Abstract
The mammalian immune system is equipped with unconventional T cells that respond to microbial molecules such as glycolipids and small-molecule metabolites, which are invisible to conventional CD4 and CD8 T cells. Unconventional T cells include invariant natural killer T (iNKT) cells and mucosa-associated invariant T (MAIT) cells, which are involved in a wide range of infectious and non-infectious diseases, such as cancer and autoimmunity. In addition, their high conservation across mammals, their restriction by non-polymorphic antigen-presenting molecules, and their immediate and robust responses make these ‘innate’ T cells appealing targets for the development of one-size-fits-all immunotherapies. In this review, we discuss how iNKT and MAIT cells directly and indirectly detect the presence of and respond to pathogenic and commensal microbes. We also explore the current understanding of the bidirectional relationship between the microbiota and innate T cells, and how this crosstalk shapes the immune response in disease.
Similar content being viewed by others
Introduction – Unconventional T cells and their functions
Several populations of unconventional T cells have been identified in mammals that possess both innate and adaptive characteristics, including γδT cells (not discussed here), invariant natural killer T (iNKT) cells and mucosa-associated invariant T (MAIT) cells.1 Unlike conventional T cells, which typically respond to peptide antigens presented in the context of major histocompatibility complex (MHC) molecules, iNKT and MAIT cells are specialized in the recognition of glycolipid antigens and microbial vitamin derivatives, respectively, two antigenic reservoirs that are invisible to conventional T cells.1
There are several phenotypic and functional features that are strikingly similar between these two populations. iNKT and MAIT cells express restricted T cell receptor (TCR) repertoires, characterized by a canonical and invariant TCRα chain. Mouse and human iNKT cells express a TRAV11-TRAJ18- and TRAV10-TRAJ18-containing TCRα chain, respectively. Both mouse and human MAIT cells express a TRAV1.2-TRAJ33-containing TCRα chain. In addition, these cells are restricted by non-polymorphic MHC class I-related proteins, namely CD1d for iNKT cells2 and MR1 for MAIT cells.3 Both cell types share similar transcriptomic profiles4,5 and are generally considered tissue-resident. Finally, iNKT and MAIT cells exhibit rapid helper and cytotoxic effector functions that are reminiscent of innate responses and are often considered to be innate T lymphocytes.
iNKT cells vastly outnumber MAIT cells in mice, whereas MAIT cells are more prevalent in humans. The most notable difference between iNKT and MAIT cells lies in the biochemical nature of the antigens they recognize. iNKT cells respond to glycolipid antigens such as α-galactosylceramide (αGC) presented by CD1d,6,7 while MAIT cells respond to riboflavin (vitamin B2) metabolic derivatives, which includes 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5-OP-RU), presented in the context of MR1.8
iNKT and MAIT cells develop in the thymus and require expression of the transcription factor promyelocytic leukaemia zinc finger (PLZF).9,10,11,12 They differentiate into discrete effector subsets that correspond to CD4 helper T (TH) cell subsets13,14 and are characterized by the expression of signature transcription factors. iNKT1 cells are T-bet+ PLZFlow and preferentially produce IFN-γ, while iNKT17 are ROR-γt+ PLZFint and produce IL-17. Similarly, MAIT1 and MAIT17 cells are characterized by their respective expression of T-bet and ROR-γt. iNKT2 cells are PLZFhigh T-betneg and ROR-γtneg and produce IL-4. No TH2 functional equivalent of MAIT cells has yet been identified. Multiple factors have been shown to control iNKT and MAIT cell effector differentiation, including TCR signal strength and cytokines.15,16,17,18 It is important to note that in humans, iNKT cell subsets have not yet been identified and MAIT cells do not exhibit the clear MAIT1/17 dichotomy found in mice.13,19
The roles iNKT and MAIT cells play in health and disease have been the focus of intense work and the subject of excellent recent reviews.1,20,21,22 Briefly, these cells actively participate in anti-infectious immunity against bacteria, viruses, and other pathogens, and it is arguable that such specialized T cells have evolved in mammals in response to pathogen exposure from their environment. In addition, these cells exert potent functions in tumor immunity20,22,23,24 as well as autoimmune, allergic and inflammatory diseases such as type 1 diabetes and Crohn’s disease.25,26,27,28,29,30,31,32 In this review, we will focus on the interactions between microbes and unconventional T cells and how these interactions influence their function in disease.
The microbiota and the immune system
Mammals are hosts to a multitude of microbes that colonize their epithelial surfaces to form heterogenous, complex and dynamic ecosystems.33,34,35 The composition of the microbiota is highly personalized and acts as an individual microbial ‘fingerprint’.34 Increased microbiota diversity (or richness) and resilience (i.e., the ability to recover from perturbations) are generally associated with better health.36,37,38 Shifts in microbial composition and diversity from the steady-state—a phenomenon known as dysbiosis—have been associated with numerous diseases, such as obesity, inflammatory bowel disease, and cancer.39,40,41,42 In some instances, disease susceptibility can be transferred upon colonization with the dysbiotic microbiota.43,44,45 The microbiota influences host physiological functions, taking part in essential biological processes, such as nutrient absorption and metabolism,46 barrier function47, and immune education.48,49,50,51
The immune system and the microbiota are engaged in an intimate and interdependent relationship. The loss of microbial diversity and the presence of pathobionts have been associated with inflammation and immune activation,51 which can feed back and fuel further dysbiosis.52 Specific pro-inflammatory microbes have been described, including Clostridium difficile,53,54 certain members of Prevotellaceae,55 the murine norovirus56 and protozoa of the Tritrichomonas genus.57,58 Recent studies suggest that individual components of the innate immune system exert little influence on shaping microbiota composition at steady-state,59,60 however ongoing immune or inflammatory responses can impact microbiota communities through the recruitment and activation of immune cells (e.g., neutrophils, macrophages), the production of pro-inflammatory cytokines, antimicrobial peptides, immunoglobulin (Ig) A and reactive oxygen and nitrogen species.52
In addition, the microbiota exerts a tremendous influence on the development, maturation, and function of the immune system, including T cells. A key role that the microbiota exerts on T cells is the local control of regulatory T (TREG) and TH17 CD4 T cell populations, which balances immune tolerance and pro-inflammatory responses. For example, certain members of the microbiota (e.g., SFB) or pathogens (e.g., Citrobacter rodentium) induce the development of intestinal TH17 cells.61,62,63 Colonization of mice by the protozoa Tritrichomonas similarly exacerbates mucosal and systemic TH1 and TH17 responses.57,58 On the other hand, certain commensals such as Clostridium species have been shown to induce the generation of TREG cells.64,65,66 Finally, monocolonization of germ-free (GF) mice by Bacteroides fragilis has been shown to restore the TH1/TH2 balance.67 B. fragilis also reduced Helicobacter hepaticus-induced inflammation by suppressing TH17 cells and inducing interleukin (IL)-10-producing TREG cells.68 Although iNKT and MAIT cells exert potent antimicrobial functions, how these unconventional T cells and the microbiota influence each other is only beginning to emerge.
Unconventional T cells detect microbial signature molecules
iNKT and MAIT cells are a key component of antimicrobial responses as they detect antigens that are invisible to conventional T cells. The microbial realm contains CD1d- and MR1-binding ligands that potently activate iNKT and MAIT cells through their TCRs. Such ligands can, therefore, be viewed as microbial-associated molecular patterns (MAMPs) and drive specific immune outputs from these innate T cells to shape the resulting immune response to pathogens.
Microbial glycolipids
Several CD1d-restricted antigens have been identified in bacteria and other microbes. For example, α-galacturonosylceramides have been isolated from Sphingomonas and Ehrlichia species,69,70 while α-galactosyldiacylglycerols and α-glucosyldiacylglycerols have been isolated from Borrelia burgdorferi71 and Streptococcus pneumoniae,72 respectively. These ligands have been extensively validated through biological, biochemical, and structural approaches. Other lipid antigens capable of activating iNKT cells include glycolipids from Chlamydia muridarum73 and Helicobacter pylori,74 as well as phospholipids from Mycobacterium bovis bacillus Calmette-Guerin75 and the parasite Leishmania donovani.76 Importantly, α-galactosylceramides have been recently isolated from the cecal and colonic contents of mice77 and from the commensal Bacteroides fragilis.78,79 These findings suggest that the intestinal microbiota may represent an unexplored reservoir of ligands capable of modulating iNKT cell activation and function.
Vitamin B metabolites
Riboflavin or vitamin B2 is essential for energy generation in all organisms.80 Although mammals acquire this metabolite through their diet, bacteria are able to obtain riboflavin through import and/or de novo biosynthesis.81 Bacteria that possess riboflavin biosynthetic pathways potently activate MAIT cells through their TCR. These include S. pneumoniae, Klebsiella pneumoniae, Salmonella enterica serovar Typhimurium, Escherichia coli, Pseudomonas aeruginosa, and Mycobacterium tuberculosis,8,82,83 as well as several bacterial commensal species from the Bacteroidetes and Proteobacteria phyla.84 The genetic ablation or inhibition of the riboflavin metabolism in several of these bacteria abolished MAIT cell responses, confirming that this metabolic pathway produces MAIT cell antigens.8,85,86 Riboflavin biosynthesis, therefore, constitutes a telltale sign of microbial activity that MAIT cells can respond to, with the riboflavin derivatives 5-OP-RU and 5-(2-oxoethylideneamino)-6-D-ribitylaminouracil (5-OE-RU) being potent agonists.85 It is important to note that not all MR1-binding ligands are capable of activating MAIT cells. For example, although the folic acid (vitamin B9) metabolite 6-formylpterin (6-FP), the aspirin analog 3-formylsalicylic acid (3-FSA), and a derivative of the drug methotrexate all bind within the MR1 antigen-binding pocket, they do not stimulate MAIT cells, suggesting that non-agonist and agonist ligands may compete to regulate MAIT cell activation.8,87
Microbes regulate iNKT and MAIT cell activation
Aside from the detection of glycolipid and vitamin metabolites, the activation and response of iNKT and MAIT cells can be controlled by many microbe- and host-derived factors.
Microbe-induced pro-inflammatory cytokines are essential co-signals
iNKT and MAIT cells can indirectly detect the presence of microbes through the recognition of MAMPs. Antigen-presenting cells (APCs) activated by natural or synthetic ligands of the toll-like receptors (TLR) 2, TLR3, TLR4, TLR5, TLR8, and TLR9 release pro-inflammatory cytokines that can activate MAIT cells to produce IFN-γ.88,89,90 Similarly, stimulation of TLR4, TLR7 and TLR9, Dectin-1, Rig-I, and Nod1/2 on APCs leads to IFN-γ production by iNKT cells.70,91,92,93,94,95,96 In these instances, MAIT and iNKT cells are activated by IL-12 and/or IL-18 produced by activated APCs such as dendritic cells and monocytes/macrophages. In addition, iNKT and MAIT cells can respond to IL-1β, IL-7, IL-15, IL-18, IL-21, IL-23, IL-25, and IL-33 alone or in certain combinations.97,98,99,100,101,102,103,104 Whereas some of these cytokines have proliferative effects (e.g IL-7 and IL-15), others induce the production of helper cytokines (e.g., IL-1β, IL-23, IL-25, and IL-33). Pro-inflammatory cytokines can activate innate T cells on their own or act as co-signals to potentiate weak TCR-mediated signals90,105,106,107,108 and, at least for iNKT cells, appear to be the main activation pathway in response to bacteria.105 Pro-inflammatory signals also underpin iNKT and MAIT cell activation during viral infection and sterile inflammation, such as during cancer and autoimmunity.1,20,21,22
Microbes regulate CD1d and MR1 expression
Regulating the expression of CD1d and MR1 molecules by microbes and their by-products constitutes another mechanism to control iNKT and MAIT cell activation and potentially prevent unwanted responses towards commensals. MR1 is essentially sequestered within the endoplasmic reticulum at steady-state109,110 and its expression at the plasma membrane can, in the presence of riboflavin derivatives such as 5-OP-RU, be induced or reinforced by MAMPs and/or type I and II interferons.111,112,113,114 In addition, viruses such as herpes virus can downregulate surface MR1 levels and lead to impaired TCR-mediated activation.115 CD1d is expressed quite ubiquitously and its expression can be increased by type I and II interferons, MAMPs such as LPS, all-trans retinoic acid, the short-chain fatty acid butyrate, and potentially vitamin D.116,117,118,119,120,121,122,123 Viruses such as herpes virus or human immunodeficiency virus-1 are also able to regulate CD1d expression, perhaps as a mechanism for immune evasion.118,124,125
Bile acids and microbial by-products may influence iNKT and MAIT cell responses
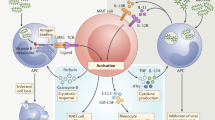
Some microbes may regulate iNKT and MAIT cell activation and function through their production of metabolites as well as modulation of bile acids within the intestine (Fig. 1). Primary bile acids are secreted into the small intestine during digestion and metabolized by the gut microbiota into secondary bile acids upon reaching the large intestine. Lithocholic acid (LCA) is a secondary bile acid produced by Clostridium and Eubacterium species that inhibits the function of TH1 CD4 T cells by binding to the vitamin D receptor.126 Since the vitamin D receptor is expressed by both iNKT and MAIT cells and is required for the normal development and function of iNKT cells,4,122,127 it is possible that LCA similarly modulates iNKT and MAIT cell function.
Lithocholic acid (LCA), a by-product of bile acid metabolism, is a ligand for the vitamin D receptor (VDR), which is expressed by iNKT and MAIT cells. Binding of LCA to VDR may inhibit their production of TH1 cytokines (e.g., IFN-γ and TNF-α). Indole is a tryptophan catabolite that may regulate IL-17 and IL-22 production by unconventional T cells through interaction with the transcription factor RORγt. The short-chain fatty acid (SCFA) butyrate regulates gene expression through inhibition of histone deacetylases (HDAC). Butyrate may inhibit HDAC3 in iNKT cells, which has been shown to inhibit their cytokine secretion. Other tryptophan catabolites may regulate iNKT cell cytokine response through interaction with the aryl hydrocarbon receptor (AhR). Finally, inhibition of AhR within intestinal epithelial cells (IEC) alters interactions with iNKT cells. This results in the loss of IL-10 production by IECs and exacerbated IL-13/IFN-γ responses in iNKT cells, which leads to intestinal inflammation.
Indole is a catabolite of tryptophan produced by Gram-negative and Gram-positive microbes containing tryptophanase, including E. coli, Bacteroides, Clostridium, and Vibrio species.128 Indole inhibits TH17 CD4 T cell differentiation through interactions with RORγt,129 and may similarly inhibit the differentiation and/or function of iNKT17 and MAIT17 cells. Other tryptophan catabolites such as tryptamine, indolelactic acid, and indoleacetic acid are ligands for the aryl hydrocarbon receptor (AhR). AhR is expressed by human iNKT cells and has been shown to regulate their IL-17 and IL-22 production,130 and AhR deficiency results in increased IFN-γ production by iNKT cells and exacerbates ConA-induced liver injury in mice.131
Oxazoles are heterocyclic aromatic organic compounds that are found in certain diets as well as in microcins, which are antimicrobial peptides produced by E. coli. Oxazoles can induce tryptophan metabolism and AhR activation in intestinal epithelial cells, and result in exacerbated pro-inflammatory responses of iNKT cells and colitis.132
Butyrate is a short-chain fatty acid derived from the fermentation of indigestible food by certain bacterial taxa belonging to Firmicutes and Bacteroidetes.133,134,135 Butyrate has gained increasing attention in recent years due to its capacity to regulate diseases such as inflammatory bowel disease and colorectal cancer.136,137 The primary mechanism of action of butyrate is the epigenetic regulation of gene expression via inhibition of histone deacetylase (HDAC),138 among which HDAC3 is required for iNKT cell development139 and has been shown to inhibit cytokine secretion by iNKT cells in a model of antibody-induced arthritis.140 Therefore, butyrate-producing microbes can participate in the control of iNKT cells via the butyrate-HDAC3 axis.
Although relatively little is known compared to conventional T cells,141,142 the emerging data suggest that iNKT and MAIT cells respond to a complex network of microbe-derived molecules and their by-products to fine tune ensuing immune responses. Specific interactions between the microbiota and unconventional T cells are discussed below.
Regulation of the microbiota by unconventional T cells
iNKT cell/CD1d-mediated shaping of the microbiota
Analysis of the gut microbiota through 16S rRNA sequencing using TRAJ18−/− mice (lacking iNKT cells) and CD1d−/− mice (lacking all CD1d-restricted T cells, including iNKT cells) has revealed that CD1d and/or iNKT cells may influence the composition of the microbiota (Table 1).143,144,145,146,147,148 Two studies have reported similar alterations in CD1d−/−143 and TRAJ18−/− mice,144 namely an increased prevalence of the Bacteroidetes, Deferribacteres, Proteobacteria, and TM7 phyla and several genera including Lactobacillus, Bacteroides, Mucispirillum, and Prevotella relative to wild-type (WT) mice. In a third study, bacteria from the phylum Tenericutes and genera Bifidobacterium, Prevotella, and Olsenella were found to be enriched in TRAJ18−/− compared to WT mice, while the Deferribacteres and Actinobacteria phyla were markedly decreased.147 It is important to note that environmental factors, such as diet, housing conditions, and maternal transmission are known to have a major impact on microbiota composition and should, therefore, be carefully controlled in all microbiota studies.33,34,149,150,151,152,153 Although co-housing of mice after weaning has been widely used in efforts to normalize the microbiota in various experimental settings, a recent study demonstrated that this method is ineffective at normalizing mucosa-associated microbes and that only littermate mice from heterozygous breeding should be used to minimize these confounding effects.152 In support of this, we found that adjacently bred non-littermate CD1d−/− and WT mice had major microbiota alterations, whereas CD1d+/+, CD1d+/− and CD1d−/− littermate mice had similar bacterial composition.143 Two of the studies discussed in this section lacked information on the experimental designs used and likely compared non-littermate mice, which puts the relevance of the findings into question.144,147 In addition, the TRAJ18−/− used in these studies were recently shown to have a less diverse T cell population and to completely lack MAIT cells as an artifact of the genetic targeting method employed.154,155 Several new strains of TRAJ18−/− have been generated to alleviate the unintended consequences of the genetic manipulation, which should help clarify the biological functions of iNKT cells in disease and in shaping the intestinal microbiota.156,157,158
An initial study found that CD1d−/− mice had altered microbial communities and increased bacterial translocation compared to WT littermates, which was attributed to altered Paneth cell function.145 However, this study contained no statistical analyses and it is unclear how much the genotype contributed to community dissimilarity. Additionally, a more recent study reported microbial alterations between CD1d−/− and CD1d+/− littermates, between CD1dfl/fl CD11c-cre (which conditionally lack CD1d only on CD11c-expressing cells) and CD1dfl/fl littermates, and following oral αGC administration in WT mice.146 However, it is unclear whether the genotype or other factors such as maternal transmission, caging or sex primarily explained the observed shifts in bacterial composition. In sum, it is presently unclear whether the presence and/or activation of iNKT cells exert a strong influence in shaping the commensal microbial communities in mice.
Influence of MAIT cells and MR1 on the microbiota
Only a few studies have addressed whether MAIT cells or MR1 shape the microbiota thus far. Recent studies showed that MR1−/− mice exhibited differences in the relative abundance of several bacterial families, including Bacteroidaceae and Lactobacilliaceae, compared to WT mice, which conferred resilience to antibiotic perturbations and was associated with increased production of bile acids and resistance to Clostridium difficile infection.159,160 However, it is important to note that these authors initially compared MR1−/− mice bred in house with commercially obtained WT mice, while additional analyses were performed on mice that were co-housed for four weeks post-weaning (Table 1). As discussed above, co-housing is not a reliable method to normalize environmental differences.152 In addition, the relative contribution of genotype and other factors to the observed variation in microbiota composition was not analyzed. An important message that can be drawn from these studies is that littermate-controlled experiments carefully designed to minimize variability and robust statistical methods are needed in order to draw biologically meaningful conclusions on the immune-mediated shaping of the gut microbiota.59
Modulation of innate T cells by the microbiota
Microbial colonization impacts innate T cell development and homeostasis
The microbiota is important for the education of the adaptive immune system, and early life exposure ensures proper T and B cell maturation, differentiation, and tolerance.49,161,162 This also appears to be true for iNKT and MAIT cells. Although the development and tissue homing capabilities of iNKT cells are largely unaffected by the absence of the microbiota in GF mice, their prevalence is increased in the lungs, small intestine and colon.27,163,164,165 In addition, iNKT cells in GF mice have an altered TCRβ repertoire and are overall less mature and hyporesponsive to αGC stimulation.163 Similar defects are also observed in mice harboring a restricted microbiota characterized by a reduced prevalence of Sphingomonas species, which are known to produce activating iNKT cell ligands.164 However, whether these changes are specific to certain iNKT cell subsets and their tissue distribution is not known. Interestingly, mono-colonization of GF mice with Sphingomonas yanoikuyake, but not E. coli, corrected the defects, suggesting that the commensal-derived antigenic load directly participates in iNKT cell homeostasis and/or function.163 Furthermore, the accumulation of iNKT cells in the lungs and intestines of GF mice can be reversed by microbiota colonization early in life but not after weaning.27
Tthe development of MAIT cells is profoundly affected by the absence of the microbiota in GF mice3,10,13,82,166 (Fig. 2). Although colonization of GF mice with a complex human microbiota is able to restore MAIT cell numbers to that of specific pathogen-free (SPF) mice, they are unable to reach the higher levels normally found in humans.10 Similarly, mono-association of GF mice with Enterobacter cloacae, Lactobacillus casei, Bacteroides thetaiotaomicron, Bifidobacterium animalis, but not E. faecalis, also restores MAIT cell numbers to SPF levels.82 In addition, early-life exposure to riboflavin-synthesizing bacteria, such as Enterobacteriaceae, can restore MAIT cell development in GF mice, whereas colonization of adult GF mice has no effect.100 Together, these findings suggest that riboflavin-synthesizing commensals can stimulate MAIT cell development and/or homeostasis (Fig. 2). Likewise, topical application of 5-OP-RU to the skin of GF mice early in life, but not in adulthood, is sufficient to restore MAIT cell numbers.100 Another recent study in mice has suggested that microbial metabolites have the potential to translocate to the thymus, upon which they can be captured by MR1-expressing cells and presented to developing thymocytes to induce MAIT cell maturation.166 In humans, MAIT found in the umbilical cord blood are immature, whereas some mature MAIT cells can be found in peripheral blood after birth and accumulate with age.167,168 This suggests that MAIT cell maturation likely involves microbial antigens and appears to be fine-tuned by the TCRβ chain.167,168 Collectively, these findings suggest that early-life colonization by the microbiota has a profound and long-lasting impact on the development and homeostasis of both iNKT and MAIT cells.
MAIT cells are virtually absent in germ-free (GF) mice. Early-life colonization of GF mice with diverse microbiota from mice or humans restores MAIT cell development. Similarly, oral, intraperitoneal, or topical administration of riboflavin-producing bacteria or the MAIT ligand 5-OP-RU restores MAIT cell development and homeostasis. Interestingly, exogenous 5-OP-RU can traffic to the thymus to stimulate MAIT cell ontogeny.
The microbiota influences innate T cell function in disease
The gut microbiota modulates iNKT cell function in disease. The accumulation of iNKT cells in the lungs and intestines of GF mice was correlated with exacerbated ovalbumin-driven asthma and oxazolone-induced colitis, which could be improved by administration of neutralizing anti-CD1d antibodies.27 The authors of this study went on to show that GF mice mono-colonized with B. fragilis from birth were protected from chemically induced colitis in adulthood as a result of normalized abundance of colonic iNKT cells79 1. The protective effect of B. fragilis was dependent on the expression of the glycosphingolipid GSL-Bf717, as GF mice colonized with mutant B. fragilis lacking GSL-Bf717 were not protected.143,169,170 Although iNKT cell functional response (e.g., cytokine production) was not directly assessed, these studies demonstrated that early-life microbial colonization is necessary to tune down the pro-inflammatory functions of iNKT cells at mucosal sites.
Additional hints on how the gut microbiota functionally impacts iNKT cells and their ability to regulate disease can be gleaned from a few other studies. For example, glycolipid-mediated activation of iNKT cells during the course of dextran sulfate sodium (DSS)-induced colitis has shown inconsistent results between studies.143,169,170 The discrepancies may be attributed to different experimental designs, including the dose, frequency, and route of glycolipid injection, as well as environmental factors such as housing conditions, diet, and microbiota differences between animal facilities. In a previous study, we demonstrated that WT mice born from dams transplanted with two discrete microbial communities have different clinical outcomes in response to the synthetic iNKT cell ligand OCH9, which suggests that iNKT cell responses and/or their ability to modulate immune and inflammatory responses are regulated by the intestinal microbiota.143 Short-term broad spectrum antibiotic treatment in adult mice increased colonic iNKT cell numbers, and subsequent reconstitution with a dysbiotic microbiota (isolated from DSS-treated mice) skewed the cells towards a pro-inflammatory phenotype.171 This microbiota-driven skewing of iNKT cell phenotype was also observed in ulcerative colitis patients.28 Antibiotic treatment also had similar effects on liver iNKT cells. In mice, accumulation of IFN-γ-producing iNKT cells in the liver was driven by the upregulation of CXCL16 in liver sinusoidal endothelial cells as a result of the reduction in microbiota-mediated primary to secondary bile acid conversion in the gut, and in this case was associated with protective function against intrahepatic tumor growth.172 Taken together, these studies suggest that the gut microbiota has the capacity to regulate iNKT cell homeostasis in certain tissues, as well as their functional response during inflammation and tumor development (Fig. 3).
iNKT cells in GF mice are pro-inflammatory and fuel allergen-induced airway inflammation and colitis. Early-life colonization with a complex microbiota, or the sphingolipid-producing commensal Bacteroides fragilis restores normal iNKT cell functions and limits inflammation. Bile acids produced by the liver are modified by the microbiota in the gut and recirculate back to the liver where they recruit iNKT cells via the chemokine CXCL16. Accumulated iNKT cells provide antitumor immunity in the liver.
MAIT cells have also been shown to play a role in multiple infectious and sterile diseases, however to what extent and how the microbiota regulates their function remains unclear. MAIT cells provide initial immunity against Mycobacterium tuberculosis infection due to their robust granzyme B production.173 Interestingly, their capacity to produce granzyme B correlates with the abundance of specific bacterial species, namely B. ovatus and P. merdae of the class Bacteroidia. Furthermore, antibiotic-induced dysbiosis was found to increase early infection levels of M. tuberculosis in mice, which correlated with reduced MAIT cell numbers in the lungs.174 The inoculation of a complex microbiota into antibiotic-treated mice restored MAIT cell numbers and conferred protection, suggesting that the microbiota modulates MAIT cell proliferation and activity in response to infection. In addition, the loss of intestinal epithelial integrity and bacterial translocation in patients with alcohol-related liver disease led to reduced circulating MAIT cell numbers and impaired IL-17 and granzyme B production.175 Similar MAIT cell defects were found in children with type 1 diabetes, which is associated with a loss of intestinal barrier integrity.25 Furthermore, MR1−/− non-obese diabetic (NOD) mice have greater intestinal permeability, decreased expression of tight-junction proteins, abnormal mucus distribution, and accelerated disease progression compared to MR1+/− NOD mice.25 Therefore, there is mounting evidence that the microbiota can modulate MAIT cell function during infectious and inflammatory diseases.
Concluding remarks
While our understanding of the biology of iNKT and MAIT cells has seen tremendous progress in recent years, it has for the most part focused on how host-derived factors control the development and function of these innate T cells, with the influence of environmental factors, such as the microbiota being mainly overlooked. Whereas it remains unclear whether innate T cells influence the composition of the microbiota in mammals, the influence of the microbiota on iNKT and MAIT cells has now come into clearer focus. These findings raise several fundamental and practical questions: Can the heterogeneity observed in innate T cell compartments between individuals and populations be explained, at least in part, by environmental differences such as microbiota composition? Are microbiota-innate T cell interactions relevant to human disease and therapies targeting these cells? What are the microbial determinants that modulate innate T cell homeostasis and functional properties? And most importantly, can we manipulate the gut microbiota or harness their by-products in order to modulate iNKT and MAIT cell function in disease? Innate T cells constitute an integral component of the immunologist’s toolkit to develop novel targeted immunotherapies,24 and prospects of their selective manipulation learned through their dialogue with the gut microbiota are certainly worthy of careful examination.
References
Godfrey, D. I., Uldrich, A. P., McCluskey, J., Rossjohn, J. & Moody, D. B. The burgeoning family of unconventionalT cells. Nat. Immunol. 16, 1114–1123 (2015).
Bendelac, A. et al. CD1 recognition by mouse NK1+ T lymphocytes. Science 268, 863–865 (1995).
Treiner, E. et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169 (2003).
Hinks, T. S. C. et al. Activation and in vivo evolution of the MAIT cell transcriptome in mice and humans reveals tissue repair functionality. Cell Rep. 28, 3249–3262.e5 (2019).
Salou, M. et al. A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J. Exp. Med. 216, 133–151 (2019).
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997).
Burdin, N. et al. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J. Immunol. 161, 3271–3281 (1998).
Kjer-Nielsen, L. et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723 (2012).
Rahimpour, A. et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 212, 1095–1108 (2015).
Cui, Y. et al. Mucosal-associated invariant T cell–rich congenic mouse strain allows functional evaluation. J. Clin. Invest. 125, 4171–4185 (2015).
Savage, A. K. et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity 29, 391–403 (2008).
Kovalovsky, D. et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol. 9, 1055–1064 (2008).
Koay, H.-F. et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat. Immunol. 17, 1300–1311 (2016).
Lee, Y. J., Holzapfel, K. L., Zhu, J., Jameson, S. C. & Hogquist, K. A. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 14, 1146–1154 (2013).
Koay, H.-F., Godfrey, D. I. & Pellicci, D. G. Development of mucosal-associated invariant T cells. Immunol. Cell Biol. https://doi.org/10.1111/imcb.12039 (2018).
Gapin, L. Development of invariant natural killer T cells. Curr. Opin. Immunol. 39, 68–74 (2016).
Wang, H. & Hogquist, K. A. How lipid-specific T cells become effectors: the differentiation of iNKT subsets. Front Immunol. 9, 1450 (2018).
Lantz, O. & Legoux, F. MAIT cells: programmed in the thymus to mediate immunity within tissues. Curr. Opin. Immunol. 58, 75–82 (2019).
Dusseaux, M. et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259 (2011).
Godfrey, D. I., Koay, H.-F., McCluskey, J. & Gherardin, N. A. The biology and functional importance of MAIT cells. Nat. Immunol. 20, 1110–1128 (2019).
Krovi, S. H. & Gapin, L. Invariant natural killer T cell subsets-more than just developmental intermediates. Front Immunol. 9, 1393 (2018).
Toubal, A., Nel, I., Lotersztajn, S. & Lehuen, A. Mucosal-associated invariant T cells and disease. Nat. Rev. Immunol. 19, 643–657 (2019).
Wolf, B. J., Choi, J. E. & Exley, M. A. Novel approaches to exploiting invariant NKT cells in cancer immunotherapy. Front Immunol. 9, 384 (2018).
Godfrey, D. I., Le Nours, J., Andrews, D. M., Uldrich, A. P. & Rossjohn, J. Unconventional T cell targets for cancer immunotherapy. Immunity 48, 453–473 (2018).
Rouxel, O. et al. Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat. Immunol. 18, 1321–1331 (2017).
Heller, F., Fuss, I. J., Nieuwenhuis, E. E., Blumberg, R. S. & Strober, W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 17, 629–638 (2002).
Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 (2012).
Burrello, C. et al. Mucosa-associated microbiota drives pathogenic functions in IBD-derived intestinal iNKT cells. Life Sci. Alliance 2, e201800229 (2019).
Beaudoin, L., Laloux, V., Novak, J., Lucas, B. & Lehuen, A. NKT cells inhibit the onset of diabetes by impairing the development of pathogenic T cells specific for pancreatic beta cells. Immunity 17, 725–736 (2002).
Akbari, O. et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 9, 582–588 (2003).
Pichavant, M. et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J. Exp. Med. 205, 385–393 (2008).
Lisbonne, M. et al. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 171, 1637–1641 (2003).
Spencer, S. P., Fragiadakis, G. K. & Sonnenburg, J. L. Pursuing human-relevant gut microbiota-immune interactions. Immunity 51, 225–239 (2019).
Gilbert, J. A. et al. Current understanding of the human microbiome. Nat. Med. 24, 392–400 (2018).
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Blaser, M. J. & Falkow, S. What are the consequences of the disappearing human microbiota? Nat. Rev. Micro 7, 887–894 (2009).
Sonnenburg, E. D. & Sonnenburg, J. L. The ancestral and industrialized gut microbiota and implications for human health. Nat. Rev. Micro 17, 383–390 (2019).
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Zitvogel, L. et al. Cancer and the gut microbiota: an unexpected link. Sci. Transl. Med 7, 271ps1–271ps1 (2015).
Ni, J., Wu, G. D., Albenberg, L. & Tomov, V. T. Gut microbiota and IBD: causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 14, 573–584 (2017).
Gomes, A. C., Hoffmann, C. & Mota, J. F. The human gut microbiota: metabolism and perspective in obesity. Gut Microbes 9, 308–325 (2018).
Irrazabal, T., Belcheva, A., Girardin, S. E., Martin, A. & Philpott, D. J. The multifaceted role of the intestinal microbiota in colon cancer. Mol. Cell 54, 309–320 (2014).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1131 (2006).
Arrieta, M.-C. et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med 7, 307ra152–307ra152 (2015).
Palm, N. W. et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010 (2014).
Oliphant, K. & Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7, 91–15 (2019).
Natividad, J. M. M. & Verdu, E. F. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol. Res. 69, 42–51 (2013).
Belkaid, Y. & Harrison, O. J. Homeostatic immunity and the microbiota. Immunity 46, 562–576 (2017).
Gensollen, T., Iyer, S. S., Kasper, D. L. & Blumberg, R. S. How colonization by microbiota in early life shapes the immune system. Science 352, 539–544 (2016).
Chen, F. & Stappenbeck, T. S. Microbiome control of innate reactivity. Curr. Opin. Immunol. 56, 107–113 (2019).
Levy, M., Kolodziejczyk, A. A., Thaiss, C. A. & Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 17, 219–232 (2017).
Zeng, M. Y., Inohara, N. & Núñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 10, 18–26 (2016).
Rodemann, J. F., Dubberke, E. R., Reske, K. A., Seo, D. H. & Stone, C. D. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 5, 339–344 (2007).
Issa, M. et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 5, 345–351 (2007).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecologyand risk for colitis. Cell 145, 745–757 (2011).
Cadwell, K. et al. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell 141, 1135–1145 (2010).
Chudnovskiy, A. et al. Host-protozoan interactions protect from mucosal infections through activation of the inflammasome. Cell 167, 444–456.e14 (2016).
Escalante, N. K. et al. The common mouse protozoa Tritrichomonas muris alters mucosal T cell homeostasis and colitis susceptibility. J. Exp. Med. 213, 2841–2850 (2016).
Wullaert, A., Lamkanfi, M. & McCoy, K. D. Defining the impact of host genotypes on microbiota composition requires meticulous control of experimental Variables. Immunity 48, 605–607 (2018).
Robertson, S. J., Goethel, A., Girardin, S. E. & Philpott, D. J. Innate immune influences on the gut microbiome: lessons from mouse models. Trends Immunol. 39, 992–1004 (2018).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Ivanov, I. I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 4, 337–349 (2008).
Geddes, K. et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat. Med. 17, 837–844 (2011).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Geuking, M. B. et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34, 794–806 (2011).
Mazmanian, S. K., Liu, C. H., Tzianabos, A. O. & Kasper, D. L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118 (2005).
Mazmanian, S. K., Round, J. L. & Kasper, D. L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625 (2008).
Kinjo, Y. et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434, 520–525 (2005).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Kinjo, Y. et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 7, 978–986 (2006).
Kinjo, Y. et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat. Immunol. 12, 966–974 (2011).
Peng, Y. et al. The glycolipid exoantigen derived from Chlamydia muridarum activates invariant natural killer T cells. Cell. Mol. Immunol. 9, 361–366 (2012).
Ito, Y. et al. Helicobacter pylori cholesteryl α-glucosides contribute to its pathogenicity and immune response by natural killer T cells. PloS one 8, e78191 (2013).
Fischer, K. et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl Acad. Sci. U. S. A. 101, 10685–10690 (2004).
Amprey, J. L. et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J. Exp. Med. 200, 895–904 (2004).
von Gerichten, J. et al. Bacterial immunogenic α-galactosylceramide identified in the murine large intestine: dependency on diet and inflammation. J. Lipid Res. https://doi.org/10.1194/jlr.RA119000236 (2019)
Brown, L. C. W. et al. Production of α-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol. 11, e1001610 (2013).
An, D. et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156, 123–133 (2014).
Powers, H. J., Corfe, B. M. & Nakano, E. in Water Soluble Vitamins: Clinical Research and Future Application (ed. Stanger, O.) 229–245 (Springer Netherlands, Dordrecht, 2012).
García-Angulo, V. A. Overlapping riboflavin supply pathways in bacteria. Crit. Rev. Microbiol. 43, 196–209 (2017).
Le Bourhis, L. et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 11, 701–708 (2010).
Gold, M. C. et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 8, e1000407 (2010).
Tastan, C. et al. Tuning of human MAIT cell activation by commensal bacteria species and MR1-dependent T-cell presentation. Mucosal Immunol. 11, 1591–1605 (2018).
Corbett, A. J. et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509, 361–365 (2014).
Soudais, C. et al. In vitro and in vivo analysis of the Gram-negative bacteria-derived riboflavin precursor derivatives activating mouse MAIT cells. J. Immunol. 194, 4641–4649 (2015).
Eckle, S. B. G. et al. A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J. Exp. Med. 211, 1585–1600 (2014).
Leng, T. et al. TCR and inflammatory signals tune human MAIT cells to exert specific tissue repair and effector functions. Cell Rep. 28, 3077–3091.e5 (2019).
Chen, Z. et al. Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol. 10, 58–68 (2017).
Ussher, J. E. et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur. J. Immunol. 44, 195–203 (2014).
Paget, C. et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 27, 597–609 (2007).
Paget, C. et al. Potential role of invariant NKT cells in the control of pulmonary inflammation and CD8+ T cell response during acute influenza A virus H3N2 pneumonia. J. Immunol. 186, 5590–5602 (2011).
Brigl, M., Bry, L., Kent, S. C., Gumperz, J. E. & Brenner, M. B. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 4, 1230–1237 (2003).
Salio, M. et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc. Natl Acad. Sci. USA 104, 20490–20495 (2007).
Cohen, N. R. et al. Innate recognition of cell wall b-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe 10, 437–450 (2011).
Selvanantham, T. et al. Nod1 and Nod2 enhance TLR-mediated invariant NKT cell activation during bacterial infection. J. Immunol. 191, 5646–5654 (2013).
Sattler, A., Dang-Heine, C., Reinke, P. & Babel, N. IL-15 dependent induction of IL-18 secretion as a feedback mechanism controlling human MAIT-cell effector functions. Eur. J. Immunol. 45, 2286–2298 (2015).
Leeansyah, E. et al. Arming of MAIT cell cytolytic antimicrobial activity is induced by IL-7 and defective in HIV-1 Infection. PLoS Pathog. 11, e1005072–23 (2015).
Martínez-Barricarte, R. et al. Human IFN-γ immunity to mycobacteria is governed by both IL-12 and IL-23. Sci. Immunol. 3, eaau6759 (2018).
Constantinides, M. G. et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 366, eaax6624–16 (2019).
Terashima, A. et al. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J. Exp. Med. 205, 2727–2733 (2008).
Rachitskaya, A. V. et al. Cutting edge: NKT cells constitutively express IL-23 receptor and ROR t and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J. Immunol. 180, 5167–5171 (2008).
Coquet, J. M. et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J. Immunol. 178, 2827–2834 (2007).
Bourgeois, E. et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur. J. Immunol. 39, 1046–1055 (2009).
Brigl, M. et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 208, 1163–1177 (2011).
Slichter, C. K. et al. Distinct activation thresholds of human conventional and innate-like memory T cells. JCI Insight 1, 717–717 (2016).
Holzapfel, K. L., Tyznik, A. J., Kronenberg, M. & Hogquist, K. A. Antigen-dependent versus -independent activation of invariant NKT cells during infection. J. Immunol. 192, 5490–5498 (2014).
Nagarajan, N. A. & Kronenberg, M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J. Immunol. 178, 2706–2713 (2007).
Huang, S. et al. MR1 uses an endocytic pathway to activate mucosal-associated invariant T cells. J. Exp. Med. 205, 1201–1211 (2008).
McWilliam, H. E. G. et al. The intracellular pathway for the presentation of vitamin B–related antigens by the antigen-presenting molecule MR1. Nat. Immunol. 17, 531–537 (2016).
Liu, J. & Brutkiewicz, R. R. The Toll-like receptor 9 signalling pathway regulates MR1-mediated bacterial antigen presentation in B cells. Immunology 152, 232–242 (2017).
Ussher, J. E. et al. TLR signaling in human antigen-presenting cells regulates MR1-dependent activation of MAIT cells. Eur. J. Immunol. 46, 1600–1614 (2016).
Lamichhane, R. et al. Type I interferons are important co-stimulatory signals during T cell receptor mediated human MAIT cell activation. Eur. J. Immunol. https://doi.org/10.1002/eji.201948279 (2019)
Seach, N. et al. Double-positive thymocytes select mucosal-associated invariant T cells. J. Immunol. 191, 6002–6009 (2013).
McSharry, B. P. et al. Virus-mediated suppression of the antigen presentation molecule MR1. Cell Rep. 30, 2948–2962.e4 (2020).
Raghuraman, G., Geng, Y. & Wang, C.-R. IFN-β-mediated up-regulation of CD1d in bacteria-infected APCs. J. Immunol. 177, 7841–7848 (2006).
Colgan, S. P. et al. IFN-gamma modulates CD1d surface expression on intestinal epithelia. Am. J. Physiol. 271, C276–C283 (1996).
Raftery, M. J. et al. Inhibition of CD1 Antigen Presentation by Human Cytomegalovirus. J. Virol. 82, 4308–4319 (2008).
Chen, Q. & Ross, A. C. Retinoic acid regulates CD1d gene expression at the transcriptional level in human and rodent monocytic cells. Exp. Biol. Med. (Maywood) 232, 488–494 (2007).
Allan, L. L. et al. CD1d and CD1c expression in human B cells is regulated by activation and retinoic acid receptor signaling. J. Immunol. 186, 5261–5272 (2011).
Berntman, E., Rolf, J., Johansson, C., Anderson, P. & Cardell, S. L. The role of CD1d-restricted NK T lymphocytes in the immune response to oral infection with Salmonella typhimurium. Eur. J. Immunol. 35, 2100–2109 (2005).
Yu, S. & Cantorna, M. T. The vitamin D receptor is required for iNKT cell development. Proc. Natl Acad. Sci. USA 105, 5207–5212 (2008).
Nascimento, C. R., Freire-de-Lima, C. G., da Silva de Oliveira, A., Rumjanek, F. D. & Rumjanek, V. M. The short chain fatty acid sodium butyrate regulates the induction of CD1a in developing dendritic cells. Immunobiology 216, 275–284 (2011).
Chen, N. et al. HIV-1 down-regulates the expression of CD1d via Nef. Eur. J. Immunol. 36, 278–286 (2006).
Sanchez, D. J., Gumperz, J. E. & Ganem, D. Regulation of CD1d expression and function by a herpesvirus infection. J. Clin. Invest. 115, 1369–1378 (2005).
Pols, T. W. H. et al. Lithocholic acid controls adaptive immune responses by inhibition of Th1 activation through the vitamin D receptor. PLoS ONE 12, e0176715 (2017).
Yu, S., Zhao, J. & Cantorna, M. T. Invariant NKT Cell Defects in Vitamin D Receptor Knockout Mice Prevents Experimental Lung Inflammation. J. Immunol. 187, 4907–4912 (2011).
Lee, J.-H. & Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 34, 426–444 (2010).
Yang, T. et al. Discovery of tertiary amine and indole derivatives as potent RORγt inverse agonists. ACS Med. Chem. Lett. 5, 65–68 (2014).
Moreira-Teixeira, L. et al. Proinflammatory environment dictates the IL-17-producing capacity of human invariant NKT cells. J. Immunol. 186, 5758–5765 (2011).
Abe, H. et al. Aryl hydrocarbon receptor plays protective roles in ConA-induced hepatic injury by both suppressing IFN-γ expression and inducing IL-22. Int. Immunol. 26, 129–137 (2014).
Iyer, S. S. et al. Dietary and microbial oxazoles induce intestinal inflammation by modulating aryl hydrocarbon receptor responses. Cell 173, 1123–1134.e11 (2018).
Louis, P., Young, P., Holtrop, G. & Flint, H. J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 12, 304–314 (2010).
Van den Abbeele, P. et al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 7, 949–961 (2013).
Rivière, A., Selak, M., Lantin, D., Leroy, F. & De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 7, 979 (2016).
Machiels, K. et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63, 1275–1283 (2014).
Belcheva, A. et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 158, 288–299 (2014).
Chriett, S. et al. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci. Rep. 9, 742 (2019).
Thapa, P., Romero Arocha, S., Chung, J. Y., Sant’angelo, D. B. & Shapiro, V. S. Histone deacetylase 3 is required for iNKT cell development. Sci. Rep. 7, 5784 (2017).
Lee, S., Koh, J., Chang, Y., Kim, H. Y. & Chung, D. H. Invariant NKT cells functionally link microbiota-induced butyrate production and joint inflammation. J. Immunol. 203, 3199–3208 (2019).
McCoy, K. D., Ronchi, F. & Geuking, M. B. Host-microbiota interactions and adaptive immunity. Immunological Rev. 279, 63–69 (2017).
Belkaid, Y., Bouladoux, N. & Hand, T. W. Effector and memory T cell responses to commensal bacteria. Trends Immunol. 34, 299–306 (2013).
Selvanantham, T. et al. NKT cell–deficient mice harbor an altered microbiota that fuels intestinal inflammation during chemically induced colitis. J. Immunol. 197, 4464–4472 (2016).
Maricic, I. et al. Differential activation of hepatic invariant NKT cell subsets plays a key role in progression of nonalcoholic steatohepatitis. J. Immunol. 201, 3017–3035 (2018).
Nieuwenhuis, E. E. S. et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J. Clin. Invest. 119, 1241–1250 (2009).
Saez de Guinoa, J. et al. CD1d-mediated lipid presentation by CD11c+ cells regulates intestinal homeostasis. EMBO J 37, e97537 (2018).
Shen, S. et al. Invariant natural killer T cells shape the gut microbiota and regulate neutrophil recruitment and function during intestinal inflammation. Front Immunol. 9, 182–15 (2018).
de Aguiar, C. F. et al. Fecal IgA levels and gut microbiota composition are regulated by invariant natural killer T cells. Inflamm. Bowel Dis. 26, 697–708 (2020).
Kolodziejczyk, A. A., Zheng, D. & Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Micro 13, 260–12 (2019).
Friswell, M. K. et al. Site and strain-specific variation in gut microbiota profiles and metabolism in experimental mice. PLoS ONE 5, e8584 (2010).
Ericsson, A. C. et al. The influence of caging, bedding, and diet on the composition of the microbiota in different regions of the mouse gut. Sci. Rep. 8, 4065 (2018).
Robertson, S. J. et al. Comparison of co-housing and littermate methods for microbiota standardization in mouse models. Cell Rep. 27, 1910–1919.e2 (2019).
Ubeda, C. et al. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J. Exp. Med. 209, 1445–1456 (2012).
Bedel, R. et al. Lower TCR repertoire diversity in Traj18-deficient mice. Nat. Immunol. 13, 705–706 (2012).
Xie, J. et al. Deficiency of mucosal-associated invariant T cells in TCRJα18 germline knockout mice. Immunohorizons 3, 203–207 (2019).
Zhang, J. et al. Mutation of the Traj18 gene segment using TALENs to generate Natural Killer T cell deficient mice. Sci. Rep. 6, 1–10 (2016).
Chandra, S. et al. A new mouse strain for the analysis of invariant NKT cell function. Nat. Publ. Group 16, 799–800 (2015).
Dashtsoodol, N. et al. Generation of novel Traj18-deficient mice lacking Vα14 natural killer T cells with an undisturbed T cell receptor α-chain repertoire. PLoS ONE 11, e0153347 (2016).
Smith, A. D. et al. Microbiota of MR1 deficient mice confer resistance against Clostridium difficile infection. PLoS ONE 14, e0223025–21 (2019).
Sun, J. et al. Bile acid profile and its changes in response to Cefoperazone treatment in MR1 deficient mice. Metabolites 10, 127–12 (2020).
McCoy, K. D., Burkhard, R. & Geuking, M. B. The microbiome and immune memory formation. Immunol. Cell Biol. 97, 625–635 (2019).
Beura, L. K. et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016).
Wingender, G. et al. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology 143, 418–428 (2012).
Wei, B. et al. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J. Immunol. 184, 1218–1226 (2010).
Park, S. H., Benlagha, K., Lee, D., Balish, E. & Bendelac, A. Unaltered phenotype, tissue distribution and function of Valpha14(+) NKT cells in germ-free mice. Eur. J. Immunol. 30, 620–625 (2000).
Legoux, F. et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science 366, 494–499 (2019).
Ben Youssef, G. et al. Ontogeny of human mucosal-associated invariant T cells and related T cell subsets. J. Exp. Med. 215, 459–479 (2018).
Gherardin, N. A. et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol. Cell Biol. 96, 507–525 (2018).
Saubermann, L. J. et al. Activation of natural killer T cells by alpha-galactosylceramide in the presence of CD1d provides protection against colitis in mice. YGAST 119, 119–128 (2000).
Ueno, Y. et al. Single dose of OCH improves mucosal T helper type 1/T helper type 2 cytokine balance and prevents experimental colitis in the presence of valpha14 natural killer T cells in mice. Inflamm. Bowel Dis. 11, 35–41 (2005).
Burrello, C. et al. Short-term oral antibiotics treatment promotes inflammatory activation of colonic invariant natural killer t and conventional CD4+ T cells. Front. Med. 5, 478–12 (2018).
Ma, C. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018).
Vorkas, C. K. et al. Mucosal-associated invariant and γδ T cell subsets respond to initial Mycobacterium tuberculosis infection. JCI Insight 3, e121899 (2018).
Dumas, A. et al. The host microbiota contributes to early protection against lung colonization by Mycobacterium tuberculosis. Front Immunol. 9, 2656 (2018).
Riva, A. et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut 67, 918–930 (2018).
Acknowledgements
We wish to apologize to our colleagues whose work could not be cited due to space constraints. The authors would like to thank Dr. Christophe Paget for his critical review of this paper. Illustrations were generated with BioRender.com. T.M. is funded by the Canadian Institutes of Health Research (363664), a John Evans Leaders Fund infrastructure grant from the Canadian Foundation for Innovation and Ontario Research Fund (37069) and a Tier 2 Canada Research Chair in NKT cell Immunobiology. D.J.P. is funded by the Canadian Institutes of Health Research (FDN-14333 and THC-135234) and the National Science and Engineering Research Council (300001). MK is supported by an Ontario Graduate Scholarship.
Author information
Authors and Affiliations
Contributions
Q.L., M.K., D.J.P., and T.M. wrote the paper and T.M., M.K., and Q.L. prepared the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, Q., Kuypers, M., Philpott, D.J. et al. The dialogue between unconventional T cells and the microbiota. Mucosal Immunol 13, 867–876 (2020). https://doi.org/10.1038/s41385-020-0326-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-020-0326-2
This article is cited by
-
Unconventional immune cells in the gut mucosal barrier: regulation by symbiotic microbiota
Experimental & Molecular Medicine (2023)
-
Mechanisms of microbe-immune system dialogue within the skin
Genes & Immunity (2021)
-
Does exercise attenuate age- and disease-associated dysfunction in unconventional T cells? Shining a light on overlooked cells in exercise immunology
European Journal of Applied Physiology (2021)