Abstract
IL-10-expressing regulatory B cells (B10 cells) are dysfunctional in patients with many immune disorders. The underlying mechanism remains to be further elucidated. Glutamine is an essential nutrient for cell metabolism. This study aims to elucidate the role of glutaminolysis in maintaining the immune regulatory capacity in B10 cells. Peripheral blood samples were collected from 50 patients with allergic rhinitis and 50 healthy control subjects. B cells were isolated from blood samples by cell sorting with flow cytometry. The role of glutaminolysis in regulating B10 cell activities was assessed by immunological and biochemical approaches. The results showed that B cells from patients with allergic rhinitis expressed low levels of the transporter of glutamine and neutral amino acid. Glutaminolysis was required in the IL-10 expression in B cells. The glutamine catabolism was required in B10 cell generation. The mTOR activation mediated the glutaminolysis-associated B10 cell induction, and the suppression of the B cell glycogen synthase kinase-3 (GSK3) activation. GSK3 activation suppressed IL-10 expression in B cells. Inhibition of GSK3 enhanced IL-10 expression in B cells and alleviated experimental allergic rhinitis by generating immune competent type 1 regulatory T cells.
Similar content being viewed by others
Introduction
IL-10+ regulatory B cells (B10 cell, in short) are a fraction of immune regulatory cells in the body. By releasing immune regulatory cytokines, such as interleukin (IL)−10, B10 cells suppress other immune cell activities, that contributes to restricting immune responses within a proper range to avoid inducing unnecessary tissue injury1. The B10 cell dysfunction has been noted that plays an important role in the pathogenesis of many immune disorders, such as allergic diseases (allergic rhinitis (AR), asthma, dermatitis, etc.) and autoimmune diseases (diabetes, rheumatic arthritis, multiple sclerosis, etc.)2. The underlying mechanism of B10 cell dysfunction is not fully understood yet. Current therapies for B10 cell dysfunction are limited and not satisfactory3.
It has been noted that nutritional factors are associated with the pathogenesis of immune diseases4. The cell fuel supply, mainly including glutamine and glucose, is required in maintaining cell activities. L-glutamine is an amino acid involving immune regulation and rapidly provide fuel for cell activities5. ASCT2 (alanine, serine, cysteine-preferring transporter 2) is a cell surface solute-carrying transporter on up taking neutral amino acids including glutamine6. Although glutamine is not an essential amino acid, it is important for cells by providing anabolic substrates for biosynthesizing macromolecules and catabolic substrates for ATP production7. Published data indicate that glutamine has a close relationship with immune responses8. Yet, whether the glutamine metabolism is associated the pathogenesis of allergic disorders, such as AR and asthma, remains to be investigated.
Both glutamine and glucose are metabolized in cells through the tricarboxylic acid (TCA) cycle. In TCA, glutamine and glucose can activate the mammalian target of rapamycin (mTOR). mTOR can be activated by many extracellular signals, including growth factors, insulin, nutrients, amino acids, glucose and other stimuli. It is mainly through the PI3K/Akt/mTOR signal pathway to achieve the regulation of cell growth, cell cycle and other physiological functions of cells. Comparing with glucose, glutamine mainly activates mTORc1, that can be inhibited by rapamycin9. mTOR activities are associated with the pathogenesis of many immune disorders10 including AR11. Thus, we hypothesize that glutamine metabolic disorders may be involved in the pathogenesis of immune deregulation. In this study, we analyzed the glutamine metabolism in B cells isolated from blood samples collected from AR patients. The results showed that the low expression of ASCT2 was found in AR B cells, that resulted less mTORc2 signals, and consequently, resulted in high glycogen synthase kinase activation, that inhibited the IL-10 expression in B cells.
Results
B cells from AR patients express low levels of ASCT2
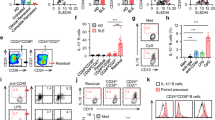
To assess the nutrient consuming profiles in B cells, PBMCs were isolated from blood samples collected from 50 AR patients and 50 HC subjects, and analyzed by fluorescence-activated cell sorting (FACS). The results showed that the B cell frequency in PBMCs was not significantly different between the AR group [in median (IQR), 10.95% (7.08%, 12.96%) and the HC group [10.27% (7.57%, 12.94%); p = 0.76, AR vs HC] (Fig. 1a–c), in which B10 cell (CD19+ IL-10+ B cell) frequency was 11.83% (9.03%, 14.79%) in the HC group while only 3.29% (1.01%, 4.85%) in the AR group (Fig. 1d, e). Further analysis showed that the ASCT2 (the glutamine transporter) expression in IL-10− B cells was 51.6 (35.2, 68.9; the mean fluorescence intensity, MFI) in the HC group, and 13.19 (5.02, 19.84) in the AR group. In B10 cells, the ASCT2 expression was 54.4 (35.2, 69.6) in the HC group, and 11.14 (5.03, 19.87) in the AR group. The expression of Glut1 (the glucose transporter) was detected in more than 80% of IL-10− B cells and B10 cells; no significant difference was detected between the HC group and the AR group, or between IL-10− B cells and B10 cells (Fig. 1h, i). The results demonstrate that the ASCT2 expression is lower in AR B cells, including both IL-10− B cells and B10 cells.
PBMCs were isolated from blood samples collected from AR patients (n = 50) and HC patients (n = 50) and analyzed by FACS. a The FSC/SSC plots. b Gated FACS plots show CD19+ B cell frequency. c Boxplots show summarized CD19+ B cell counts. d, e Gated FACS plots show IL-10+ CD19+ B cells (B10 cells); boxplots show summarized B10 cell counts. f, g Gated FACS histograms show ASCT2+ IL-10− B cells and B10 cells; boxplots show MFI of ASCT2 in IL-10− B cells and B10 cells against the total MFI. h, i Gated FACS histograms show Glut1+ IL-10− B cells and B10 cells; boxplots show MFI of Glut1 in IL-10− B cells and B10 cells. ***p < 0.001 (Mann Whitney-U test), compared with the HC group. Each dot in boxplots presents data obtained from one sample (samples were tested in triplicate). The FACS plots are from one experiment, that represent 50 independent tests. The group labels of g, i are the same as that in FACS histograms on the left side.
glutamine is required in IL-10 expression in B cells
As ASCT2 is the major transporter of glutamine12, the results of Fig. 1 show that B cells from AR patients express lower IL-10 levels, we inferred that glutamine plays a role in the IL-10 expression in B cells. To test this, we collected blood samples from HC subjects; B cells were isolated from the samples by cell sorting. As B cells do not spontaneously express IL-10, we cultured B cells in the presence of CD40L and CpG (CpG can induce IL-10 expression in B cells13) for 24 h. The expression of IL-10 by B cells were analyzed by RT-qPCR and enzyme-linked immunosorbent assay (ELISA). We found that exposure to CD40L and CpG significantly increased the IL-10 expression by B cells. Removal of glutamine, but not glucose removal, from culture medium prevented the expression of IL-10 by B cells (Fig. 2a, b), that did not alter the TNF-α expression in B cells (Fig. 2c, d). The results demonstrate that glutamine, but not glucose, plays a crucial role in the IL-10 expression in B cells. The cell viability was greater than 99% as assessed by Trypan blue exclusion assay after culture in the absence of either glutamine or glucose (Fig. 2e). After exposure to CD40L/CpG in culture for 24 h, B cells proliferated by 0.1–0.4 folds, which was not affected by removal of either glutamine or glucose from culture medium (Fig. 2f). Notably, we also observed less IL-10 expression in B cells isolated from AR patients than that from HC subjects in response to CD40L/CpG stimulation in the culture (Fig. 2a, b, d).
Naive B cells were isolated from blood samples collected from 20 HC subjects and 20 AR patients; cells were cultured (106 cells/sample) for 24 h with the conditions denoted on the x-axis of boxplots. Saline: Saline was added to culture. CD40L/CpG: CD40L (1 µg/ml)/CpG (10 µg/ml) were added to culture to activate B cells. glutamine-d: The glutamine-deficient culture medium. Glucose-d: The glucose-deficient culture medium. a IL-10 mRNA levels in B cells. b IL-10 levels in culture supernatant. c TNF-α mRNA levels in B cells. d TNF-α protein levels in culture supernatant. e Cell viability (assessed by trypan blue exclusion assay). f Cell counts. Each dot in boxplots presents data obtained from one sample. *p < 0.05, ***p < 0.001, compared with the saline group. ###p < 0.001, compared with the HC B cell/CD40L/CpG group. Statistical method: ANOVA + Dunnett’s test. The experiments were repeated five times.
Glutamine catabolism is required in B10 cell induction
The expression of IL-10 is the cornerstone of B10 cell generation1. As glutamine is required in the IL-10 expression in B cells as shown by Fig. 2, we tested the role of glutaminolysis in the B10 cell generation. Naive B cells were isolated from HC subject blood samples by cell sorting and cultured in the presence of CD40L and CpG. After 3-day culture, we detected that about 13% B cells expressed IL-10 and CD35, this is in line with previous reports14, indicating that a portion of B cells were induced into B10 cells. To test the role of glutaminolysis in B10 cell induction, we added BPTES (an inhibitor of glutaminase as that is the enzyme to convert glutamine to glutamate; the latter enters the TCA) to the culture. Indeed, the B10 cell induction was abolished. The results demonstrate that the glutamine catabolism is required in B10 cell induction. To verify the results, we added DMOG (1 mM15; an analogue of α-ketoglutarate, α-KG) into the culture of BPTES (10 μM16)-treated B cells (α-KG is the form of glutamate enters TCA). The addition of DMOG rescued the B10 cell induction in the presence of BPTES (Fig. 3a, b). The changes of IL-10 mRNA were also detected in B cells that were in parallel to the IL-10+ B cell number changes (Fig. 3c); similar changes were also detected in IL-10 protein in culture supernatant (Fig. 3d). It is noteworthy that the absence of glucose in the culture did not affect the B10 cell generation by CD40L/CpG. Additionally, apart from the portion of IL-10+ B cells expressing CD35, there are more cells expressing CD35 (Fig. 3a), which may be other leukocytes17. The results demonstrate that the glutamine catabolism is crucial in B10 cell development.
B cells isolated from HC subjects were cultured in the conditions denoted above each FACS plot for 3 days. CD40L: 1 µg/ml. CpG: 10 µg/ml. BPTES: 10 µM. DMOG: 1 mM. glutamine-d: glutamine-deficient medium. Glucose-d: Glucose-deficient medium. a Gated FACS plots show CD35+ IL-10+ B cell frequency. b Boxplots show B10 cell counts from ten independent experiments. Boxplots show IL-10 mRNA in B cells (c) and IL-10 protein in culture supernatant (d). ***p < 0.001 (ANOVA followed by the Dunnett’s test), compared with group a. The group labels of b are the same as those in a. Each dot in boxplots presents data obtained from one sample. The FACS plots of a are from one experiment that represent six independent experiments. Exposure to BPETS solvent, DMSO (0.5 μl/ml), did not alter the IL-10 expression in B cells (data not shown). Cell viability at the end of culture was greater than 99%.
mTORc1 activation mediates the glutamine-induced B10 cell generation
As mTORc1 can be activated by glutaminolysis9, we inferred that mTORc1 might be the factor mediating the effects of glutamine in B10 cell development. To test this, after exposure to glutamine in the culture, B cells were isolated from blood samples collected from HC subjects and AR patients, and exposed to CD40L/CpG in the culture with or without glutamine in the culture overnight. B cells were collected at the end of culture, and processed to assess the levels of mTORc1 and S6 phosphorylation (S6 is a component of the downstream of mTORc1 signal pathway)18. We found that the presence of glutamine was required in the increase in the phosphor mTORc1 levels and S6 phosphorylation in HC B cells induced by CD40L/CpG, while AR B cells did not show increase in the IL-10 expression in response to CD40L/CpG in the culture (Fig. 4a–d), that were positively correlated with the IL-10 levels in culture supernatant (Fig. 4e–g). The results indicate that the mTORc1 pathway is involved in the glutamine-induced IL-10 expression in B cells. To verify this, we added rapamycin (an inhibitor of mTORc1) to culture medium. Indeed, the glutamine-induced B cell IL-10 expression was abolished (Fig. 4e). The results demonstrate that the mTORc1 signal pathway mediates the glutamine-induced IL-10 expression in B cells. In addition, the absence of glutamine did not show appreciable effects on the non-phosphor mTORc1 or S6 levels in B cells (Fig. 4a–d).
a–d B cells were isolated from blood samples collected from HC subjects and AR patients, and exposed to reagents listed below the immunoblots of a. Glutamine: glutamine in the culture medium. Glutamine-d: glutamine-deficient culture medium. Rapa: Rapamycin in the culture (10 nM). CD40L: 1 µg/ml. CpG: 10 µg/ml. The immunoblots show phosphor mTORc1 (p-mTORc1) (a) and phosphor S6 (pS6) (c) in B cells. The scatter plots show integrated density of p-mTORc1 blots (b) and pS6 blots (d). e The scatter dot plots show the IL-10 levels in culture supernatant. The boxplots show positive correlation between the IL-10 levels in supernatant and the pmTORc1 levels (f) and the pS6 levels (g) in B cells. ***p < 0.001 (ANOVA followed by the Dunnett’s post hoc test), compared with the saline group. The data represent ten independent experiments.
GSK3 (glycogen synthase kinase-3) activation suppresses IL-10 expression in B cells
The data of Figs. 1–4 show that AR B cells express lower ASCT2 levels, that results in less glutaminolysis and less mTORc1 phosphorylation in B cells. As mTORc1 plays a critical role in restricting the GSK3 activities19, GSK3 inhibits the IL-10 expression in B cells20, the results implicate that GSK3 may be involved in the signal pathway by restricting the glutamine-induced IL-10 expression in B cells. To this end, we firstly assessed the GSK3 activation status in AR B cells. The results showed that GSK3 was indeed highly phosphorylated in AR B cells (Fig. 5a–d). We then activated HC B cells with CD40L/CpG in the culture in the presence of glutamine, and either BPTS (an inhibitor of glutaminase) or rapamycin (an inhibitor of mTORc1). The results showed that the presence of glutamine inhibited GSK3 phosphorylation, that was abolished by the presence of either BPTS or rapamycin (Fig. 5e–j). We also found that the IL-10 levels in culture supernatant were reversely correlated with GSK3 phosphorylation in B cells (Fig. 5k), suggesting that the GSK3 activation plays a crucial role in restricting the IL-10 expression. Additionally, B cells were isolated from AR patient blood samples, and cultured in glutamine-deficient medium in the presence of CD40L/CpG and with or without a GSK3 inhibitor. The results showed that the presence of CD40L/CpG/GSK3 inhibitor (SB216763) significantly induced the IL-10 production by AR B cells, while the presence of CD40L/CpG (without GSK3 inhibitor) did not induce the IL-10 production by AR B cells (Fig. 5k). The results suggest that the inhibition of GSK3 restores the IL-10 production in AR B cells.
a–d B cells were isolated from blood samples of HC subjects (n = 10) and AR patients (n = 10), and immediately analyzed by western blotting. The immunoblots show the GSK3 phosphorylation status in B cells; boxplots show blot density. e–k Naive B cells were treated with the reagents in the culture as denoted below each panel. CD40L: 1 µg/ml. Glutamine: The presence of glutamine in the culture. CpG: 10 µg/ml. BPTES: 10 µM. GSK3 inhibitor (SB216763, 10 µM). The immunoblots show the GSK3 phosphorylation status in B cells (e, h); boxplots show blot density (f, g, i, j). k Boxplots show IL-10 levels in culture supernatant. The data in boxplots are presented as median (IQR). ***p < 0.001, compared with the HC group (c) or the saline group (i, j, k). Statistical methods: ANOVA + Bonferroni test (c) or Dunnett’s test (i, j, k). The data of a, c, e, h are from one experiment that represent ten independent experiments. The cell viability was greater than 99% at the end of experiments.
Inhibition of GSK3 alleviates experimental allergic rhinitis (AR) by generating type 1 regulatory T cells
The data of Fig. 5 suggest that inhibition of GSK3 may alleviate allergic disorders. Following published procedures21, we prepared an AR mouse model with ovalbumin (OVA) as a specific antigen (Fig. 6a). One day after completing the sensitization, AR mice were treated with GSK3 inhibitor (SB216763, SB, in short) or saline through nasal instillation daily for 1 week. One day after completing the SB-therapy, mice were challenged with OVA (the specific antigen) through nasal instillation. AR mice showed AR-like response, including nasal itch (scratching the nasal skin), sneezing, profound nasal discharge (Fig. 6b–d), high levels of mouse mast cell protease-1, EPX (eosinophil peroxidase) in nasal secretions (Fig. 6e, f), and specific IgE, Th2 cytokines and IL-10 in nasal tissue protein extracts (Fig. 6g–k). The AR-response was markedly attenuated in the SB-therapy group, that was abolished by administration of an IL-10 inhibitor AS101 (Fig. 6b–k). In addition, single cells were prepared with the nasal tissues, from which we also found that the ASCT2 expression was significantly lower in AR mouse nasal mucosal B cells than that in naive control B cells (Fig. 6l); the frequency of eosinophils and mast cells were increased in the AR group as compared with that in the NC group. Treating AR mice with SB reduced the frequency of eosinophils and mast cells in the AR nasal mucosa, that was abolished in the presence of AS101 (Fig. 6l–o). In addition, treating AR mice with AS101 alone exacerbated the AR response (data not shown).
a A schematic of AR mouse model preparation. b–k AR mice were treated with NS (saline) or SB or SBi (both SB and AS101, an inhibitor of IL-10) in nasal instillation (AS101 = 1 mg/ml; SB = 1 mg/ml) daily for 1 week. Scatter dot plots show AR symptoms (nasal scratches, sneezing, and nasal discharges; b–d), Mcp1 and EPX (e, f) in nasal secretions, specific IgE (sIgE), Th2 cytokines and IL-10 in nasal tissue protein extracts (g–k). l–n Single cells were isolated from the nasal mucosal tissues, and analyzed by RT-qPCR, western blotting and FACS. l B cells were isolated from the single cells by cell sorting. Immunoblots show ASCT2 protein, boxplots show the integrated density of immunoblots or ASCT2 mRNA levels in B cells. m, n Gated FACS plots show eosinophil (Eo) counts and mast cell counts; scatter plots show Eo frequency and mast cell frequency. MCC mast cell chymase. The data are presented as mean ± SD; each dot presents data obtained from one mouse. **p < 0.01, ***p < 0.001 (ANOVA followed by the Bonferroni post hoc test), compared with the NS-treated AR group. NC naive control mice (no treatment). Each group consists of six mice. Each experiment was performed three times (two mice each time).
Furthermore, by isolating B cells from the mouse nasal mucosa by cell sorting and analyzed by FACS, we found that the B10 cell frequency was lower in the AR group than that of the naive control group. After the SB-therapy, the B10 cell frequency in the AR nasal mucosa was significantly increased (Fig. 7a, b), that was reversely associated with the changes of GSK3 phosphorylation in B cells (Fig. 7c, d), indicating that the SB-therapy suppresses the GSK3 activation to restore the B10 cell generation in the nasal mucosa.
An AR mouse model was prepared as described in Fig. 6. Single cells were isolated from the nasal mucosal tissues and analyzed by FACS, RT-qPCR, and western blotting. a, b Gated FACS plots show IL-10+ B cell (B10) counts. Dot plots show B10 cell frequency. Dot plots show integrated density of immunoblots. c, d B cells were isolated from the nasal tissues, and analyzed by western blotting. Immunoblots show phosphorylated GSK3 levels. Scatter plots show integrated density of immunoblots. e CD3+ CD4+ T cells were gated. f Gated FACS plots show Tr1 cell counts (the LAG3+ IL-10+ cells were also CD49b+, not shown). g Scatter dot plots show Tr1 cell frequency. The data of b, e are presented as mean ± SD. ***p < 0.001 (ANOVA followed by the Bonferroni post hoc test), compared with the NS-treated AR group. Each dot presents data obtained from one mouse. Each group consists of six mice. The data of a, c, e, f are from one experiment, that represent three independent experiments. SBi: Mice were treated with both SB and IL-10 inhibitor AS101 (the “i” stands for “inhibitor”). Final concentration in nasal instillation: AS101 = 1 mg/ml; SB = 1 mg/ml).
In the cells isolated from the nasal mucosa, we also detected that type 1 regulatory T cells (Tr1 cell, characterized expressing CD3, CD4, LAG3, CD49b and IL-1022), that were markedly increased in AR mice with SB-therapy (Fig. 7e–g). The results implicate that the SB-therapy also increases Tr1 cell development by inhibiting GSK3 activities in B cells. To verify this, we primed AR B cells (B cells isolated from AR patients) with CD40L and CpG in the presence or absence of SB in the culture for 24 h. The B cells were washed with fresh medium and cocultured with naive CD4+ T cells [isolated from blood samples collected from HC subjects and the same AR patients (self-AR CD4+ T cells), respectively] for 3 days. Indeed, SB-primed B cells induced about 25% Tr1 cells from HC CD4+ T cells and self-AR CD4+ T cells, while only about 1% Tr1 cells were detected in those exposed to non-primed AR B cells (Fig. 8a–c). The induced Tr1 cells could suppress CD4+ T cell proliferation (Fig. 8d, e). The results demonstrate that GSK3 inhibition can restore the B cell tolerogenic properties, that can induce immune competent Tr1 cells.
B cells (from AR patients) were primed with CD40L/CpG in the culture in the presence or absence of SB (10 µM) for 24 h, and cultured with naive CD4+ T cells (from HC subjects, n = 3, and AR subjects, n = 3) at ratio of 1:10 (B cell:T cell) for 3 days. Cells were then analyzed by FACS. a CD4+ T cells were gated. b Gated cells show Tr1 cell counts. c Dot plots show Tr1 cell frequency from three independent experiments. d, e CD4+ CD25¯ CD62L+ T cells (Naive CD4+ T cells; from HC subjects; labeled with CFSE) were cultured with Tr1 cells (generated as described in b) at ratio of 1:10 (Tr1:Teff) in the presence of anti-CD3/CD28 Abs for 3 days. Teff proliferation was assessed by FACS. Gated histograms show proliferating Teff counts. Dot plots show proliferating Teff frequency. ***p < 0.001 (ANOVA followed by the Dunnett’s post hoc test), compared with group a (c) or group b (e). NS no stimulation group. Group labels of c, e are the same as FACS plots on the left side. The data of a, b, d are from one experiment, that represent three independent experiments.
Discussion
The present data show that glutamine catabolism, but not glucose catabolism, plays a crucial role in B10 cell generation. We found that the ASCT2 levels were lower in peripheral B cells isolated from AR patients as compared with that of the HC subjects. The low ASCT2 expression was correlated with the lower IL-10 expression in B cells. Since ASCT2 is the transporter of glutamine, the data indicate that less glutamine can enter B cells of AR patients. This was verified by in vitro experiments; we found that the induction of the IL-10 expression in B cells was compromised in the absence of glutamine in culture, that was not affected by the absence of glucose. The results emphasize that glutamine is required in the induction of IL-10 in B cells. The results showed that the GSK3 activation played a key role in the inhibition of IL-10 in B cells. The GSK3 inhibition could be induced by glutaminolysis. Additionally, we also found that inhibition of GSK3 could promote the IL-10 expression in B cells, that consequently, inhibited experimental AR by the generation of immune competent Tr1 cells.
B10 cells are one of the major cellular parts in the immune regulatory system. The B10 cell dysfunction or reduction of B10 cell numbers has been found playing an important role in the pathogenesis of many immune disorders, such as AR23, allergic asthma24 and allergic dermatitis25. The immune regulatory function of B10 cell is mediated primarily through the IL-10 production26. Thus, the induction of IL-10 expression in B cells has caught much attention. In fact, many attempts have been tried to induce B cells to produce IL-10, such as IL-10 itself, lipopolysaccharide, CpG, etc27. The present study found that the IL-10 induction in B cells could be naturally achieved. In other words, in a physiological environment, one of the major nutrients, glutamine, serves as an inducer of IL-10 in B cells.
The data show that glutaminolysis in B cells maintains the IL-10 expression by restricting GSK3 activation. glutamine and glucose are the two major nutritional sources for the cellular energy suppling. Although the catabolism of both glutamine and glucose can activate the mTOR signal pathway, mTORc1 can inhibit GSK3 activation. Glutaminolysis mainly activates mTORc19. The less ASCT2 expression implicates the less glutaminolysis in B cells, and thus, results in less mTORc1 activation. Such a condition was mimicked by culturing B cells in glutamine-deficient culture medium. The results indeed showed less mTORc1 phosphorylation in B cells, and consequently, the GSK3 phosphorylation was markedly promoted, and the IL-10 production was diminished.
It has been recognized that GSK3 is a multitasking kinase. The elevation of GSK3 activity is associated with a number of diseases including asthma, chronic inflammation, bipolar disorder, arthritis, cancer, and diabetes28. The present data add novel information to this study spot by showing that B cells isolated from patients with AR, one of the allergic diseases, have high GSK3 levels that are associated with the incompetent in the IL-10 production; this is presumably because of the decrease in ASCT2. It is naturally conceivable that the inhibition of GSK3 may reconcile the condition of immune deregulation in AR subjects. The inference was verified in an AR animal model study. By administrating a GSK3 inhibitor, AR response was efficiently alleviated, in which the generation of Tr1 cells played a crucial role. On the other hand, besides produced by B10 cells, IL-10 can be produced by other immune cells, such as tolerogenic DCs; the DC-derived IL-10 also induce Tr1 cells29. Whether the ASCT2-insufficient or -deficient in DCs compromises the Tr1 cell induction through the diminished glutaminolysis and over-activation of GSK3 pathway is an interesting topic to be investigated.
To our knowledge, this is the first report to describe the abnormal glutaminolysis and GSK3 activities in AR B cells. The data demonstrate that the insufficient glutaminolysis results in the GSK3 over activation; the latter, in turn, inhibits the IL-10 expression in B cells; this may be one of the reasons inducing B10 cell dysfunction and reducing B10 cell development. It has been noted that the B10 cell dysfunction is associated with many immune diseases30. Our data reveal that the energy metabolism may be abnormal in immune regulatory cells; this may contribute to the pathogenesis of immune disorders by causing immune regulatory cell dysfunction. Importantly, the data show that the inhibition of GSK3 can restore the IL-10 expression in AR B cells; the latter can induce immune competent Tr1 cells from HC CD4+ T cells as well as self-AR CD4+ T cells. On the other hand, we did not perform the experiments to observe whether GSK3 reversely had any effects on regulating the activities of mTORc1 in the present study, which may be investigated in the future. Others also observed GSK3 in CD4+ T cells; inhibition of GSK3 promoted the induction of Tr1 cells31. This further emphasizes the translation potential of the GSK3 inhibition in the treatment of immune disorders.
We observed that the ASCT2 levels were lower in B cells of AR patients as well as in B cells isolated from the AR mouse nasal tissues. The present data do not provide the causative factors or mechanism for this result. Further investigation is warranted to elucidate the etiology of ASCT2 dysfunction in B cells of AR patients. To find the way increasing ASCT2 expression in AR B cells may be an efficient approach to improving B10 cell function. As ASCT2 is involved in cell activities of many cells, whether ASCT2 is involved in the cell activities of other immune cells, such as DCs and macrophages, is to be studied. In addition, there are inhibitors of ASCT2, such as L-gamma-Glutamyl-p-nitroanilide (GPNA)32, whether administration of ASCT2 inhibitors facilitates the induction of AR in animal models is an interesting point; this may be investigated in the future.
In summary, the present data show that B cells of AR patients have low ASCT2 levels, that results in low glutaminolysis in B cells, high GSK3 activities and low IL-10 expression. Inhibition of GSK3 can alleviate experimental AR, suggesting that inhibiting GSK3 can be a novel therapeutic remedy for the treatment of allergic disorders, such as AR.
Materials and methods
Reagents
Antibodies (Ab) of mTORc1 (Clone#: 30), phosphor-mTORc1 (59.Ser 2448), GSK-3α/β (1H8), p-GSK-3α/β (6D3), mTOR (30), p-mTOR (59), LAG3 (D-8, AF 488), CD49b (C-9; AF 594), CD3 (PS1, AF 488), CD4 (5B4, AF 594), CD25 (C-9, AF 647), CD62L (B-8, AF 488), IL-10 (3C12C12, AF 790), CD19 (B-1, AF 546), mast cell chymase (CC1, AF790) and CD35 (H-2, AF 594) were purchased from Santa Cruz Biotech (Santa Cruz, CA). EPX Ab (AF700) was purchased from Biocompare (South San Francisco, CA). S6 ribosomal protein (54D2) and phospho-S6 ribosomal protein (Ser235/236) (D57.2.2E) Abs were purchased from Cell Signaling (Danvers, MA). SB216763, BPTES, rapamycin, was purchased from Sigma Aldrich (St. Louis, MO). ASCT2 Ab and GLUT1 Ab were purchased from Biorbyt Ltd. (Cambridge, UK). CD40L, ELISA kits of IL-10 (DY417, D1000B), IL-4 (M4000B), IL-5 (M5000), and IL-13 (M1300MCB) were purchased from R&D Systems (Minneapolis, MN). ELISA kits of EPX (F20146) and Mcp1 (CSB-E07430m-1) were purchased from DakeweBioMart (Shenzhen, China). CpG (ODN 2006) was purchased from InvivoGen (San Diego, CA). DMOG was purchased from MCE (New Jersey). Reagents and materials for RT-qPCR and western blotting were purchased from Invitrogen (Carlsbad, CA).
Human subjects
Patients with perennial AR and healthy control (HC) subjects were recruited into this study at Longgang ENT Hospital affiliated to Shenzhen University and Shenzhen Hospital of Southern Medical University from May 2019 to October 2020. The diagnosis and management of AR were carried out by our doctors following our routine procedures that also can be found elsewhere33. Briefly, AR patients have AR history more than 2 years; paroxysmal sneezing, nasal congestion, and profound nasal discharge. Air borne allergen skin prick test positive and positive specific IgE in the serum. The general data of human subjects are presented in Table 1. Patients have any of the following conditions were excluded, including autoimmune diseases, severe organ diseases, cancers, and during treatment of immune suppressors for any reasons. AR patients during allergen specific immunotherapy were excluded. All the recruited AR patients did not use any anti-allergy treatment at least for 3 days before the sample collection. The experimental procedures were approved by the Human Ethical Committee at Shenzhen University. A written informed consent was obtained from each human subject.
Isolation peripheral blood mononuclear cells (PBMC)
Blood samples (20 ml per person) were collected from human subjects through the ulnar vein puncture. PBMCs were isolated from the samples by Percoll gradient density centrifugation.
Cell culture
Cells were cultured in RPMI1640 medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin and 2 mM glutamine. For B cell culture, CD40L (1 µg/ml) was added to the culture to avoid B cell apoptosis. Viability of cells was determined by the Trypan blue exclusion assay, that was greater than 99% before handling over for further experiments.
Fluorescence-activated cell sorting (FACS)
In the surface staining, cells were stained with fluorescence-conjugated Abs (Ab types are detailed in figures) or isotype IgG in 1:100 dilution for 30 min at 4 °C, washed with PBS three times and analyzed with a flow cytometer (BD FACSCanto II). In the intracellular staining, brefeldin A (1 mM) was added to the culture in last 2 h. Cells were fixed with 1% paraformaldehyde (containing 0.05% Triton X-100 to increase the cell membrane permeability) for 1 h at room temperature, washed with PBS, and processed with the same procedures of surface staining. The FACS data were analyzed with the Flowjo software package (TreeStar Inc., Ashland, OR) with the data obtained from isotype IgG staining as gating references.
Immune cell purification by cell sorting with FACS
Cells were stained with fluorescence-labeled Abs (detailed in figures) and sorted by FACS (BD FACS Aria). Purity of isolated cells was checked by FACS. If purity did not reach 95%, purification was repeated.
Assessment of IL-10 mRNA levels in B cells by real-time quantitative RT-PCR (RT-qPCR)
RNA was extracted from B cells with TRIzol reagents, and converted to cDNA with a reverse transcription kit following the manufacturer’s instruction. The cDNA samples were amplified in a qPCR device (Bio Rad CFX96 Touch qPCR Detection System) with SYBR Green Master Mix and in the presence of relevant primers, including IL-10 (gccaagccttgtctgagatg and aagaaatcgatgacagcgcc) and TNF-α (gtcaacctcctctctgccat and ccaaagtagacctgcccaga). The data were processed by the 2−∆∆Ct method and presented as relative expression.
Enzyme-linked immunosorbent assay (ELISA)
Cytokine levels in culture supernatant and the nasal secretion were determined by ELISA with commercial reagent kits following the manufacturer’s instruction.
Assessment of the role of glutamine or glucose in IL-10 expression in B cells
To test the role of glutamine in the IL-10 expression in B cells, glutamine-deficient or glucose-deficient culture medium was employed for cell culture. The medium was prepared by Sigma Aldrich.
Induction of IL-10 expression in B cells
B cells were cultured in the presence of CD40L (1 µg/ml) and CpG (ODN 2006, 10 µg/ml) for 24 h. The expression of IL-10 in B cells was assessed by RT-qPCR with RNA extracts from B cells. The IL-10 levels in culture supernatant were determined by ELISA.
Western blotting
Proteins were extracted from cells, fractioned by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis), and transferred onto a PVDF (polyvinylidene fluoride) membrane. The membrane was blocked with 5% skim milk for 30 min, incubated with primary Abs (diluted to 1:300–500; Ab types are detailed in figures) overnight at 4 °C, washed with TBST (Tris-buffered saline containing 0.05% Tween 20) three times, incubated with horseradish peroxidase-labeled second Abs (diluted to 1:5000) for 2 h at room temperature, washed with TBST three times. Immunoblots on the membrane were developed with the enhanced chemiluminescence and photographed in the ChemiDoc imaging system (UVP, Cambridge, UK). The integrated density of immunoblots was determined by ImageJ software (NIH).
Development of experimental AR in a murine model
BALB/c mice (6–8-week-old) were purchased from the Guangdong Experimental Animal Center (Guangzhou, China). Mice were maintained in a specific pathogen-free facility and allowed to access food and water freely. The animal experimental procedures were approved by the Animal Ethical Committee at Shenzhen University. Following published procedures21 that included 4 phases. In phase I, mice were sensitized by injecting with OVA (0.1 mg/mouse) mixed in 0.1 ml Alum (Sigma Aldrich) on the back skin on day 0 and day 7, respectively. In phase II, from day 13 to day 21, mice were treated with OVA-containing nasal instillation (30 µl/nostril; 1 mg OVA/ml) daily. In phase III, from day 28 to day 35, mice were undergone the therapy of nasal instillation containing SB (30 µl/nostril; 1 mg SB/ml), or AS101 (an inhibitor of IL-1034; 30 µl/nostril; 1 mg AS101/ml), or saline, daily. Phase IV (challenge with OVA) was performed on day 43 (see below).
Assessment of AR response in mice
One day after completing the 1-week SB-therapy, mice were challenged with OVA-containing nasal instillation (30 µl/nostril; 5 mg OVA/ml). During 30 min after nasal instillation, the counts of scratching the nasal skin (nasal itch) and sneezing counts were recorded, and nasal discharge (nasal secretion) was collected from the nostrils. The mice were then sacrificed by cervical dislocation. The nasal cavities were opened longitudinally immediately after the sacrifice. All nasal tissues were collected with a curet. Because of small volume, the nasal tissue from five mice were pooled as one sample in the present study. A small part of the nasal tissues was used to extract proteins to be analyzed in cytokine assessment by ELISA.
Isolation immune cells from the mouse nasal tissues
The nasal tissues were cut into small pieces (2 × 2 × 2 mm), digested by incubating with collagenase IV (0.5 mg/ml) for 30 min at 37 °C. Single cells in the lysates were filtered through a cell strainer (70 µm first, then 40 µm). Cells were then resuspended in culture medium for immune cell purification by cell sorting with FACS.
Induction of IL-10 expression in B cells
B cells were purified from PBMCs and cultured in the presence of CD40L (1 µg/ml) and CpG (10 µg/ml). For B cells collected from AR patients, SB216763 (SB, 1 µg/ml) was added to the culture. Three days later, B cells were harvested and analyzed by FACS. Supernatant was analyzed by ELISA.
B10 cell isolation
After exposure to CD40L/CpG/SB in the culture for 3 days, B cells were stained with AF 488-CD19 and AF 597-CD35, and sorted by FACS. As checked by FACS, more than 96% isolated CD19+ CD35+ B cells were also IL-10+. This is in line with our previous studies 14.
Generation of Tr1 cells in vitro
CD4+ CD25− CD62L+ T cells were isolated from PBMCs collected from HC subjects by FACS. B10 cells were prepared as described above. B10 cells and CD4+ T cells were cocultured at a ratio of 1:10 in the presence of CD40L (1 µg/ml), PMA (50 ng/ml), ionomycin (100 ng/ml). Three days later, cells were stained with fluorescence-labeled LAG3 Ab and CD49b Ab, and sorted by FACS. As checked by FACS, the isolated LAG3+ CD49b+ T cells also IL-10+. This is in line with previous reports 22.
Assessment of Tr1 cells’ immune suppressive function
CD4+ CD25− CD62L+ T cells were isolated from HC PBMCs by FACS and labeled with CFSE; the cells were used as naive CD4+ T cells. Tr1 cells were prepared as described above. Tr1 cells and naive CD4+ T cells were cocultured at a ratio of 1:10 in the presence of anti-CD3/CD25 Ab (5 µg/ml of each). Three days later, the cells were analyzed by FACS (the CFSE dilution assay). CD4+ T cell proliferation was assessed and used as an indicator of Tr1 cell’s immune suppressive function.
Statistics
The difference between two groups was determined by Student t test (for normally distributed data) or Mann Whitney-U test (for non-normally distributed data). Multiple comparisons were performed with ANOVA followed by the Dunnett’s post hoc test. P < 0.05 was set as a significant criterion.
References
Rosser, E. C. & Mauri, C. Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612 (2015).
Brosseau, C. et al. CD9(+) regulatory B cells induce T cell apoptosis via IL-10 and are reduced in severe asthmatic patients. Front Immunol. 9, 3034 (2018).
Wang, L., Fu, Y. & Chu, Y. Regulatory B cells. Adv. Exp. Med Biol. 1254, 87–103 (2020).
Venter, C., Eyerich, S., Sarin, T. & Klatt, K. C. Nutrition and the immune system: a complicated tango. Nutrients 12, 3 (2020).
Newsholme, P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 131, 2515S–2522S (2001).
Nakaya, M. et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 40, 692–705 (2014).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 104, 19345–19350 (2007).
Cruzat, V., Macedo Rogero, M., Noel Keane, K., Curi, R. & Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients. 10, 1564 (2018).
Durán, R. V. et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol. Cell 47, 349–358 (2012).
Perl, A. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann. N. Y Acad. Sci. 1346, 33–44 (2015).
Nian, J. B. et al. Epithelial cells expressed IL-33 to promote degranulation of mast cells through inhibition on ST2/PI3K/mTOR-mediated autophagy in allergic rhinitis. Cell Cycle 19, 1132–1142 (2020).
Zhang, Z. et al. ASCT2 (SLC1A5)-dependent glutamine uptake is involved in the progression of head and neck squamous cell carcinoma. Br. J. Cancer 122, 82–93 (2020).
Imbrechts, M. et al. IFN-γ stimulates CpG-induced IL-10 production in B cells via p38 and JNK signalling pathways. Eur. J. Immunol. 48, 1506–1521 (2018).
Zhang, H. P. et al. TSP1-producing B cells show immune regulatory property and suppress allergy-related mucosal inflammation. Sci. Rep. 3, 3345 (2013).
Zhu, T., Park, H. C., Son, K. M. & Yang, H. C. Effects of dimethyloxalylglycine on wound healing of palatal mucosa in a rat model. BMC Oral. Health 15, 60 (2015).
Chen, L. et al. Glutamine deprivation plus BPTES alters etoposide- and cisplatin-induced apoptosis in triple negative breast cancer cells. Oncotarget 7, 54691–54701 (2016).
Hammad, R. et al. Immunoregulatory complement receptor-1 and leukocyte-associated Ig-like receptor-1 expression on leukocytes in Psoriasis vulgaris. Innate Immun. 26, 683–692 (2020).
Krencz, I. et al. In situ analysis of mTORC1/2 and cellular metabolism-related proteins in human Lymphangioleiomyomatosis. Hum. Pathol. 79, 199–207 (2018).
Wang, H., Brown, J. & Martin, M. Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine 53, 130–140 (2011).
Hu, X. et al. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity 24, 563–574 (2006).
Xue, J. M. et al. Survivin facilitates T-helper 2-biased inflammation in the airway. Int Forum Allergy Rhinol. 9, 656–664 (2019).
Huang, W., Solouki, S., Carter, C., Zheng, S. G. & August, A. Beyond Type 1 Regulatory T Cells: Co-expression of LAG3 and CD49b in IL-10-Producing T Cell Lineages. Front Immunol. 9, 2625 (2018).
Kim, A. S. et al. Regulatory B cells and T follicular helper cells are reduced in allergic rhinitis. J. Allergy Clin. Immunol. 138, 1192–1195.e1195 (2016).
Wiest, M. et al. Phenotypic and functional alterations of regulatory B cell subsets in adult allergic asthma patients. Clin. Exp. Allergy 49, 1214–1224 (2019).
Yoshihara, Y. et al. IL-10-producing regulatory B cells are decreased in patients with atopic dermatitis. J. Invest Dermatol 139, 475–478 (2019).
Cerqueira, C., Manfroi, B. & Fillatreau, S. IL-10-producing regulatory B cells and plasmocytes: molecular mechanisms and disease relevance. Semin Immunol. 44, 101323 (2019).
Liu, Z. et al. Toll-like receptor agonists Porphyromonas gingivalis LPS and CpG differentially regulate IL-10 competency and frequencies of mouse B10 cells. J. Appl Oral. Sci. 25, 90–100 (2017).
Noori, M. S. et al. Identification of a novel selective and potent inhibitor of glycogen synthase kinase-3. Am. J. Physiol. Cell Physiol. 317, C1289–c1303 (2019).
Gori, S. et al. Decidualization process induces maternal monocytes to tolerogenic IL-10-producing dendritic cells (DC-10). Front Immunol. 11, 1571 (2020).
Meffre, E. & O’Connor, K. C. Impaired B-cell tolerance checkpoints promote the development of autoimmune diseases and pathogenic autoantibodies. Immunol. Rev. 292, 90–101 (2019).
Cheng, H. et al. Cutting Edge: Inhibition of Glycogen Synthase Kinase 3 Activity Induces the Generation and Enhanced Suppressive Function of Human IL-10(+) FOXP3(+)-Induced Regulatory T Cells. J. Immunol. 205, 1497–1502 (2020).
Corti, A. et al. γ-Glutamyltransferase enzyme activity of cancer cells modulates L-γ-glutamyl-p-nitroanilide (GPNA) cytotoxicity. Sci. Rep. 9, 891 (2019).
Wise, S. K. et al. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. Int Forum Allergy Rhinol. 8, 108–352 (2018).
Kalechman, Y. et al. Anti-IL-10 therapeutic strategy using the immunomodulator AS101 in protecting mice from sepsis-induced death: dependence on timing of immunomodulating intervention. J. Immunol. 169, 384–392 (2002).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (32090052, 81870706, 82071807, 82004046, 81900914, 81700888), Guangdong Provincial Key Laboratory of Regional Immunity and Diseases (2019B030301009), Guangdong Natural Science Foundation (2021A1515010971), the Shenzhen Science, Technology and Innovation Committee (KQJSCX20180328095619081, KQTD20170331145453160), and Shenzhen Key Medical Discipline Construction Fund (No. SZXK039).
Author information
Authors and Affiliations
Contributions
J.Q.L., X.R.G., T.Y.H., L.H.M., X.Q.L., S.Y.Q., D.B.L., Z.G.L., and J.B.S. performed experiments, analyzed data and reviewed the manuscript. Z.Q.L. and P.C.Y. organized the study and supervised experiments. P.C.Y. designed the project and prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, JQ., Geng, XR., Hu, TY. et al. Glutaminolysis is required in maintaining immune regulatory functions in B cells. Mucosal Immunol 15, 268–278 (2022). https://doi.org/10.1038/s41385-021-00481-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-021-00481-9
This article is cited by
-
A glutamine tug-of-war between cancer and immune cells: recent advances in unraveling the ongoing battle
Journal of Experimental & Clinical Cancer Research (2024)