfections
In a previous section of this review the issue of the immune responses stimulated by a helminth infection could potentially be a strategy of the established worm to protect its host from further infection by other competing parasites. Mouse models using Trichinella spiralis demonstrate that the parasite induces a systemic response in distal tissues with increased mucus production observed in the lungs of mice infected with T. spiralis, which is dependent on ILC2-derived IL-13. Similarly, intestinal-dwelling helminths H. polygyrus or Hymenolepis microstoma showed a similar increase of mucin production in the lung152, and this mucus production is thought to reduce subsequent infections by N. brasiliensis152. Co-infection models indicate that intestinal helminths can protect the host from other types of microbial infections. Using H. polygyrus, Filbey et al. showed in H. polygyrus infected mice that infective lung migrating N. brasiliensis and T. muris larvae were killed by a mechanism involving IL-33 activated CD4+ T cells and IL-5 production153. In another model using respiratory syncytial virus (RSV), H. polygyrus was able to induce protection through expression of type I interferon genes and interferon-stimulated genes in the lungs, by a process involving the microbiota but independent from adaptive and TH2 immunity58.
Helminth induced immune responses in the gut a focus on mucus
The metaphorically elegant term “weep-and-sweep” is often used to describe the effector mechanisms associated with localized gut immunity to intestinal helminths. N. brasiliensis, H. polygyrus, and T. muris are widely used to study immune responses in the gut. IL-4 and IL-13 are key players of this response, inducing smooth muscle hypercontractility with the involvement of enteric nerves and immune cells such as alternative activated macrophages32,154,155. Several other immune cells and cytokines are also involved in the anti-helminth type 2 immune responses in the gut. The intestinal environment undergoes significant tissue remodeling during parasitic infections, with the expansion and hyperplasia of goblet cells observed in multiple nematode infections156,157,158,159. Goblet cells are the main source of mucins, the major family of glycoproteins that comprise the mucus barrier and provide its viscoelastic properties160. MUC2 is the major mucin of the intestinal mucus gel, forming insoluble net-like structures mediated by covalent linkages between different mucin monomers160. The mucus barrier plays an important role in homeostasis and defense mechanisms, it protects epithelial cells from pathogens, and modifications are associated with pathologies such as cancer and ulcerative colitis161. In mice, the loss of mucins or their components, induced mice to develop spontaneous colitis or inflammation that resembled colitis162,163. This indicates an important role for mucins in the pathophysiology of certain diseases. In the response against parasites, the mucus layer is believed to be involved in trapping parasites, reducing motility and nutrient uptake, blocking the parasite to establish infection in the gastrointestinal tract160. The expulsion of T. muris is dependent on Muc2 expression164 with Hasnain et al. showed that the absence of Muc2 delayed worm expulsion, leading to an increase in the immune properties of the mucus barrier164. Similarly, the cytokines IL-4 and IL-13 have been shown to stimulate goblet cells to produce resistin-like molecule β (RELM-β) that has a protective effect against N. brasiliensis and H. polygyrus but no protective effect is observed against T. muris infection165,166. IL-13 is predominantly produced by ILC2s and TH2 cells that can be activated by several host-derived factors such as IL-33. ILC2s have been identified as a rapid innate source of IL-13 at steady state. During N. brasiliensis and H. polygyrus infections, TH2 cells that express the epidermal growth factor receptor (EGFR) can be activated by IL-33 in a TCR-independent manner to release IL-13 and play a role in host protection against infection167.
Helminth induced immune responses in the gut, a focus on alarmins and leukotrienes
Recently the role of IL-33 in gut immunity has been investigated with Shimokawa et al. showing that IL-33 can be released by dead cells in the intestine, with the concomitant release of ATP from damaged cells activating mast cells to release IL-33 leading to increased ILC2 activation and IL-13 production168 (Fig. 4). Recently, a novel tissue specific role for IL-33 has been identified in immunity to helminth infection with a deficiency of IL-33 in intestinal epithelial cells (IECs) resulting in delayed parasite clearance and reduced number of ILC2s in the small intestine when challenged by N. brasiliensis infection169. Interestingly, mice lacking IL-33 in the DC compartment showed enhanced type 2 immune responses, with a lower parasite burden after both N. brasiliensis and H. polygyrus infection. DC-derived IL-33 was required to induce ST2+Foxp3+ TREG that suppress type 2 immune responses169 (Fig. 4). The authors showed an important mechanism in the induction/regulation of type 2 immune responses, observing that the release of IL-33 is highly tissue context dependent, and this can have a strong influence in the generation of immune responses. Another host factor that has recently been a focus in helminth infections is the alarmin IL-25 produced by tuft cells in the intestine13,14,170. Intestinal tuft cells are specialized IECs that act as secondary chemosensory cells, with apical microvilli, and express a variety of structural markers, taste receptors, and enzymes for prostaglandins and leukotrienes production171. Chemosensory cells express common transcriptional factors and have been identified in several tissues including the intestine, thymus, gallbladder, and airways172. Furthermore, they have been identified in the ovine stomach, where they expand during helminth infections, indicating that the expansion of tuft cells is a conserved mammalian mechanism that allows a host to be aware of, and respond appropriately to parasite infection strategies173. In the airways, tuft cells have been shown to play a role in allergic type 2 immune responses with their activation by allergens triggering the production of IL-25 and leukotrienes. This in turn activates ILC2s and DCs to drive eosinophil recruitment, CD4+ T cells expansion, production of IL-13, and goblet cell hyperplasia in the lung172. Similarly, leukotrienes can be produced by tuft cells after H. polygyrus and N. brasiliensis infection in the intestine. Tuft cells can sense the parasite and rapidly produce IL-25 and leukotrienes that activate ILC2s (Fig. 4). The absence of leukotrienes synthesis in tuft cells has been shown to result in delayed parasite clearance174. Additionally, at steady state IL-25 has been shown to regulate homeostatic IL-13 expression, as IL-25-deficient mice have a reduction in IL-13 expression14. IL-13 produced by immune cells also acts in a positive feedback loop, as it increases goblet cell and tuft cells numbers170 (Fig. 4). Drurey et al. have recently shown that H. polygyrus can reduce expansion of tuft cells in a N. brasiliensis co-infection model, and H. polygyrus E/S products were able to block the effects of IL-4/IL-13 in tuft cells and goblet cells gene expression and expansion175. In this study, authors showed that H. polygyrus infection downregulated several genes involved in cell differentiation of goblet cells, Paneth cells, and endocrine cells, suggesting that the parasite modulated several cells involved in helminth defense175. IL-13 is a key cytokine involved in mucus production and IL-13-deficiency prevented goblet cell hyperplasia in the lung152. In addition, IL-13 has been shown to have a critical role in resistance to intestinal helminth infection147,176. ILC2-derived IL-13 has been shown to drive intestinal goblet cell hyperplasia upon IL-33 injection177, however there is a lack of research on the role of IL-13 and goblet cell hyperplasia in the gut during helminth infections. Using N. brasiliensis and T. muris, Turner et al. showed that IL-22-deficiency impaired anti-helminth immunity despite a strong induction of IL-5, IL-13, and IL-4 in the intestine178. IL-22 deficient mice showed a reduction in goblet cells and delayed worm expulsion, suggesting that IL-22 acts directly on epithelial cells to induce mucin expression178. IL-22 is a member of the IL-10 cytokine family, and it mediates epithelial defense, tissue repair, and wound healing processes. Increased levels of IL-22 have been reported during N. americanus infection179 and IL-22+ CD4+ T cells have been observed during T. trichiura infection180. Turner et al. reported a novel role for IL-22 in the induction of goblet cells and mucus production, following previous studies showing IL-4/IL-13 independent goblet cell hyperplasia during infection with the nematode Syphacia obvelata181.
Adult hookworm can establish chronic infection in the intestinal tract. Several factors contribute to the activation of intestinal ILC2s. IL-33 released from epithelial cells and from ATP-activated mast cell can directly activate ILC2s. IL-25 and leukotrienes (LTs) produced by tuft cells, and NMU by enteric neurons induce production of IL-13. IL-13 induces hyperplasia of goblet cells and mucus production, and it acts on progenitor intestinal cells to promote goblet cells and tuft cells development. In contrast, IL-33 released from DCs induces expansion of ST2+ TREG that suppress ILC2s activation, reducing type 2 immune responses.
Helminth infection induced regulation of type 2 immune responses in the gut
An important feature of the host response to helminth infection is that the magnitude of inflammation and activation of immune cells must be tightly regulated to enable efficient killing and removal of harmful levels of parasites and their products, whilst not going overboard and limiting host tissue damage. This is true for the induction and regulation of Type 2 immune responses, as well as Type 1 immune responses. Several factors have been shown able to dampen Type 2 immune responses, like IL-10, TGFβ, IL-13 decoy receptor (IL-13Rα2), and RELMα. Goblet cells and tuft cells express CRTH2, the receptor for prostaglandin D2 (PGD2). Prostaglandins are eicosanoids, bioactive lipids with pro- and anti-inflammatory activity, derived from arachidonic acid27,182. The pro-inflammatory role of PGD2 has been shown in the lungs, and intestinal tuft cells have the potential to produce PGD2 with the possibility to be involved in anti-helminth immunity. Oyesola et al. showed that in the intestine the PGD2-CRTH2 pathway limited Type 2 immune responses. Mice lacking CRTH2 showed enhanced N. brasiliensis clearance which was associated with increased goblet cell hyperplasia, indicating a novel role for PGD2-CRTH2 pathway in influencing epithelial cells responses during type 2 immunity183. As mentioned in the previous section on helminth induced lung immune responses, neurotransmitters and neuropeptides have been shown able to induce and regulate type 2 immune responses. The intestinal environment is highly innervated, and several immune cells are co-localized with enteric neurons184. A typical example is the subset of enteric neurons which express NMU and co-localize with ILC2s, providing a rapid activator of ILC2s and promoting intestinal type 2 immune responses26,31 (Fig. 4). In contrast to NMU, the β2-adrenergic receptor (β2AR) pathway has been shown to downregulate Type 2 immune responses. ILC2s express β2AR, and deficiency of β2AR in mice enhanced type 2 immune responses but did not affect ILC2 development185. Increased eosinophilia and goblet cells hyperplasia were observed following N. brasiliensis infection in β2AR-deficient mice, with enhanced worm expulsion185. These findings indicate that the nervous system has evolved adrenergic and cholinergic neurons that respectively downregulate and activate intestinal ILC2s26,31,185, and have the potential to play an important role in inflammatory responses and tissue repair during both helminth infection and host immunopathologic diseases. Another consideration is the important role enteric neurons play in regulating homeostatic and physiological mechanisms in the intestine (e.g. intestinal motility). Enteric neurons need to be protected from damage due to their reduced proliferation and regeneration capacity with Ahrends et al. recently identifying that IL-4 and IL-13 produced during S. venezuelensis infection were required to induce neuroprotection. Helminth-induced neuroprotection was dependent on IL-5-mediated recruitment of duodenal eosinophils, and their IL-4/IL-13 production186. Long-term neuroprotection was induced due to modification in the progenitor compartments in the bone marrow after S. venezuelensis infection. This study identified a novel role for eosinophil-derived IL-4 and IL-13 in intestinal neuroprotection186. In addition, a recent study by Progatzky et al. described a novel role for IFNγ in promoting homeostasis and tissue repair after intestinal damage by H. polygyrus infection, highlighting the role of enteric glial cells (EGCs) in immunity and repair187. Disrupting the IFNs pathway increased eosinophils, neutrophils, and monocytes inflammation, which was associated with a delay in granulomas resolution. The authors identified that EGCs produced CXCL10, which was required for tissue repair after infection187.
Challenges to studying gut immune responses during helminth infections
One of the biggest challenges when studying immune responses in the gut tissue during helminth infection has been isolating viable immune cells from the lamina propria (LP)188,189. These difficulties are largely due to the infection induced changes to intestinal physiology, with increased mucus production and thickening of the gut wall189. Immune responses in the intestine have been mainly performed by studying cells in the mLN or peritoneal lavage. However, two protocols have been recently optimized to obtain viable cells from the lamina propria of mice infected with H. polygyrus enabling in depth interrogation of cells by high-dimensional flow cytometry188,189. Focusing on the inflammatory infiltrate, Webster et al. reported IL-13+ and IL-5+ CD4+ T cells in the LP of mice infected with H. polygyrus, increasing by day 7 post infection, in parallel to an increase in neutrophils and DCs. Similarly, Ferrer-Font et al. showed that several immune cells are increased by day 7 post infection. Neutrophils are recruited to the intestine but return to normal levels by day 14. In contrast, on day 7 and 14 eosinophils are still infiltrating the LP, as are RELMα+ macrophages188. These protocols are useful to enable investigation of local immune responses against helminths, and in combination with the use of transgenic mice, they can help clarify the role of immune cells in anti-helminthic immune responses.
Even more challenging is the study of local tissue specific immune responses in parasitized humans. Human hookworm studies have been performed in the past, as well as clinical trials for the treatment of inflammatory disorders190. Studies in individuals infected with N. americanus showed a transient increase of Th2 cytokines in whole blood cultures, while low levels of TH1 cytokines or IL-10 were detected191. In a controlled human infection using 50 N. americanus larvae, blood eosinophilia was observed in the two individuals examined, and cytokine/chemokine release followed a pattern that allowed authors to distinguish the infective phase of the parasite. A slight increase in IL-10, CCL17, and IL-13 were observed during the larvae migratory phase, with an increase of IL-10 in the pre-patency phase when the parasites start reaching the gut192. However, this study focused on systemic immune responses, the authors did not examine local immune responses. Gaze et al. characterized systemic and mucosal responses during N. americanus infection in humans. PBMCs and biopsies from the duodenum from infected individuals showed increased production of type 2 cytokines such as IL-4, IL-13, IL-5, IL-9, as well as IL-10, and TGF-β, in response to N. americanus excretory/secretory (NaES) products179. Some parasitic infections are well tolerated by their host, leading to the trial of parasites as therapeutic agents for pathologies like ulcerative colitis (UC) and Crohn’s disease. T. trichuria has shown potential therapeutic effect in both Crohn’s disease and UC193,194. Broadhurst et al. provided a comprehensive study from an individual affected by UC. Tissue from an UC lesion showed a prominent inflammatory infiltrate, with T helper cells producing IL-17 and increased pro-inflammatory genes. Infection with T. trichiura induced the emergence of IL-22+ T cells in the mucosa. The disease went into remission, possibly due to the promotion of TH2 immune responses and IL-22 production180.
Concluding remarks
Experimental helminth infection models have begun to help decipher the role of immune cells and cytokines involved in tissue specific Type 2 responses both in the context of immunity, tissue repair and tolerance of the parasite by the host. Significant differences in these responses have been observed depending on the parasite species and route of administration and the tissues involved in the infection. The stage is now set to ask what are the next questions needed for better understanding the immune responses induced in the host by helminth infections? Firstly, we believe that a better understanding of the local responses during chronic helminth infection is required. Although inflammatory and morphological changes are associated with chronic helminth infection there is not the same degree of pathology as seen in allergic inflammation in atopic disease, possibly due to the immunomodulatory effect that adult parasites have on cells of the host immune system. Another key area that needs to be further explored and characterized is the effect of helminth infection on the host microbiota. Metabolites produced by the microbiota are known to have a positive effect on suppressing inflammation and promote health, possibly not only at the site of infection but also at distal tissue sites such as skin, lung and gut. Furthermore, in recent years the phenomenon of “trained innate immunity” has come to the fore. Studies involving Fasciola hepatica products suggest that macrophages and monocytes can be trained to have an anti-inflammatory phenotype195,196. Although, the question of whether infection with helminths can induce changes on the myeloid compartment or bone marrow stem cells remain unanswered to date future studies need to address whether helminth infection in early life translates to more permanent effects during adulthood. Finally, an important goal is to identify whether the immunomodulatory effects of helminth parasites can be translated into therapies for treating human disease. Safety concerns associated with live parasitic infection and high immunological variability among human populations limit clinical trials. However, a better understanding of the effect of helminth infections in human is needed, and this can be achieved by using safe infection models, with controlled conditions and standardized sample collection methods. In the past few decades, the world of immunology has rapidly evolved. Recent technological advances allowed the very detailed phenotyping of immune cells including the analysis of their metabolic state, and a snapshot of gene expression at single-cell level. The ability to block expression of specific genes in mice through either transient or stable gene deletion at germline level has enabled Type 2 immune responses to parasite infections to be studied in the absence of specific immune cells or cytokines. This has much improved understanding of the mechanisms involved in disease settings as well as anti-helminth immunity. The improvement of protocols to study local immune responses in the intestine of infected mice and humans in combination with technological advances for studying changes to the microbiome will help build a comprehensive picture of the role of different immune cells in type 2 immunity, and the possibility of discovering new immune cell subsets and pathways involved in inflammation, homeostasis, and tissue repair.
References
Liston A., Humblet-Baron S., Duffy D., Goris A. Human immune diversity: from evolution to modernity. Nat Immunol. https://doi.org/10.1038/s41590-021-01058-1 (2021).
Tao, L. & Reese, T. A. Making mouse models that reflect human immune responses. Trends Immunol. 38, 181–193 (2017).
Harris, N. L. & Loke, P. Recent advances in type-2-cell-mediated immunity: insights from helminth infection. Immunity 47, 1024–1036 (2017).
Fumagalli, M. et al. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J. Exp. Med 206, 1395–1408 (2009).
Loukas, A., Maizels, R. M. & Hotez, P. J. The yin and yang of human soil-transmitted helminth infections. Int J. Parasitol. 51, 1243–1253 (2021).
Pullan, R. L., Smith, J. L., Jasrasaria, R. & Brooker, S. J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors 7, 1–19 (2014).
Lavelle E. C., Ward R. W. Mucosal vaccines — fortifying the frontiers. Nat Rev Immunol. Published online. https://doi.org/10.1038/s41577-021-00583-2 (2021).
Buys, J., Wever, R., van Stigt, R. & Ruitenberg, E. J. The killing of newborn larvae of Trichinella spiralis by eosinophil peroxidase in vitro. Eur. J. Immunol. 11, 843–845 (1981).
Capron, M., Torpier, G. & Capron, A. In vitro killing of S. mansoni schistosomula by eosinophils from infected rats: role of cytophilic antibodies. J. Immunol. 123, 2220–2230 (1979).
Fallon, P. G. et al. Identification of an interleukin (IL)-25–dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med 203, 1105–1116 (2006).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Hm, R. et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science (80-) 351, 1329–1333 (2016).
von Moltke, J., Ji, M., Liang, H.-E. & Locksley, R. M. Tuft-cell-derived IL-25 regulates an intestinal ILC2–epithelial response circuit. Nature 529, 221–225 (2016).
Grainger, J. R. et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med 207, 2331–2341 (2010).
Layland, L. E. et al. Pronounced phenotype in activated regulatory T cells during a chronic helminth infection. J. Immunol. 184, 713–724 (2010).
Blankenhaus, B. et al. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J. Immunol. 186, 4295–4305 (2011).
Hussaarts, L., Van Der Vlugt, L. E. P. M., Yazdanbakhsh, M. & Smits, H. H. Regulatory B-cell induction by helminths: Implications for allergic disease. J. Allergy Clin. Immunol. 128, 733–739 (2011).
Henry, E. K., Inclan-Rico, J. M. & Siracusa, M. C. Type 2 cytokine responses: regulating immunity to helminth parasites and allergic inflammation. Curr. Pharm. Rep. 3, 346–359 (2017).
Nutman, T. B. Evaluation and differential diagnosis of marked, persistent eosinophilia. Immunol. Allergy Clin. North Am. 27, 529–549 (2007).
Saluzzo, S. et al. First-breath-induced type 2 pathways shape the lung immune environment. Cell Rep. 18, 1893–1905 (2017).
Odegaard, J. I. et al. Perinatal licensing of thermogenesis by IL-33 and ST2. Cell 166, 841–854 (2016).
Mayer J. U. et al. Homeostatic IL-13 in healthy skin directs dendritic cell differentiation to promote T(H)2 and inhibit T(H)17 cell polarization. Nat Immunol. https://doi.org/10.1038/s41590-021-01067-0 (2021).
Moretti S., Renga G., Oikonomou V., … CG-N, 2017 undefined. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. nature.com. Accessed Dec 8, 2021. https://www.nature.com/articles/ncomms14017?sf52360125=1
Wallrapp A., Riesenfeld S., Burkett P., Nature RA-, 2017 undefined. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. nature.com. Accessed Dec 8, 2021. https://www.nature.com/articles/nature24029
Klose, C. S. N. et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nat 2017 5497671 549, 282–286 (2017).
Wojno E., Monticelli L., Tran S., … TA-M, 2015 undefined. The prostaglandin D 2 receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. nature.com. Accessed Dec 8, 2021. https://www.nature.com/articles/mi201521
Moltke J von, O’Leary C., … NB-J of E, 2017 undefined. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. rupress.org. Accessed Dec 8, 2021. https://rupress.org/jem/article-abstract/214/1/27/42225
Cephus J., Stier M., Fuseini H., Yung J., reports ST-C, 2017 undefined. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Elsevier. Accessed Dec 8, 2021. https://www.sciencedirect.com/science/article/pii/S2211124717315905
Roberts L. B. et al. Acetylcholine production by group 2 innate lymphoid cells promotes mucosal immunity to helminths. Sci Immunol. 6. https://doi.org/10.1126/SCIIMMUNOL.ABD0359/SUPPL_FILE/ABD0359_SM.PDF (2021).
Cardoso, V. et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nat 2017 5497671 549, 277–281 (2017).
Finkelman, F. D. et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 201, 139–155 (2004).
Urban, J. F. et al. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8, 255–264 (1998).
Min, B. et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J. Exp. Med 200, 507–517 (2004).
O’Neill, C. A., Monteleone, G., McLaughlin, J. T. & Paus, R. The gut-skin axis in health and disease: a paradigm with therapeutic implications. Bioessays 38, 1167–1176 (2016).
Ochoa-Repáraz, J. et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 183, 6041–6050 (2009).
Sudo, N. et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 558, 263–275 (2004).
Richards, P., Thornberry, N. A. & Pinto, S. The gut–brain axis: Identifying new therapeutic approaches for type 2 diabetes, obesity, and related disorders. Mol. Metab. 46, 101175 (2021).
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013).
Johnston, C. J. C. et al. A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat. Commun. 8, 1741 (2017).
Vacca F. et al. A helminth-derived suppressor of ST2 blocks allergic responses. Elife. 9. https://doi.org/10.7554/eLife.54017 (2020).
Osbourn, M. et al. HpARI protein secreted by a helminth parasite suppresses interleukin-33. Immunity 47, 739–751.e5 (2017).
Navarro, S. et al. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci. Transl. Med 8, 362ra143 (2016).
Hewitson, J. P. et al. Proteomic analysis of secretory products from the model gastrointestinal nematode Heligmosomoides polygyrus reveals dominance of Venom Allergen-Like (VAL) proteins. J. Proteom. 74, 1573–1594 (2011).
Camberis, M., Le Gros, G. & Urban, J. Jr Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr. Protoc. Immunol. 55, 19.12.1–19.12.27 (2003).
Reynolds, L. A., Filbey, K. J. & Maizels, R. M. Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin Immunopathol. 34, 829–846 (2012).
Breloer, M. & Abraham, D. Strongyloides infection in rodents: immune response and immune regulation. Parasitology 144, 295–315 (2017).
Antignano F., Mullaly S. C., Burrows K., Zaph C. Trichuris muris infection: a model of type 2 immunity and inflammation in the gut. J Vis Exp. 2774 https://doi.org/10.3791/2774 (2011).
Bancroft, A. J., Else, K. J. & Grencis, R. K. Low‐level infection with Trichuris muris significantly affects the polarization of the CD4 response. Eur. J. Immunol. 24, 3113–3118 (1994).
Mayer, J. U. et al. Different populations of CD11b(+) dendritic cells drive Th2 responses in the small intestine and colon. Nat. Commun. 8, 15820 (2017).
Lundie, R. J. et al. A central role for hepatic conventional dendritic cells in supporting Th2 responses during helminth infection. Immunol. Cell Biol. 94, 400–410 (2016).
Maizels, R. M., Smits, H. H. & McSorley, H. J. Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity 49, 801–818 (2018).
George, P. J. et al. Helminth infections coincident with active pulmonary tuberculosis inhibit mono- and multifunctional CD4+ and CD8+ T cell responses in a process dependent on IL-10. PLoS Pathog. 10, e1004375 (2014).
Elias, D., Britton, S., Aseffa, A., Engers, H. & Akuffo, H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-β production. Vaccine 26, 3897–3902 (2008).
Cooper, P. J., Espinel, I., Paredes, W., Guderian, R. H. & Nutman, T. B. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J. Infect. Dis. 178, 1133–1138 (1998).
Rolot, M. et al. Helminth-induced IL-4 expands bystander memory CD8+ T cells for early control of viral infection. Nat. Commun. 2018 91 9, 1–16 (2018).
Scheer, S. et al. S. mansoni bolsters anti-viral immunity in the murine respiratory tract. PLoS One 9, e112469 (2014).
McFarlane, A. J. et al. Enteric helminth-induced type I interferon signaling protects against pulmonary virus infection through interaction with the microbiota. J. Allergy Clin. Immunol. 140, 1068–1078.e6 (2017).
Furze, R. C., Hussell, T. & Selkirk, M. E. Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis. Infect. Immun. 74, 1924–1932 (2006).
Endara, P. et al. Long-term periodic anthelmintic treatments are associated with increased allergen skin reactivity. Clin. Exp. Allergy 40, 1669–1677 (2010).
Cooper, P. J. et al. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J. Allergy Clin. Immunol. 111, 995–1000 (2003).
de Ruiter K. et al. Helminth infections drive heterogeneity in human type 2 and regulatory cells. Sci Transl Med. Published online 2020. https://doi.org/10.1126/scitranslmed.aaw3703.
van den Biggelaar, A. H. J. et al. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J. Infect. Dis. 189, 892–900 (2004).
van den Biggelaar, A. H. et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet (Lond., Engl.) 356, 1723–1727 (2000).
Wolday, D. et al. Effect of co-infection with intestinal parasites on COVID-19 severity: A prospective observational cohort study. EClinicalMedicine 39, 101054 (2021).
Wilson M. S. et al. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. Published online 2005. https://doi.org/10.1084/jem.20042572
White M. P. J. et al. The Helminth Parasite Heligmosomoides polygyrus Attenuates EAE in an IL-4Rα-Dependent Manner. Front Immunol. Published online 2020. https://doi.org/10.3389/fimmu.2020.01830
Filbey K. J. The Gastrointestinal Helminth Heligmosomoides bakeri Suppresses Inflammation in a Model of Contact Hypersensitivity. Front Immunol. Published online 2020. https://doi.org/10.3389/fimmu.2020.00950
Ryan S. M., Eichenberger R. M., Ruscher R., Giacomin P. R., Loukas A. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLoS Pathog. 16. https://doi.org/10.1371/JOURNAL.PPAT.1008508 (2020).
Palmer L. J. et al. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Respir Crit Care Med. 165, 1489–1493 (2012).
Hawlader, M. D. H. et al. Ascaris lumbricoids infection as a risk factor for asthma and atopy in rural Bangladeshi children. Trop. Med Health 42, 77 (2014).
Leonardi-Bee J., Pritchard D., Britton J. Asthma and current intestinal parasite infection. Am J Respir Crit Care Med. 174, 514–523 (2012).
Jõgi N. O. et al. Ascaris exposure and its association with lung function, asthma, and DNA methylation in Northern Europe. J Allergy Clin Immunol. 0. https://doi.org/10.1016/J.JACI.2021.11.013/ATTACHMENT/C954FD41-3B84-42B8-AE83-8AA49C196655/MMC1.DOCX (2020).
Salgame, P., Yap, G. S. & Gause, W. C. Effect of helminth-induced immunity on infections with microbial pathogens. Nat. Immunol. 2013 1411 14, 1118–1126 (2013).
Desai, P. et al. Enteric helminth coinfection enhances host susceptibility to neurotropic flaviviruses via a tuft cell-IL-4 receptor signaling axis. Cell 184, 1214–1231.e16 (2021).
Reese, T. A. et al. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science (80-) 345, 573–577 (2014).
Heath, W. R. & Carbone, F. R. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 14, 978–985 (2013).
Bouchery, T. et al. Hookworms evade host immunity by secreting a deoxyribonuclease to degrade neutrophil extracellular traps. Cell Host Microbe 27, 277–289.e6 (2020).
Ehrens, A. et al. Eosinophils and neutrophils eliminate migrating strongyloides ratti larvae at the site of infection in the context of extracellular DNA trap formation. Front Immunol. 12, 3115 (2021).
Bonne-Année S. et al. Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microbes Infect. 16, 502–511 (2014).
Weller, P. F. & Spencer, L. A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017 1712 17, 746–760 (2017).
Daly, C. M., Mayrhofer, G. & Dent, L. A. Trapping and immobilization of Nippostrongylus brasiliensis larvae at the site of inoculation in primary infections of interleukin-5 transgenic mice. Infect. Immun. 67, 5315–5323 (1999).
Dent, L. A. et al. Interleukin-5 transgenic mice show enhanced resistance to primary infections with Nippostrongylus brasiliensis but not primary infections with Toxocara canis. Infect. Immun. 67, 989–993 (1999).
Knott, M. L. et al. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J. Parasitol. 37, 1367–1378 (2007).
Huang, L. et al. Eosinophils mediate protective immunity against secondary nematode infection. J. Immunol. 194, 283–290 (2015).
Huang, L. et al. Eosinophil-derived IL-10 supports chronic nematode infection. J. Immunol. 193, 4178–4187 (2014).
Capron, M., Torpier, G. & Capron, A. In vitro killing of S. Mansoni Schistosomula by eosinophils from infected rats: role of cytophilic antibodies. J. Immunol. 123, 2220 LP–2222230, http://www.jimmunol.org/content/123/5/2220.abstract (1979).
Spencer, L. A. et al. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J. Leukoc. Biol. 85, 117–123 (2009).
Gessner, A., Mohrs, K. & Mohrs, M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J. Immunol. 174, 1063–1072 (2005).
Goh, Y. P. S. et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc. Natl Acad. Sci. USA 110, 9914–9919 (2013).
Inclan-Rico, J. M. & Siracusa, M. C. First responders: innate immunity to helminths. Trends Parasitol. 34, 861–880 (2018).
Peng, J. & Siracusa, M. C. Basophils in antihelminth immunity. Semin Immunol. 53, 101529 (2021).
Obata-Ninomiya, K. et al. The skin is an important bulwark of acquired immunity against intestinal helminths. J. Exp. Med 210, 2583–2595 (2013).
van Panhuys, N. et al. Basophils are the major producers of IL-4 during primary helminth infection. J. Immunol. 186, 2719–2728 (2011).
Schwartz, C. et al. Basophil-mediated protection against gastrointestinal helminths requires IgE-induced cytokine secretion. Proc. Natl Acad. Sci. USA 111, E5169–E5177 (2014).
Mukai K., Karasuyama H., Kabashima K., Kubo M., Galli S. J. Differences in the importance of mast cells, basophils, IgE, and IgG versus that of CD4+ T cells and ILC2 cells in primary and secondary immunity to Strongyloides venezuelensis. Infect Immun. 85, https://doi.org/10.1128/IAI.00053-17/SUPPL_FILE/ZII999092030S1.PDF (2017).
Reitz, M., Brunn, M. L., Voehringer, D. & Breloer, M. Basophils are dispensable for the establishment of protective adaptive immunity against primary and challenge infection with the intestinal helminth parasite Strongyloides ratti. PLoS Negl. Trop. Dis. 12, e0006992 (2018).
Kim, B. S. et al. Basophils promote innate lymphoid cell responses in inflamed skin. J. Immunol. 193, 3717–3725 (2014).
Pellefigues, C. et al. Basophils promote barrier dysfunction and resolution in the atopic skin. J. Allergy Clin. Immunol. 148, 799–812.e10 (2021).
He, Y. X., Chen, L. & Ramaswamy, K. Schistosoma mansoni, S. haematobium, and S. japonicum: early events associated with penetration and migration of schistosomula through human skin. Exp. Parasitol. 102, 99–108 (2002).
Angeli, V. et al. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J. Exp. Med 193, 1135–1148 (2001).
Cook, P. C. et al. Multiple helminth infection of the skin causes lymphocyte hypo-responsiveness mediated by Th2 conditioning of dermal myeloid cells. PLoS Pathog. 7, e1001323 (2011). Wynn TA, ed.
Bourke, C. D. et al. Epidermal keratinocytes initiate wound healing and pro-inflammatory immune responses following percutaneous schistosome infection. Int J. Parasitol. 45, 215–224 (2015).
Kim B. S. et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 5, (2013). https://doi.org/10.1126/SCITRANSLMED.3005374/SUPPL_FILE/5-170RA16_SM.PDF (2013).
Smith, K. A. et al. Chronic helminth infection promotes immune regulation in vivo through dominance of CD11cloCD103− dendritic cells. J. Immunol. 186, 7098–7109 (2011).
Feng, X. et al. Atrophy of skin-draining lymph nodes predisposes for impaired immune responses to secondary infection in mice with chronic intestinal nematode infection. PLOS Pathog. 14, e1007008 (2018).
Classon C. H. et al. Intestinal helminth infection transforms the CD4+ T cell composition of the skin. Mucosal Immunol. 1–11. https://doi.org/10.1038/s41385-021-00473-9 (2021).
Hogg, K. G., Kumkate, S. & Mountford, A. P. IL-10 regulates early IL-12-mediated immune responses induced by the radiation-attenuated schistosome vaccine. Int Immunol. 15, 1451–1459 (2003).
Sanin, D. E., Prendergast, C. T., Bourke, C. D. & Mountford, A. P. Helminth infection and commensal microbiota drive early IL-10 production in the skin by CD4+ T cells that are functionally suppressive. PLOS Pathog. 11, e1004841 (2015). Wynn TA, ed.
Saraiva, M. et al. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity 31, 209–219 (2009).
Ramaswamy, K., Kumar, P. & He, Y.-X. A role for parasite-induced PGE2 in IL-10-mediated host immunoregulation by skin stage schistosomula of Schistosoma mansoni. J. Immunol. 165, 4567–4574 (2000).
Colombo, S. A. P. & Grencis, R. K. Immunity to soil-transmitted helminths: evidence from the field and laboratory models. Front Immunol. 11, 1286 (2020).
Glover, M., Colombo, S. A. P., Thornton, D. J. & Grencis, R. K. Trickle infection and immunity to Trichuris muris. PLOS Pathog. 15, e1007926 (2019).
Paterson S., Wilkes C., Bleay C., Viney M. E. Immunological responses elicited by different infection regimes with Strongyloides ratti. PLoS One. 3. https://doi.org/10.1371/JOURNAL.PONE.0002509 (2008).
Weatherhead, J. E. et al. Ascaris larval infection and lung invasion directly induce severe allergic airway disease in mice. Infect. Immun. 86, 533–551 (2018).
Nabeya, D. et al. Pulmonary strongyloidiasis: assessment between manifestation and radiological findings in 16 severe strongyloidiasis cases. BMC Infect. Dis. 17, 1–9 (2017).
Reece, J. J., Siracusa, M. C. & Scott, A. L. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect. Immun. 74, 4970–4981 (2006).
Heitmann, L. et al. TGF-β–responsive myeloid cells suppress type 2 immunity and emphysematous pathology after hookworm infection. Am. J. Pathol. 181, 897–906 (2012).
Hung, L. Y. et al. Trefoil factor 2 promotes type 2 immunity and lung repair through intrinsic roles in hematopoietic and nonhematopoietic cells. Am. J. Pathol. 188, 1161–1170 (2018).
Marsland, B. J., Kurrer, M., Reissmann, R., Harris, N. L. & Kopf, M. Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur. J. Immunol. 38, 479–488 (2008).
Chapman, P. R., Giacomin, P., Loukas, A. & McCarthy, J. S. Experimental human hookworm infection: a narrative historical review. PLoS Negl. Trop. Dis. 15, e0009908 (2021).
Sutherland, T. E. et al. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat. Immunol. 2014 1512 15, 1116–1125 (2014).
Chen, F. et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med 18, 260–266 (2012).
Rieu, P., Ueda, T., Haruta, I., Sharma, C. P. & Arnaout, M. A. The A-domain of beta 2 integrin CR3 (CD11b/CD18) is a receptor for the hookworm-derived neutrophil adhesion inhibitor NIF. J. Cell Biol. 127, 2081–2091 (1994).
Chen, F. et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol. 15, 938–946 (2014).
Mbow, M. et al. T-helper 17 cells are associated with pathology in human schistosomiasis. J. Infect. Dis. 207, 186–195 (2013).
Choy D. F. et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 7. https://doi.org/10.1126/SCITRANSLMED.AAB3142 (2015).
Ajendra, J. et al. IL-17A both initiates, via IFNγ suppression, and limits the pulmonary type-2 immune response to nematode infection. Mucosal Immunol. 13, 958 (2020).
Newcomb, D. C. et al. A functional IL-13 receptor is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J. Immunol. 182, 5317–5321 (2009).
McSorley, H. J. & Smyth, D. J. IL-33: a central cytokine in helminth infections. Semin Immunol. 53, 101532 (2021).
Cayrol, C. & Girard, J. P. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 31, 31–37 (2014).
de Kleer, I. M. et al. Perinatal activation of the interleukin-33 pathway promotes type 2 immunity in the developing lung. Immunity 45, 1285–1298 (2016).
Snelgrove R. J. et al. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 134. https://doi.org/10.1016/J.JACI.2014.02.002 (2014)
Poulsen, N. N. et al. Airway Interleukin-33 and type 2 cytokines in adult patients with acute asthma. Respir. Med 140, 50–56 (2018).
Chen, Y. L. et al. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci. Transl. Med 11, 2945 (2019).
Chu C. et al. The ChAT-acetylcholine pathway promotes group 2 innate lymphoid cell responses and anti-helminth immunity. Sci Immunol. 6. https://doi.org/10.1126/SCIIMMUNOL.ABE3218 (2021)
Pavón-Romero, G. F., Serrano-Pérez, N. H., García-Sánchez, L., Ramírez-Jiménez, F. & Terán, L. M. Neuroimmune Pathophysiology in Asthma. Front Cell Dev. Biol. 9, 1174 (2021).
Gori, S. et al. Acetylcholine polarizes dendritic cells toward a Th2-promoting profile. Allergy 72, 221–231 (2017).
Barner, M., Mohrs, M., Brombacher, F. & Kopf, M. Differences between IL-4Rα-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr. Biol. 8, 669–672 (1998).
Grencis A. J., Bancroft A. N. J., Mckenzie R. K. Intestinal Nematode Infection A Critical Role for IL-13 in Resistance to. Published online 1998. Accessed Dec 9, 2021. http://www.jimmunol.org/content/160/7/3453
Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl Acad. Sci. USA 107, 11489–11494 (2010).
Wilson, M.S., et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 207, 535–552 (2010).
Gieseck, R. L., Wilson, M. S. & Wynn, T. A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 18, 62–76 (2018).
Chenery A. L. et al. IL-13 deficiency exacerbates lung damage and impairs epithelial-derived type 2 molecules during nematode infection. Life Sci Alliance. 4. https://doi.org/10.26508/LSA.202001000 (2021).
Zurawsli, S. M., Vega, F., Huyghe, B. & Zurawski, G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J. 12, 2663 (1993).
Bosurgi, L. et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science (80-) 356, 1072–1076 (2017).
Oeser, K., Schwartz, C. & Voehringer, D. Conditional IL-4/IL-13-deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunol. 2015 83 8, 672–682 (2014).
Hogan, S. P. et al. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J. Immunol. 161, 1501–1509 (1998).
Grünig, G. et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science (80-) 282, 2261–2263 (1998).
Minutti, C. M. et al. Local amplifiers of IL-4Ra-mediated macrophage activation promote repair in lung and liver. Science (80-) 356, 1076–1080 (2017).
Chen, F. et al. Helminth resistance is mediated by differential activation of recruited monocyte-derived alveolar macrophages and arginine depletion. Cell Rep. 38, 110215 (2022).
Campbell, L. et al. ILC2s mediate systemic innate protection by priming mucus production at distal mucosal sites. J. Exp. Med 216, 2714 (2019).
Filbey, K. J. et al. Intestinal helminth infection promotes IL-5- and CD4 + T cell-dependent immunity in the lung against migrating parasites. Mucosal Immunol. 12, 352–362 (2019).
Zhao, A. et al. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology 135, 217–225.e1 (2008).
Zhao, A. et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J. Immunol. 171, 948–954 (2003).
Khan, W. I. et al. Stat6 dependent goblet cell hyperplasia during intestinal nematode infection. Parasite Immunol. 23, 39–42 (2001).
Shekels, L. L. et al. Coordinated Muc2 and Muc3 mucin gene expression in Trichinella spiralis infection in wild-type and cytokine-deficient mice. Dig. Dis. Sci. 46, 1757–1764 (2001).
Hasnain, S. Z., Thornton, D. J. & Grencis, R. K. Changes in the mucosal barrier during acute and chronic Trichuris muris infection. Parasite Immunol. 33, 45–55 (2011).
Webb, R. A., Hoque, T. & Dimas, S. Expulsion of the gastrointestinal cestode, Hymenolepis diminuta by tolerant rats: evidence for mediation by a Th2 type immune enhanced goblet cell hyperplasia, increased mucin production and secretion. Parasite Immunol. 29, 11–21 (2007).
Sharpe, C., Thornton, D. J. & Grencis, R. K. A sticky end for gastrointestinal helminths; the role of the mucus barrier. Parasite Immunol. 40, e12517 (2018).
Forgue-Lafitte, M. E. et al. Abnormal expression of M1/MUC5AC mucin in distal colon of patients with diverticulitis, ulcerative colitis and cancer. Int J. Cancer 121, 1543–1549 (2007).
Heazlewood, C. K. et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLOS Med 5, e54 (2008).
Fu, J. et al. Loss of intestinal core 1–derived O-glycans causes spontaneous colitis in mice. J. Clin. Invest 121, 1657 (2011).
Hasnain S. Z. et al. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology. 138, (2010). https://doi.org/10.1053/J.GASTRO.2010.01.045
Herbert, D. R. et al. Intestinal epithelial cell secretion of RELM-β protects against gastrointestinal worm infection. J. Exp. Med 206, 2947–2957 (2009).
Nair, M. G. et al. Goblet cell-derived resistin-like molecule β augments CD4+ T cell production of IFN-γ and infection-induced intestinal inflammation. J. Immunol. 181, 4709–4715 (2008).
Minutti, C. M. et al. Epidermal growth factor receptor expression licenses type-2 helper T cells to function in a T cell receptor-independent fashion. Immunity 47, 710 (2017).
Shimokawa, C. et al. Mast cells are crucial for induction of group 2 innate lymphoid cells and clearance of helminth infections. Immunity 46, 863–874.e4 (2017).
Hung, L. Y. et al. Cellular context of IL-33 expression dictates impact on anti-helminth immunity. Sci. Immunol. 5, 6259 (2020).
Gerbe, F. et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nat 2016 5297585 529, 226–230 (2016).
Schütz, B. et al. Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract. Sci. Rep. 2019 91 9, 1–13 (2019).
Ualiyeva S. et al. Tuft cell–produced cysteinyl leukotrienes and IL-25 synergistically initiate lung type 2 inflammation. Sci Immunol. 6, (2021). https://doi.org/10.1126/SCIIMMUNOL.ABJ0474
Hildersley, K. A. et al. Tuft cells increase following ovine intestinal parasite infections and define evolutionarily conserved and divergent responses. Front Immunol. 12, 4979 (2021).
McGinty, J. W. et al. Tuft-cell-derived leukotrienes drive rapid anti-helminth immunity in the small intestine but are dispensable for anti-protist immunity. Immunity 52, 528–541.e7 (2020).
Drurey C. et al. Intestinal epithelial tuft cell induction is negated by a murine helminth and its secreted products. J Exp Med. 219, (2022). https://doi.org/10.1084/JEM.20211140/212820
Bancroft, A. J., McKenzie, A. N. & Grencis, R. K. A critical role for IL-13 in resistance to intestinal nematode infection. J. Immunol. 160, 3453–3461 (1998).
Waddell, A., Vallance, J. E., Hummel, A., Alenghat, T. & Rosen, M. J. IL-33 Induces Murine Intestinal Goblet Cell Differentiation Indirectly via Innate Lymphoid Cell IL-13 Secretion. J. Immunol. 202, 598–607 (2019).
Turner J. E., Stockinger B., Helmby H. IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection. PLoS Pathog. 9. https://doi.org/10.1371/JOURNAL.PPAT.1003698 (2013).
Gaze S. et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. Published online 2012. https://doi.org/10.1371/journal.ppat.1002520
Broadhurst M. J. et al. IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med. 2. https://doi.org/10.1126/SCITRANSLMED.3001500/SUPPL_FILE/2-60RA88_SM.PDF (2010).
Marillier, R. G. et al. IL-4/IL-13 independent goblet cell hyperplasia in experimental helminth infections. BMC Immunol. 9, 11 (2008).
Oyesola, O. O. et al. The prostaglandin D 2 receptor CRTH2 promotes IL-33–induced ILC2 accumulation in the lung. J. Immunol. 204, 1001–1011 (2020).
Oyesola O. O. et al. PGD2 and CRTH2 counteract Type 2 cytokine–elicited intestinal epithelial responses during helminth infection. J Exp Med. 218. https://doi.org/10.1084/JEM.20202178/212485 (2021).
Jacobson, A., Yang, D., Vella, M. & Chiu, I. M. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021 143 14, 555–565 (2021).
Moriyama, S. et al. β2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science (80-) 359, 1056–1061 (2018).
Ahrends, T. et al. Enteric pathogens induce tissue tolerance and prevent neuronal loss from subsequent infections. Cell 184, 5715–5727.e12 (2021).
Progatzky, F. et al. Regulation of intestinal immunity and tissue repair by enteric glia. Nat 2021 5997883 599, 125–130 (2021).
Ferrer-Font L. et al. High-dimensional analysis of intestinal immune cells during helminth infection. Elife. 9, (2020). https://doi.org/10.7554/ELIFE.51678 (2020).
Webster, H. C., Andrusaite, A. T., Shergold, A. L., Milling, S. W. F. & Perona-Wright, G. Isolation and functional characterisation of lamina propria leukocytes from helminth-infected, murine small intestine. J. Immunol. Methods 477, 112702 (2020).
Chapmanid, P. R., Giacominid, P., Loukas, A. & Mccarthyid, J. S. Experimental human hookworm infection: a narrative historical review. PLoS Negl. Trop. Dis. 15, e0009908 (2021). Cotton J, ed.
Wright V., Bickle Q. Immune responses following experimental human hookworm infection. Clin Exp Immunol. Published online 2005. https://doi.org/10.1111/j.1365-2249.2005.02945.x
Geiger, S. M., Fujiwara, R. T., Santiago, H., Corrêa-Oliveira, R. & Bethony, J. M. Early stage-specific immune responses in primary experimental human hookworm infection. Microbes Infect. 10, 1524–1535 (2008).
Summers, R. W., Elliot, D. E., Urban, J. F., Thompson, R. & Weinstock, J. V. Trichuris suis therapy in Crohn’s disease. Gut 54, 87–90 (2005).
Summers, R. W., Elliott, D. E., Urban, J. F., Thompson, R. A. & Weinstock, J. V. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 128, 825–832 (2005).
Quinn S. M. et al. Anti-inflammatory trained immunity mediated by helminth products attenuates the induction of T cell-mediated autoimmune disease. Front Immunol. 10. https://doi.org/10.3389/FIMMU.2019.01109/FULL (2010).
Cunningham, K. T., Finlay, C. M. & Mills, K. H. G. Helminth imprinting of hematopoietic stem cells sustains anti-inflammatory trained innate immunity that attenuates autoimmune disease. J. Immunol. 206, 1618–1630 (2021).
Acknowledgements
We would like to express our gratitude to Jodie Chandler for manuscript editing and the Health Research Council of New Zealand and Marjorie Barclay Trust for their support.
Author information
Authors and Affiliations
Contributions
F.V. wrote the manuscript. G.L.G provided feedback and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vacca, F., Le Gros, G. Tissue-specific immunity in helminth infections. Mucosal Immunol 15, 1212–1223 (2022). https://doi.org/10.1038/s41385-022-00531-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-022-00531-w
This article is cited by
-
The Relationship of Parasite Allergens to Allergic Diseases
Current Allergy and Asthma Reports (2023)
-
Lessons from helminths: what worms have taught us about mucosal immunology
Mucosal Immunology (2022)