Abstract
Memory T cells are fundamental to maintain immune surveillance of the human body. During the past decade, it has become apparent that non-recirculating resident memory T cells (TRMs) form a first line memory response in tissues to tackle re-infections. The fact that TRMs are essential for local immunity highlights the therapeutic potential of targeting this population against tumors and infections. However, similar to other immune subsets, TRMs are heterogenous and may form distinct effector populations with unique functions at diverse tissue sites. Further insight into the mechanisms of how TRM function and respond to pathogens and malignancies at different mucosal sites will help to shape future vaccine and immunotherapeutic approaches. Here, we review the current understanding of TRM function and biology at four major mucosal sites: gastrointestinal tract, lung, head and neck, as well as female reproductive tract. We also summarize our current knowledge of how TRM targets invading pathogens and developing tumor cells at these mucosal sites and contemplate how TRMs may be exploited to protect from infections and cancer.
Similar content being viewed by others
Introduction
The human body, and in particular mucosal surfaces, are constantly exposed to a wide-range of pathogens and developing malignancies that the immune system needs to keep in check. The adaptive immune system serves here a critical role to mediate a memory response that rapidly could re-encounter pathogens and malignancies to limit disease propagation. In this regard, memory T cells are utterly pivotal and are mainly categorized into two different subsets with distinct functions. Memory CD4 + T cells surveil peripheral tissues for extracellular and intracellular pathogens and provide essential helper functions that augment other arms of the immune system. Memory CD8 + T cells are primarily known for their abilities to eradicate neoplastic cells and intracellular pathogens through direct lysis via cytotoxic mechanisms. As such, both memory CD4 + and CD8 + T cells coordinate immunity at mucosal surfaces to rapidly promote disease protection.
We know today that many T cells continuously migrate between tissues and blood. Early work in rats showed that T cells migrate from non-lymphoid tissues (NLTs) and secondary lymphoid tissues (SLTs) to the blood in a unidirectional manner mainly via the big lymphatic vessel thoracic duct1,2,3. A series of elegant studies in rats and sheep between the 1960s and the 1980s reported that most cells egressing from tissues are lymphocytes and mainly consist of T cells1,4,5,6. Much or our current usage of memory T cell classifications have been ramified from this early work. For instance, we still commonly separate memory T cells into central memory T cell (TCM) and effector memory T cell (TEM) subsets based on their differential capacities to home to SLTs and NLTs, respectively7. Also, CD4 + T cells are commonly classified using the TEM and TCM nomenclature, but these T cells are generally more plastic and are therefore commonly subdivided based on their functions (i.e., regulatory T cells, T helper 1 (Th1), Th2, Th17 and T follicular helper (Tfh) cells). However, many of these classifications are binary in nature and simplified—of obvious reason to not complicate memory classifications too much—and based on intravascular T cell phenotypes and functions. In reality, we know today from both studies in murine models and humans that circulating TEM cells can be divided into two distinct subsets: (i) cytotoxic blood-confined CX3CR1 + TEM cells and (ii) early-differentiated CX3CR1- TEM with certain stem-like properties8,9,10. These studies demonstrate a greater complexity of memory CD8 + T cell differentiation in the blood than caught through the common TCM and TEM model.
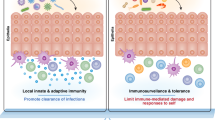
The complexity of classifying T cells based on blood is just the tip of the iceberg and do not take into consideration the fact that certain memory T cells do not leave tissues and circulate to blood. From seminal work for more than a decade ago, it became apparent that many memory T cells in NLTs are not re-circulating TEM cells, but instead stable resident populations11,12,13,14. These T cells are now known as tissue-resident memory T cells (TRMs) and form sentinel CD4 + and CD8 + T cell subsets in most tissues (reviewed in15). We now believe that residency typifies tissues for most immune lineages, where TRMs specifically dominate the total pool of virus-specific CD8 + T cells16 and their unique positioning within tissues allows them to generate immediate site-specific effector responses after secondary challenge to promote rapid protection11.
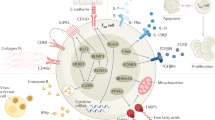
In this review we summarize our current knowledge of human TRM homeostasis and parallel their characteristics to studies derived from murine models. We focus in on four different mucosal sites—gastrointestinal (GI) tract, lung, head/neck and female reproductive tract (FRT) —and discuss the knowns and unknowns of TRM biology and differential functions in relation to intracellular pathogens (Fig. 1) and malignancies (Fig. 2).
TRMs are found in mucosal tissues following different infections. They are known to express variable levels of the tissue retention markers CD69 and CD103. Following IAV infection CD8 + TRMs infiltrate the lung epithelium and induce cell death in the targeted cells through perforin/granzyme delivery as well asl FasL/Fas pathways and produce IFNγ, TNF, IL-2, and other cytokines and chemokines to enhance inflammation and immune activation in the infected lung tissue. HIV-specific CD8 + TRMs express CD69 and intermediate levels of CD103 in the gut mucosal epithelium, where they secrete granzyme A, IFNγ and TNF against HIV-infected cells. In the tonsil, EBV-specific CD8 + TRMs localize at the lymphoepithelial barrier, where most EBV + B cells are found. Tonsillar EBV-specific CD8 + TRMs are polyfunctional and produce IFN-γ among other cytokines. After intravaginal infections HSV-specific CD8 + TRMs are established in the lower FRT, where they persist in the dermo-epidermal junction and rely on perforin/granzyme and cytokine secretion (mostly IFNγ, but also TNF and RANTES expression) for clearance of infected cells.
TRMs expressing CD103 and CD69 have been identified in numerous cancerous tissues. The presence of TRMs within and around tumor tissues are often associated with improved patient outcomes. Their favorable positioning within the tissues makes TRMs rapid first-line effectors that may suppress and eliminate local tumor growths. In an MHC-I dependent fashion, TRMs can directly lyse tumor cells via release of cytotoxic molecules, such as granzyme B and perforin. Alternatively, TRMs may also influence the local tumor microenvironment (TME) through their enhanced expression of inflammatory cytokines i.e., IFNγ, TNF and IL-2. Expression of inhibitory receptors such as PD-1, CTLA-4, LAG-3, TIM-3 and TIGIT have also found to be elevated on TRMs making them attractive targets for checkpoint blockade therapies. Indeed, tumor-specific TRMs targeted by anti-PD-1 show enhanced reactivity toward autologous tumor samples. Local signals within the TME such as TGF-b or VCAM-1 signaling may also shape TRMs phenotype and survival by influencing TRM markers such as CD103, thus improving retention within tumor tissues.
Human tissue-resident memory T cells (Trms)
Since the discovery of TRMs about a decade ago, multiple studies using parabiosis experiments, intravascular labeling, tissue grafting, and migration blockade have helped to elucidate the non-circulatory nature of memory T cells in several tissues of the mouse (reviewed in17). TRMs rapidly secrete cytokines and chemokines upon restimulation and offer increased protection from pathogen reinfections and tumor challenges, in comparison to re-circulating T cells18,19. Mechanistic demonstration of tissue residency is more difficult to prove in humans. As such, most human studies rely on using surface phenotypes and transcriptional signatures to designate resident lymphocytes. However, during the past years several innovative experiments have provided compelling evidence that TRMs most likely exist in human tissues. For instance, patients with cutaneous T cell lymphomas receiving alemtuzumab (anti-CD52 depleting antibody) experience complete depletion of recirculating TEM and TCM cells from blood and skin. Closer examination revealed that T cells with a conventional TRM phenotype (CD69 + CD62L−CCR7−) were largely unaffected multiple weeks after treatment20,21. Likewise, transplantation of HLA miss-matched organs, which allow the tracking of donor-derived TRMs from re-circulating recipient TCM and TEM cells, have demonstrated that donor-derived T cells, with a TRM phenotype, can be observed for 1–2 years after GI, lung and skin transplantation22,23,24. These studies particularly found that donor-derived CD69 + CD103 + TRMs were preserved for an extensive period, indicating that certain TRM populations may experience differential resident longevity. Pioneering studies by Farber et al. on human organ donors have further shown that virtually all organs have T cells with a TRM phenotype (CD69 + CCR7−) throughout a lifetime25,26. CD69 directly hinders T cells from migrating from tissues to blood through its binding to the egress receptor, S1PR1, on the cell surface27. This leads to the internalization and degradation of S1PR1, and the incapacity of T cells to respond to the S1P gradients in lymphatic vessels. Residency in humans is now, as such, usually inferred by high expression of CD69 and other retention markers and integrins (CD103 and CD49a), and low expression of egress markers (S1PR1, CD62L, and CCR7). The CD69 + CCR7− expression pattern is observed in a majority of human memory CD4 + and CD8 + T cells in SLTs and NLTs25,26, whereas memory T cells in the blood and lymph do not express CD69 at steady-state28,29. In early models, the protection afforded by TRMs was thought to primarily involve perforin-mediated transfer of serine proteases (granzymes), so called cytotoxic activity13. However, this paradigm does not necessarily apply to all TRMs across different tissue24,28,30, which instead exhibit a unique transcriptional profile compared to circulating cells31 and are thought to work partly as innate-like sensors32. As such, it is tempting to speculate that human TRMs might act through both cytotoxic mechanisms and recruitment of other immune subsets, to maintain full protection from pathogen invasion and malignancy development.
Lung
The lung forms an important immunological barrier between the host and the external environment. Thus, TRMs act as important long-lived sentinels that protect against invading pathogens and survey tissues for malignant cells. As highlighted above, data from human lung transplants have shown that donor-specific CD4 + and CD8 + TRMs within transplanted tissue show up to 1 year maintenance within recipients23. Overtime, an accumulation of host-specific T cells can be seen within donor-tissue which acquire a TRM phenotype, suggesting de novo generation occurs via seeding of tissues by circulating T cells. Further, analysis of the TCR repertoire of CD4 + and CD8 + TRMs from human organ donors spanning over 50 years show stable TCR clonal maintenance suggesting these cells are constantly being replenished and maintained within the tissue as we age33. During homeostasis, both CD4 + and CD8 + TRMs within lung tissue display high baseline levels of mRNA encoding inflammatory markers such as IFNγ, granzyme B and TNF without need for stimulation34,35, emphasizing their role as fast acting mediators of immunity.
Lung cancer
Current research on the role of TRMs in lung cancers have been focused on non-small-cell lung carcinoma (NSCLC), which accounts for 85% of all lung cancers in the US36. Infiltrating “TRM-like” CD4 + and CD8 + CD103 + cells have been detected within the TILs in NSCLCs, where studies have specifically demonstrated that CD8 + TRMs are associated with enhanced survival37,38,39,40,41. Although studies can transcriptionally confirm the phenotype of such T cells to have a TRM phenotype, i.e., through co-expression of CD69 or other inflammatory markers, it remains difficult in humans to delineate bona fide TRMs which arise during inflammation. This is because CD69 may be upregulated during TCR stimulation, hypoxia or type I interferon signaling and CD103 in presence of TGFb42,43,44,45. However, based on the fact that tumor-specific clonotypes are preferentially localized within the tumor microenvironment, and to a less degree in the peripheral blood, it seems likely that most CD103 + tumor-specific CD8 + T cells are TRMs. Within tumor tissue, lung CD8 + TRMs also display high levels of cytotoxic effector molecules, proliferation and express high levels of co-inhibitory markers PD-1, CTLA-4, LAG-3 and Tim-337,39,46, making them attractive targets for checkpoint inhibitor therapy. Indeed, CD8 and CD103 co-expression has been shown to be a predictive marker in positive response to anti-PD-L1 treatment in NSCLC47. Moreover, ex vivo stimulation of lung-resident CD8 + TRMs with anti-PD-1 enhances their IFNγ secretion and their capacity to kill autologous tumor cells37,46. Thus, accumulating evidence suggest that TRMs contribute to tumor control in NSCLC (Fig. 2).
Influenza A virus (IAV)
IAV is a highly contagious virus which accounted for an estimated 9.5 million hospitalizations in 201748, and its consequent respiratory disease, the ‘flu’, has significant mortality rates among immunocompromised individuals. Several human studies have described the detection of CD4 + and CD8 + TRMs within lung tissue after infection with IAV49,50,51,52,53. Ex vivo stimulation of IAV-specific CD8 + TRMs results in proliferation and polyfunctional inflammatory cytokine production, including IFNγ, TNF, and IL-254 (Fig. 1). Subsequent re-infection of individuals with seasonal variations of IAV has posed the question whether TRMs offer heterosubtypic protection after seeding the lung tissue. Indeed, both murine and human studies indicate that CD4 + and CD8 + TRMs play a significant role in heterosubtypic immunity providing protection upon secondary infection55,56. Interestingly, unlike their other mucosal counterparts, murine studies have also shown that IAV-specific CD8 + TRM numbers appear to diminish overtime, thought to be in part contributed by increased apoptosis57. Recently, this phenomenon has been also described in human lung tissue spanning 22–68 years of age; compared to other T cell subpopulations, CD8 + TRMs show the most drastic decline in cell numbers associated with age58. Moreover, in vitro cultures of lung tissue from these tissues with IAV have revealed a reduced capacity of CD8 + TRMs to produce IFNγ with age58. There data indicate that the loss of functional CD8 + IAV-specific TRMs overtime may contribute to diminished long-term heterosubtypic immunity in elderly indivduals.
Coronaviruses
Severe acute respiratory syndrome coronavirus (SARS-CoV), middle east respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) represent different examples of severe coronaviruses that have emerged during the past two decades from zoonotic sources. All three viruses cause acute respiratory illnesses as they infect lung cells, damaging airways and alveolar epithelial cells59. Despite the extraordinary efforts recently made into Covid-19 research (disease caused by SARS-CoV-2), it remains unknown whether TRMs can attenuate Covid-19 severity in humans60. Studies on MERS-CoV and SARS-CoV, have reported a clear role of respiratory T cells for the protection against severe disease in the lung59,61. There is now also evidence that SARS-CoV-2-specific CD4 + and CD8 + T cells with TRM characteristics are found in lung lavages from convalescent patients62. Likewise, a new study recently demonstrated that pre-existing SARS-CoV-2-reactive CD8 + TRMs are present in tonsils from a large proportion of unexposed individuals63. As such, cross-reactive TRMs might contribute to rapid sentinel responses to SARS-CoV-2 in newly exposed donors. Analyses of vaccine-induced TRMs have been rare due to limited access to patient samples, however intranasal delivery of a ChAd-SARS-CoV-2-S vaccine in mice incudes localized CD8 + TRM responses, which correlate with reduced viral replication and shedding in comparison to systemic delivery64. As such, it remains unclear if intramuscular injection with the current Covid-19 vaccines will induce local cell-mediated immunity, including CD4 + and CD8 + TRM responses, in the respiratory tract.
Tuberculosis
Tuberculosis (TB) is one of the leading causes of death worldwide and is caused by the infectious agent (Mycobacterium tuberculosis, Mtb). Although many studies have demonstrated on the importance of T cells to protect from active TB infection, the major obstacle in development of an effective vaccine is a lack of defined immune correlates. Nevertheless, numerous murine studies have deciphered the immune responses generated by the only licensed TB vaccine: Mycobacterium bovis-derived Bacillus Calmete Guérin (BCG)65. BCG protects infants, but demonstrate poor protection of pulmonary TB in adolescents and adults66. Studies have shown that blockade of lymphocyte tissues egress, by FTY720 administration, after BCG vaccination does not negatively impact outcome after TB challenge67,68, indicating that lung CD4 + and/or CD8 + TRMs are sufficient for protection. The accumulating body of evidence that TRMs may mediate protection to TB have guided current vaccine efforts to generate TRMs via vaccination. Through various strategies such as prime-boost, adjuvanted and viral vectors of Mtb antigens and/or BCG, several groups have been able to effectively generate both CD4 + and CD8 + lung TRMs that confer enhanced protection against TB challenge69,70,71,72,73. Similar induction of lung CD4 + and CD8 + TRM have been obtained in studies with non-human primates using BCG or subunit vaccines delivered intravenously or intranasally74,75,76,77,78. In one of these recent studies78, the authors found that intravenous immunization with BCG induced high levels of CD4 + and CD8 + TRMs across the entire lung parenchyma, where nine of ten animals demonstrated durable protection from Mtb infection. Collectively, vaccine studies in murine and non-human primates suggest that induction of robust CD4 + and CD8 + TRM responses through specific routes might become a rationale to induce an effective vaccine response against TB.
Gastrointenstinal (GI) Tract
The GI tract form an essential interface to the external environment and maintain constant balance between protection against invading pathogens and tolerance of nonpathogenic bacteria and food antigens. The GI tract consists of the esophagus, stomach, pancreas, liver, and intestinal tissues. In humans, all these tissues have been reported to harbor a large proportion of CD4 + and CD8 + TRMs25,79,80,81. However, for the purposes of this review, we will focus on the lower GI tract, namely the gastric and intestinal tissues which contain a diverse population of immune cells where a large proportion of the CD4 + and CD8 + T cell compartment (up to 90%) display phenotypic features of TRMs31,82. These cells seed the intestinal mucosa and in contrast to other T cell subsets, are stably maintained over a lifetime24,26,83,84. Studies from both human intestinal allografts and non-human primate allogeneic hematopoietic stem cell transplants demonstrate that circulating CD4 + and CD8 + T cells may enter the intestinal mucosa and acquire a TRM phenotype85,86, which is thought to be largely contributed by constitutive expression of TGF-b within the intestinal epithelium that drives CD103 expression87,88,89,90.
Gastric adenocarcinoma
Gastric adenocarcinoma, which makes up 95% of all gastric cancers, is the fifth most diagnosed and third most lethal cancer worldwide91. Several recent studies have shown that CD8 + TRMs readily infiltrate gastric cancers, produce high levels of inflammatory cytokines and are associated with better prognosis92,93,94. One in vitro study showed that CD8 + CD103 + T cells produce higher levels of IFNγ and TNF when co-cultured with tumor cells and had higher expression of PD-1, TIGIT and CD39 in comparison to CD103- T cells92. Survival of CD8 + TRMs was dependent on fatty-acid metabolism where tumor cells outcompeted CD8 + TRMs leading to cell death. However, co-culture with anti-PD-L1 reverse this phenotype, resulting in enhanced fatty-acid uptake in CD8 + TRMs and enhanced survival, again demonstrating TRMs as an attractive target for checkpoint blockade therapies. Indeed, subsequent studies have shown that patients with high infiltrating CD103 + CD8 + T cells receiving anti-PD-1 have higher overall survival compared to total CD103- CD8 + T cell and were highly associate with tertiary lymphoid structures93,94. Collectively, these data suggest that CD8 + TRMs might be attractive targets for future immunotherapies in gastric cancer.
Colorectal cancer
Colorectal cancer (CRC) ranks as the 3rd leading cause of cancer-related deaths worldwide95. Colorectal cancers (CRCs) can be roughly divided based on presence or deficiency of the patient’s mismatch repair system (MMR). Around 10–20% of patients with CRC will be deficient for MMR96,97 and these patients have a higher survival rate which is thought to be contributed by a higher mutational burden of up to 100-fold98,99, leading to enhanced immune infiltration within the tumor100,101. Few human studies have investigated the role of TRMs within CRCs, however, recently CD8 + TRMs were shown to be enriched within MMR-deficient CRC tissues compared to healthy mucosa or MMR-stable CRC tissues102. Treatment with anti-PD-1 therapy improves survival in patients with MMR101, and CD4 + and CD8 + TRM-like cells with high expression of PD-1 can be detected within MMR-deficient CRC tissues102. No study has yet evaluated whether TRMs within CRC are the cells that are reactive to anti-PD-1 therapy and convey protection, however, their reactivity within other cancer types including gastric cancer, highly suggest that these cells play a key role in checkpoint blockade therapy in CRC (Fig. 2).
HIV
The human immunodeficiency virus (HIV) infects primarily memory CD4 + T cells, leading to a diminished capacity of the immune system to defend against otherwise relatively harmless infections. Without antiretroviral therapy (ART), most humans eventually progress to acquired immunodeficiency syndrome (AIDS). Although most HIV studies have been conducted on peripheral blood, new data on non-human primate studies suggest that the GI tract harbors 98% of the total viral reservoirs during ART103. This is most likely a consequence of the early seeding of CCR5 + CD4 + T cells within the gut with viral particles104. Whether CD4 + TRMs represent a cellular reservoir, similar to cervix105, remains unknown. Similar to other studies in humans, it is hard to infer phenotypes to a bona fide TRM status in HIV-infected tissues. Nevertheless, previous studies have found that HIV-specific T cells are generally present at higher magnitudes in SLTs and the GI tract compared to blood; despite the fact that most HIV-specific T cells lack homing receptors to lymphoid106,107,108. In addition, most HIV-specific CD8 + TRMs express CD69 and intermediate levels of CD103 in rectosigmoid and demonstrate polyfunctional characteristics109 (Fig. 1). HIV-specific CD8 + TRM responses are strongest in individuals naturally controlling HIV, suggesting that CD8 + TRMs mediate protection against HIV disease progression28. Recent vaccine studies in non-human primates further suggest that induction of CD8 + TRM responses may lower the threshold of neutralizing antibodies to confer durable protection after lentiviral challenge110. Overall, these data indicate that future HIV vaccine and cure regimens might benefit from inducing local TRM responses.
Female reproductive tract (FRT)
The mucosal immune system in the FRT is an early barrier against pathogenic organisms, including sexually transmitted diseases of epidemic proportions (e.g., Chlamydia, Herpes Simplex virus (HSV) or HIV)111. Two types of FRT mucosal surfaces exist: upper FRT (endometrium and endocervix) lined with a type I single columnal epithelial layer and lower FRT (vagina and ectocervix), with a type II lining compromised of several stratified squamous epithelial layers linked to a basement membrane. Mucosa-associated lymphoid tissues (MALT) are only present in the upper FRT, where T cell infiltration mainly occurs under inflammatory conditions112. Numerous murine studies have demonstrated that long-lived CD8 + TRMs can be established during infection and generated through vaccination in the FRT and can protect against infectious pathogens such as HSV113,114,115,116,117,118,119. Emerging data in the human field suggest that CD8 + TRMs expressing higher levels of CD103 and cytotoxic molecules (e.g., Granzyme B) are close to the epithelium, whereas lower levels of CD103 are present on CD8 + TRMs in the stroma120, indicating that distinct antigen-specific CD8 + TRM subsets are positioned throughout the human cervix.
Cervical cancer
Cervical cancer is the leading cause of cancer-related deaths in women, where up to 75% of cervical cancers are caused by HPV16 and HPV18 infection121. Limited human studies exist investigating the role of CD4 + and CD8 + TRMs within cervical cancers. Nevertheless a recent study found favorable prognosis in individuals with high infiltration of CD8 + CD103 + T cells122. These CD8 + TRM-like cells also expressed high levels of granzyme B and PD-1122. In the same study, irradiation combined with an E6/7 targeting vaccine delivered therapeutically was able to induce infiltrating HPV-specific CD8 + TRMs within a preclinical cervical cancer model. An earlier preclinical study also showed that intravaginal vaccination with an HPV vector could induce cervicovaginal localized CD8 + TRMs115. However, clinical trials with therapeutic vaccination against HPV-induced cervical cancers have so far yielded poor results, indicating that more work is required to address whether CD8 + TRMs can be harnessed for HPV-induced cervical cancer therapies.
Herpes simplex virus 2
HSV-2 infection is almost exclusively sexually transmitted and the main cause of genital herpes. The virus leaves most often no symptoms and remains lifelong, latent and incurable. Numerous studies in murine models have shown that HSV-specific CD8 + TRMs can be generated within the genital mucosa and surrounding tissue115,116,117,118,119. Despite not containing MALT in the steady state, both human CD4 + and CD8 + TRMs can be established in the lower FRT after intravaginal infections, and low numbers of those persist as TRMs for several months after lesion healing123. More specifically, they are located in the dermo-epidermal junction and provide immune surveillance along the nerve endings124. Thus, the severity of HSV-2 infection may depend on the CD4 + and CD8 + TRM density in the specific infection site125, where TRMs mainly rely on cytokine secretion (mostly IFNγ), rather than cytotoxicity effect for viral clearance126,127 (Fig. 1). As such, these data suggest that CD4 + and CD8 + TRM subsets in the cervix provide immune surveillance mechanisms to control HSV-2 replication120.
Head and neck
The upper aerodigestive tract consists of several distinct anatomical sites lined by mucosal epithelia including paranasal sinuses, nasal and oral cavities, pharynx, and larynx. Populations of CD4 + and CD8 + TRM can be readily detected in associated oropharyngeal lymphoid structures such as the tonsils. Moreover, studies in Epstein-Barr virus (EBV) infection show a preferential accumulation of antigen-specific CD8 + TRM within the tonsils during and after infection suggesting an essential role in control and protection against upper-respiratory tract pathogens25,128,129,130.
Head and neck cancer
Head and neck squamous cell carcinomas (HNSC) encompass several malignancies that originate in the mucosal lining of the upper aerodigestive tract. It is now widely accepted that the major risk factors for these diseases are smoking tobacco and alcohol use131,132,133. Furthermore, high-risk human papilloma virus (HPV) infection is another major risk factor, where HPV-16 strain accounts for over 90% of HPV-related HNSC. Vaccination against HPV has led to a substantial decrease in the incidence of cervical cancer across a number of countries and is known to be dependent on the formation of neutralizing antibodies against the virus134. Whether HPV vaccination can induce protection against HPV-related HNSC and if CD4 + and CD8 + TRMs may play a role, has yet to be fully studied. However, a recent murine study using mucosal vaccination with E7 protein from HPV-16 has shown that CD8 + TRM can be induced within the head and neck of mice which correlate with protection against orthotopic tumor challenge40. It is also evident from clinical studies that CD8 + TRM play a key role in immunity against HNSC as infiltration of the tumor with CD103 + TILs are a prognostic marker for enhanced survival across several HNSCs and these cells are able to kill autologous tumor cells in an MHC-I dependent manner44,135,136. Although studies have largely focus on CD8 + TRM within HNSC, interestingly, the tumor microenvironment itself may play a role in inducing and sustaining both CD4 + and CD8 + TRM populations within the tumor. A recent study has shown that mesenchymal stromal cells that accumulate within HSNC also provide survival signals and induce expression of CD4 + and CD8 + TRM surface markers through VCAM1137.
CMV
CMV persists as a latent infection and can reactivate to lytic infection at various mucosal tissues, including tonsils and salivary glands. While most individuals are asymptomatic, immunocompromised patients are more susceptible for CMV morbidity and mortality138. CMV-specific T cells are critical to control CMV reactivation, as evidenced in clinical settings using infusion of CMV-specific T cells to prevent organ dysfunction139. Furthermore, immune-deficient patients that experience uncontrolled viral replication and end-organ disease usually have impaired T cell responses140,141. Murine CMV models (MCMV) have identified MCMV-specific CD4 + and CD8 + T cells with a TRM phenotype in the salivary glands142. Most our knowledge of human CMV-specific T cell immunity is limited to peripheral blood samples, where these cells generally harbor a unique terminally-differentiated phenotype with high cytotoxic activity10. Studies derived from tissues have instead shown that CMV-specific T cells generally possess a recirculating TEM phenotype, enabling them to continuously migrate into mucosal tissues143. Recent studies using human organ donors have identified the lung as a large CMV reservoir, where CD8 + T cells are often activated, whereas tonsil and salivary glands seem to serve as long-term reservoirs of the CMV-specific T cells143. Most commonly CMV-specific CD8 + T cells display variable levels of CD69 and low CD103 expression in tonsils and SLTs, instead suggesting that these cells might preferentially rely on re-circulation mechanisms to provide protection against CMV reactivation28,144.
EBV
EBV infects more than a 90% of the worldwide population. It is usually acquired asymptomatically in childhood, establishing a latent infection of the memory B cell pool, but when acquired through saliva in adolescence, it can cause Infectious Mononucleosis disease (IM)145. However, many of the B cells downregulate antigen expression, becoming part of a latent pool of infected antigen-negative genome-positive memory B cells, which recirculates between the tonsil (pharyngeal lymphoid tissues) and blood146. The expression of both lytic and latent proteins in primary infection is immunogenic and triggers the recruitment of EBV-specific CD8 + TRMs to the tonsil. Many EBV-specific CD8 + T cells express both CD69 and CD103, and are positioned along the epithelial barrier, which could explain why EBV reactivation is often asymptomatic or sub-clinical144 (Fig. 1). A recent study from our group confirmed these results in human tonsils and additionally showed higher frequencies of EBV-specific CD8 + T cells in tonsils than blood63—probably a direct consequence of their residency status. Moreover, this study indicated that EBV-specific CD8 + TRMs are polyfunctional and express CXCR5, to directly spearhead the antiviral response against the virus in B cell follicles63.
Conclusions
The identification of TRMs have caused a paradigm shift in our understanding of T cell-mediated immunity. We know believe that TRMs, and not only re-circulating T cells, underpins immune surveillance of tissues to mediate proper protection and control of infections and malignancies. Despite the explosion of studies centered on human TRM responses, there is still a general lack of knowledge of relatively trivial pathogen-specific TRM characteristics (e.g., phenotype and functions) in contrast to blood; and although it is clear that certain TRM populations exist between mucosal sites, we still do not know if distinct TRM populations could impart differential immune pressure on tumors and pathogens within and between different sites. A further, and ultimate, goal in the future will be to translate our knowledge derived from TRM studies in mice to humans and develop new types of vaccines through mucosal routes that can induce long-lived TRM responses. This might be a future path forward, for instance in Covid-19 vaccine development, given the recent surge in variants of concern. Continual studies are as such needed to integrate mouse and human studies to harness the full potential of TRMs at mucosal surfaces.
References
GOWANS, J. L. The effect of the continuous re-infusion of lymph and lymphocytes on the output of lymphocytes from the thoracic duct of unanaesthetized rats. Br. J. Exp. Pathol. 38, 67–78 (1957).
Gowans, J. L. The recirculation of lymphocytes from blood to lymph in the rat. J. Physiol. 146, 54–69 (1959).
GOWANS, J. L. & KNIGHT, E. J. The route of re-circulation of lymphocytes in the rat. Proc. R. Soc. London. Ser. B, Contain. Pap. 159, 257–282 (1964).
Mackay, C. R., Kimpton, W. G., Brandon, M. R. & Cahill, R. N. P. Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J. Exp. Med. 167, 1755–1765 (1988).
Mackay, C. R., Marston, W. L. & Dudler, L. Naive and memory t cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 171, 801–817 (1990).
Maddox, J. F., Mackay, C. R. & Brandon, M. R. Surface antigens, SBU-14 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology 55, 739–748 (1985).
Sallusto, F., Lenig, D., Förster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Gerlach, C. et al. The Chemokine Receptor CX3CR1 Defines Three Antigen-Experienced CD8 T Cell Subsets with Distinct Roles in Immune Surveillance and Homeostasis. Immunity 45, 1270–1284 (2016).
Böttcher, J. P. et al. Functional classification of memory CD8 + T cells by CX 3 CR1 expression. Nat. Commun. 6, 8306 (2015).
Buggert, M. et al. The Identity of Human Tissue-Emigrant CD8+ T. Cells Cell 183, 1946–1961 (2020).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009).
Masopust, D. et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564 (2010).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrançois, L. Preferential localization of effector memory cells in nonlymphoid tissue. J. Immunol. 192, 845–849 (2014).
Wakim, L. M., Waithman, J., Van Rooijen, N., Heath, W. R. & Carbone, F. R. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science (80-) 319, 198–202 (2008).
Szabo, P. A., Miron, M. & Farber, D. L. Location, location, location: tissue resident memory T cells in mice and humans. Sci. Immunol. 4, eaas9673 (2019).
Steinert, E. M. et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161, 737–749 (2015).
Gebhardt, T., Palendira, U., Tscharke, D. C. & Bedoui, S. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol. Rev. 283, 54–76 (2018).
Jiang, X. et al. Skin infection generates non-migratory memory CD8 + T RM cells providing global skin immunity. Nature 483, 227–231 (2012).
Malik, B. T. et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci. Immunol. 2, eaam6346 (2017).
Clark, R. A. et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci. Transl. Med. 4, 117 (2012).
Watanabe, R. et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 7, 279 (2015).
Lian, C. G. et al. Biomarker evaluation of face transplant rejection: Association of donor T cells with target cell injury. Mod. Pathol. 27, 788–799 (2014).
Snyder, M. E. et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci. Immunol. 4, eaav5581 (2019).
Bartolomé-Casado, R. et al. Resident memory CD8 T cells persist for years in human small intestine. J. Exp. Med. 216, 2412–2426 (2019).
Sathaliyawala, T. et al. Distribution and Compartmentalization of Human Circulating and Tissue-Resident Memory T Cell Subsets. Immunity 38, 187–197 (2013).
Thome, J. J. C. et al. Spatial map of human t cell compartmentalization and maintenance over decades of life. Cell 159, 814–828 (2014).
Shiow, L. R. et al. CD69 acts downstream of interferon-α/β to inhibit S1P 1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006).
Buggert, M. et al. Identification and characterization of HIV-specific resident memory CD8+ T cells in human lymphoid tissue. Sci. Immunol. 3, eaar4526 (2018).
Vella, L. A. et al. T follicular helper cells in human efferent lymph retain lymphoid characteristics. J. Clin. Investig. 129, 3185–3200 (2019).
Pallett, L. J. et al. IL-2high tissue-resident T cells in the human liver: Sentinels for hepatotropic infection. J. Exp. Med. 214, 1567–1580 (2017).
Kumar, B. V. et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 20, 2921–2934 (2017).
Schenkel, J. M., Fraser, K. A., Vezys, V. & Masopust, D. Sensing and alarm function of resident memory CD8 + T cells. Nat. Immunol. 14, 509–513 (2013).
Miron, M. et al. Maintenance of the human memory T cell repertoire by subset and tissue site. Genome Med. 13, 100 (2021).
Hombrink, P. et al. Programs for the persistence, vigilance and control of human CD8 + lung-resident memory T cells. Nat. Immunol. 17, 1467–1478 (2016).
Oja, A. E. et al. Trigger-happy resident memory CD4 + T cells inhabit the human lungs. Mucosal. Immunol. 11, 654–667 (2018).
Molina, J. R., Yang, P., Cassivi, S. D., Schild, S. E. & Adjei, A. A. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 83, 584–594 (2008).
Djenidi, F. et al. CD8 + CD103 + Tumor–Infiltrating Lymphocytes Are Tumor-Specific Tissue-Resident Memory T Cells and a Prognostic Factor for Survival in Lung Cancer Patients. J. Immunol. 194, 3475–3486 (2015).
Koh, J. et al. Prognostic implications of intratumoral CD103+ tumor-infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget 8, 13762–13769 (2017).
Ganesan, A. P. et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol. 18, 940–950 (2017).
Nizard, M. et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat. Commun. 8, 15221 (2017).
Oja, A. E. et al. Functional heterogeneity of CD4+ tumor-infiltrating lymphocytes with a resident memory phenotype in NSCLC. Front. Immunol. 9, 2654 (2018).
Cibrián, D. & Sánchez-Madrid, F. CD69: from activation marker to metabolic gatekeeper. Eur. J. Immunol. 47, 946–953 (2017).
Labiano, S. et al. CD69 is a direct HIF-1α target gene in hypoxia as a mechanism enhancing expression on tumor-infiltrating T lymphocytes. Oncoimmunology 6, e1283468 (2017).
Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018).
Le Floc’h, A. et al. αEβ7 Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis, integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J. Exp. Med. 204, 559–570 (2007).
Wang, P. et al. CD103+CD8+ T lymphocytes in non-small cell lung cancer are phenotypically and functionally primed to respond to PD-1 blockade. Cell. Immunol. 325, 48–55 (2018).
Banchereau, R. et al. Intratumoral CD103+ CD8+ T cells predict response to PD-L1 blockade. J. Immunother. Cancer 9, e002231 (2021).
Troeger, C. E. et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 7, 69–89 (2019). 7, (2019).
Turner, D. L. et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal. Immunol. 7, 501–510 (2014).
Piet, B. et al. CD8+ T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J. Clin. Investig. 121, 2254–2263 (2011).
Koutsakos, M. et al. Human CD8 + T cell cross-reactivity across influenza A, B and C viruses. Nat. Immunol. 20, 613–625 (2019).
De Bree, G. J. et al. Selective accumulation of differentiated CD8+ Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung, T cells specific for respiratory viruses in the human lung. J. Exp. Med. 202, 1433–1442 (2005).
Teijaro, J. R. et al. Cutting Edge: Tissue-Retentive Lung Memory CD4 T Cells Mediate Optimal Protection to Respiratory Virus Infection. J. Immunol. 187, 5510–5514 (2011).
Pizzolla, A. et al. Influenza-specific lung-resident memory t cells are proliferative and polyfunctional and maintain diverse TCR profiles. J. Clin. Investig. 128, 721–733 (2018).
Wu, T. et al. Lung-resident memory CD8 T cells (T RM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 95, 215–224 (2014).
Sridhar, S. et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 19, 1305–1312 (2013).
Slütter, B. et al. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci. Immunol. 2, eaag2031 (2017).
Nguyen, T. H. O. et al. Influenza, but not SARS-CoV-2, infection induces a rapid interferon response that wanes with age and diminished tissue-resident memory CD8+ T cells. Clin. Transl. Immunol. 10, e1242 (2021).
Channappanavar, R., Fett, C., Zhao, J., Meyerholz, D. K. & Perlman, S. Virus-Specific Memory CD8 T Cells Provide Substantial Protection from Lethal Severe Acute Respiratory Syndrome Coronavirus Infection. J. Virol. 88, 11034–11044 (2014).
Karlsson, A. C., Humbert, M. & Buggert, M. The known unknowns of T cell immunity to COVID-19. Sci. Immunol. 5, eabe8063 (2020).
Channappanavar, R., Zhao, J. & Perlman, S. T cell-mediated immune response to respiratory coronaviruses. Immunologic Res. 59, 118–128 (2014).
Grau-Expósito, J. et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat. Commun. 12, 3010 (2021).
Julia, N. et al. Identification of resident memory CD8+ T cells with functional specificity for SARS-CoV-2 in unexposed oropharyngeal lymphoid tissue. Sci. Immunol. 10.1126-sciimmunol.abk0894 (2021).
Hassan, A. O. et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. Cell Rep. 36, 109452 (2021).
Calmette, A. Preventive Vaccination against Tuberculosis with BCG. J. R. Soc. Med. 24, 1481–1490 (1931).
Trunz, B. B., Fine, P. & Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367, 1173–1180 (2006).
Connor, L. M. et al. A key role for lung-resident memory lymphocytes in protective immune responses after BCG vaccination. Eur. J. Immunol. 40, 2482–2492 (2010).
Bull, N. C. et al. Induction and maintenance of a phenotypically heterogeneous lung tissue-resident CD4+ T cell population following BCG immunisation. Vaccine 36, 5625–5635 (2018).
Aguilo, N. et al. Pulmonary Mycobacterium bovis BCG vaccination confers dose-dependent superior protection compared to that of subcutaneous vaccination. Clin. Vaccin. Immunol. 21, 594–597 (2014).
Copland, A. et al. Mucosal delivery of fusion proteins with Bacillus subtilis spores enhances protection against tuberculosis by Bacillus Calmette-Guérin. Front. Immunol. 9, 346 (2018).
Hart, P. et al. Nanoparticle-Fusion Protein Complexes Protect against Mycobacterium tuberculosis Infection. Mol. Ther. 26, 822–833 (2018).
Hu, Z. et al. Sendai Virus Mucosal Vaccination Establishes Lung-Resident Memory CD8 T Cell Immunity and Boosts BCG-Primed Protection against TB in Mice. Mol. Ther. 25, 1222–1233 (2017).
Perdomo, C. et al. Mucosal BCG vaccination induces protective lung-resident memory T cell populations against tuberculosis. MBio 7, e01686–16 (2016).
Sharpe, S. et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: Protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis 101, 174–190 (2016).
Kaveh, D. A., Bachy, V. S., Hewinson, R. G. & Hogarth, P. J. Systemic BCG immunization induces persistent lung mucosal multifunctional CD4 TEM cells which expand following virulent mycobacterial challenge. PLoS ONE 6, e21566 (2011).
White, A. D. et al. Evaluation of the immunogenicity of Mycobacterium bovis BCG delivered by aerosol to the lungs of macaques. Clin. Vaccin. Immunol. 22, 992–1003 (2015).
Darrah, P. A. et al. Aerosol Vaccination with AERAS-402 Elicits Robust Cellular Immune Responses in the Lungs of Rhesus Macaques but Fails To Protect against High-Dose Mycobacterium tuberculosis Challenge. J. Immunol. 193, 1799–1811 (2014).
Darrah, P. A. et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 577, 95–102 (2020).
French, J. J. et al. T cell adhesion and cytolysis of pancreatic cancer cells: A role for E-cadherin in immunotherapy? Br. J. Cancer 87, 1034–1041 (2002).
Han, L. et al. Characterization of CD103+ CD8+ tissue-resident T cells in esophageal squamous cell carcinoma: may be tumor reactive and resurrected by anti-PD-1 blockade. Cancer Immunol. Immunother. 69, 1493–1504 (2020).
Booth, J. S. et al. Characterization and functional properties of gastric tissue-resident memory T cells from children, adults, and the elderly. Front. Immunol. 5, 294 (2014).
Thome, J. J. C. et al. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat. Med. 22, 72–77 (2016).
Senda, T. et al. Microanatomical dissection of human intestinal T-cell immunity reveals site-specific changes in gut-associated lymphoid tissues over life. Mucosal Immunol. 12, 378–389 (2019).
Bartolomé-Casado, R. et al. CD4+ T cells persist for years in the human small intestine and display a TH1 cytokine profile. Mucosal Immunol. 14, 402–410 (2021).
Zuber, J. et al. Bidirectional intragraft alloreactivity drives the repopulation of human intestinal allografts and correlates with clinical outcome. Sci. Immunol. 1, eaah3732 (2016).
Tkachev, V. et al. Spatiotemporal single-cell profiling reveals that invasive and tissue-resident memory donor CD8+ T cells drive gastrointestinal acute graft-versus-host disease. Sci. Transl. Med. 13, eabc0227 (2021).
Skon, C. N. et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 14, 1285–1293 (2013).
Sheridan, B. S. et al. Oral infection drives a distinct population of intestinal resident memory cd8+ t cells with enhanced protective function. Immunity 40, 747–757 (2014).
Zhang, N. & Bevan, M. J. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39, 687–696 (2013).
Konkel, J. E. et al. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat. Immunol. 12, 312–319 (2011).
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. & Lordick, F. Gastric cancer. Lancet 396, 635–648 (2020).
Lin, R. et al. Fatty acid oxidation controls CD8+Tissue-resident memory t-cell survival in gastric adenocarcinoma. Cancer Immunol. Res. 8, 479–492 (2020).
Li, R. et al. Identification and validation of an immunogenic subtype of gastric cancer with abundant intratumoural CD103+CD8+ T cells conferring favourable prognosis. Br. J. Cancer 122, 1525–1534 (2020).
Mori, T. et al. Tertiary lymphoid structures show infiltration of effective tumor-resident T cells in gastric cancer. Cancer Sci. 112, 1746–1757 (2021).
Rawla, P., Sunkara, T. & Barsouk, A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz. Gastroenterologiczny 14, 89–103 (2019).
Koopman, M. et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer 100, 266–273 (2009).
Goldstein, J. et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann. Oncol. 25, 1032–1038 (2014).
Timmermann, B. et al. Somatic mutation profiles of MSI and MSS colorectal cancer identified by whole exome next generation sequencing and bioinformatics analysis. PLoS ONE 5, e15661 (2010).
Muzny, D. M. et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Gatalica, Z. et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol. Biomark. Prev. 23, 2965–2970 (2014).
Le, D. T. et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
De Vries, N. L. et al. High-dimensional cytometric analysis of colorectal cancer reveals novel mediators of antitumour immunity. High-dimensional cytometric analysis of colorectal cancer reveals novel mediators of antitumour immunity. Gut 69, 691–703 (2020).
Estes, J. D. et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat. Med. 23, 1271–1276 (2017).
Veazey, R. S. et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science (80-). 280, 427–431 (1998).
Cantero-Pérez, J. et al. Resident memory T cells are a cellular reservoir for HIV in the cervical mucosa. Nat. Commun. 10, 4739 (2019).
Altfeld, M. et al. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J. Clin. Investig. 109, 837–843 (2002).
Shacklett, B. L. et al. Trafficking of Human Immunodeficiency Virus Type 1-Specific CD8 + T Cells to Gut-Associated Lymphoid Tissue during Chronic Infection. J. Virol. 77, 5621–5631 (2003).
Buggert, M. et al. T-bet and Eomes Are Differentially Linked to the Exhausted Phenotype of CD8+ T Cells in HIV Infection. PLoS Pathog. 10, e1004251 (2014).
Kiniry, B. E. et al. Detection of HIV-1-specific gastrointestinal tissue resident CD8 + T-cells in chronic infection. Mucosal Immunol. 11, 909–920 (2018).
Arunachalam, P. S. et al. T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat. Med. 26, 932–940 (2020).
Wira, C. R., Fahey, J. V., Rodriguez-Garcia, M., Shen, Z. & Patel, M. V. Regulation of mucosal immunity in the female reproductive tract: The role of sex hormones in immune protection against sexually transmitted pathogens. Am. J. Reprod. Immunol. 72, 236–258 (2014).
Wira, C. R. et al. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol. 4, 335–342 (2011).
Wijeyesinghe, S. et al. Expansible residence decentralizes immune homeostasis. Nature 592, 457–462 (2021).
Schenkel, J. M. et al. Resident memory CD8 t cells trigger protective innate and adaptive immune responses. Sci. (80-.) 346, 98–101 (2014).
Çuburu, N. et al. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J. Clin. Investig. 122, 4606–4620 (2012).
Beura, L. K. et al. Intravital mucosal imaging of CD8 + resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory article. Nat. Immunol. 19, 173–182 (2018).
Iijima, N. & Iwasaki, A. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science (80-) 346, 93–98 (2014).
Shin, H. & Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467 (2012).
Shin, H., Kumamoto, Y., Gopinath, S. & Iwasaki, A. CD301b+ dendritic cells stimulate tissue-resident memory CD8+ T cells to protect against genital HSV-2. Nat. Commun. 7, 13346 (2016).
Peng, T. et al. Distinct populations of antigen specific tissue resident CD8 T cells in human cervix mucosa. JCI Insight 6, https://doi.org/10.1172/jci.insight.149950 (2021).
Arbyn, M. et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob. Heal 8, e191–e203 (2020).
Komdeur, F. L. et al. CD103+ tumor-infiltrating lymphocytes are tumor-reactive intraepithelial CD8+ T cells associated with prognostic benefit and therapy response in cervical cancer. Oncoimmunology 6, e1338230 (2017).
O’neil, T. R. et al. The role of tissue resident memory cd4 t cells in herpes simplex viral and hiv infection. Viruses 13, 359 (2021).
Posavad, C. M. et al. Persistence of mucosal T-cell responses to herpes simplex virus type 2 in the female genital tract. Mucosal Immunol. 8, 115–126 (2015).
Schiffer, J. T. et al. A Fixed Spatial Structure of CD8 + T Cells in Tissue during Chronic HSV-2 Infection. J. Immunol. 201, 1522–1535 (2018).
Roychoudhury, P. et al. Tissue-resident T cell-derived cytokines eliminate herpes simplex virus-2-infected cells. J. Clin. Investig. 130, 2903–2919 (2020).
Koelle, D. M. et al. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Investig. 101, 1500–1508 (1998).
Wong, M. T. et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity 45, 442–456 (2016).
Hislop, A. D. et al. Tonsillar homing of Epstein-Barr virus-specific CD8+ T cells and the virus-host balance. J. Clin. Investig. 115, 2546–2555 (2005).
Woodberry, T. et al. α E β 7 (CD103) Expression Identifies a Highly Active, Tonsil-Resident Effector-Memory CTL Population. J. Immunol. 175, 4355–4362 (2005).
Argiris, A. & Eng, C. Epidemiology, staging, and screening of head and neck cancer. Cancer Treatment Res. 114, 15–60 (2003).
Szymańska, K. et al. Alcohol and tobacco, and the risk of cancers of the upper aerodigestive tract in Latin America: A case-control study. Cancer Causes Control 22, 1037–1046 (2011).
Anantharaman, D. et al. Population attributable risk of tobacco and alcohol for upper aerodigestive tract cancer. Oral. Oncol. 47, 725–731 (2011).
Harper, D. M. & DeMars, L. R. HPV vaccines – A review of the first decade. Gynecologic Oncol. 146, 196–204 (2017).
Hewavisenti, R. et al. CD103+ tumor-resident CD8+ T cell numbers underlie improved patient survival in oropharyngeal squamous cell carcinoma. J. Immunother. Cancer 8, e000452 (2020).
Mann, J. E. et al. Analysis of tumor-infiltrating CD103 resident memory T-cell content in recurrent laryngeal squamous cell carcinoma. Cancer Immunol. Immunother. 68, 213–220 (2019).
Mazzoni, A. et al. Human T cells interacting with HNSCC-derived mesenchymal stromal cells acquire tissue-resident memory like properties. Eur. J. Immunol. 50, 1571–1579 (2020).
Sinclair, J. Human cytomegalovirus: Latency and reactivation in the myeloid lineage. J. Clin. Virol. 41, 180–185 (2008).
Walter, E. A. et al. Reconstitution of Cellular Immunity against Cytomegalovirus in Recipients of Allogeneic Bone Marrow by Transfer of T-Cell Clones from the Donor. N. Engl. J. Med. 333, 1038–1044 (1995).
Lilleri, D. et al. Human cytomegalovirus-specific CD4+ and CD8+ T cell responses in primary infection of the immunocompetent and the immunocompromised host. Clin. Immunol. 131, 395–403 (2009).
Özdemir, E. et al. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood 100, 3690–3697 (2002).
Thom, J. T. & Oxenius, A. Tissue-resident memory T cells in cytomegalovirus infection. Curr. Opin. Virol. 16, 63–69 (2016).
Gordon, C. L. et al. Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J. Exp. Med. 214, 651–667 (2017).
Woon, H. G. et al. Compartmentalization of Total and Virus-Specific Tissue-Resident Memory CD8+ T Cells in Human Lymphoid Organs. PLoS Pathog. 12, e1005799 (2016).
Taylor, G. S., Long, H. M., Brooks, J. M., Rickinson, A. B. & Hislop, A. D. The immunology of epstein-barr virus-induced disease. Annu. Rev. Immunol. 33, 787–821 (2015).
Long, H. M., Meckiff, B. J. & Taylor, G. S. The T-cell Response to Epstein-Barr Virus–New Tricks From an Old Dog. Front. Immunol. 10, 2193 (2019).
Acknowledgements
We would like to express gratitude to our funders. M.B. was supported by the Swedish Research Council, the Karolinska Institutet, the Swedish Society of Medicine, the Swedish Cancer Society, the Swedish Childhood Cancer Fund, the Åke Wibergs Stiftelse, and the Jonas Söderquist Stiftelse. Figures were conducted using Biorender.
Author information
Authors and Affiliations
Contributions
M.B. conceived the review; J.L., O.R.B. and M.B. wrote the paper; M.B. edited the paper.
Corresponding author
Ethics declarations
Competing interests
M.B. is a consultant for Oxford Immunotec. All other authors report no competing financial interests, patents, patent applications, or material transfer agreements associated with this review.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lange, J., Rivera-Ballesteros, O. & Buggert, M. Human mucosal tissue-resident memory T cells in health and disease. Mucosal Immunol 15, 389–397 (2022). https://doi.org/10.1038/s41385-021-00467-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-021-00467-7
This article is cited by
-
Tissue-Resident Memory T Cells in Allergy
Clinical Reviews in Allergy & Immunology (2024)
-
Intranasal COVID-19 vaccine induces respiratory memory T cells and protects K18-hACE mice against SARS-CoV-2 infection
npj Vaccines (2023)
-
Assessing the generation of tissue resident memory T cells by vaccines
Nature Reviews Immunology (2023)
-
A spotlight on heightened T cell complexity and relevance in mucosal tissues
Mucosal Immunology (2022)
-
Immunization of preterm infants: current evidence and future strategies to individualized approaches
Seminars in Immunopathology (2022)