Abstract
Pathobiology of several chronic inflammatory disorders, including ulcerative colitis and Crohn’s disease is related to intermittent, spontaneous injury/ulceration of mucosal surfaces. Disease morbidity has been associated with pathologic release of the pro-inflammatory cytokine tumor necrosis factor alpha (TNFα). In this report, we show that TNFα promotes intestinal mucosal repair through upregulation of the GPCR platelet activating factor receptor (PAFR) in the intestinal epithelium. Platelet activating factor (PAF) was increased in healing mucosal wounds and its engagement with epithelial PAFR leads to activation of epidermal growth factor receptor, Src and Rac1 signaling to promote wound closure. Consistent with these findings, delayed colonic mucosal repair was observed after administration of a neutralizing TNFα antibody and in mice lacking PAFR. These findings suggest that in the injured mucosa, the pro-inflammatory milieu containing TNFα and PAF sets the stage for reparative events mediated by PAFR signaling.
Similar content being viewed by others
Introduction
The gastrointestinal epithelium plays a pivotal role in separating luminal antigens and pathogens from underlying tissues. Mucosal inflammatory disorders such as inflammatory bowel disease, are associated with epithelial barrier compromise and mucosal wounds. Efficient epithelial repair is important in ensuring resolution of inflammation and restoration of mucosal homeostasis.1 It is now appreciated that a compromised epithelial barrier results in spatiotemporal recruitment of immune cells that release mediators which interact with the epithelium to orchestrate mucosal repair.2
Pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNFα), have been shown to initiate the synthesis of mediators that not only inhibit the inflammatory cascade but also serve to dismantle it, resulting in restoration of tissue homeostasis.3 Several of these endogenous mediators, include proteins/peptides and bioactive lipids which activate signaling cascades downstream of G-protein coupled receptors (GPCRs) in the epithelium and immune cell populations, stimulating proliferation and migration of cells to orchestrate mucosal repair.4
Platelet activating factor receptor (PAFR) is a GPCR expressed in intestinal epithelial cells (IECs) and immune cells such as neutrophils, monocytes, and macrophages.5 The PAFR ligand, PAF is a bioactive phospholipid that is released into the injured sites early in the inflammatory response.6 Levels of PAF and PAFR are known to be elevated in the mucosa of individuals with inflammatory bowel disease (IBD) and in rodent models of colitis.7,8,9 The active mucosal inflammatory response is associated with increased pro-inflammatory cytokine TNFα. Contribution of these inflammatory mediators to mucosal repair is however not well understood. A recent study revealed that TNFα promotes mucosal healing in colitis by influencing colonic epithelial progenitor cell population.10
The role of PAFR signaling in influencing intestinal mucosal wound repair is uclear, and contradictory functional effects have been reported.11,12 Here, we show that TNFα upregulates intestinal epithelial expression of PAFR during mucosal repair, leading to enhanced PAF signaling and intestinal epithelial wound repair. Furthermore, the pro-inflammatory cytokine IFNγ potentiates TNFα effects on PAFR signaling and mucosal repair.
Results
TNFα increases PAFR expression through NF-κB signaling
Molecules involved in initiation of the resolution phase of inflammation have been implicated in activating GPCRs signaling to promote proliferation and migration of cells and wound repair. Since IFNγ and TNFα levels are elevated within actively inflamed intestinal mucosa and IFNγ has been shown to induce cell surface expression of TNFα receptors in epithelial cells,13 we examined the effects of both these cytokines on GPCR expression using PCR arrays and a model IEC line SKCO15.
As shown in Fig. 1a, the epithelial GPCR, PAFR (Gene: PTAFR for human and Ptafr for mouse) was markedly upregulated in SKCO15 after TNFα and IFNγ incubation for 24 h (121 ± 32-fold, p < 0.001). To confirm this result, two model intestinal epithelia cell lines, SKCO15 and T84 were treated with TNFα or/and IFNγ for 24 h and PTAFR mRNA expression was assessed by quantitative polymerase chain reaction (qPCR). In SKCO15 cells PTAFR was increased after incubation with TNFα (4.3 ± 0.25-fold) or IFNγ (2.77 ± 0.17-fold) compared to untreated controls. Furthermore, a marked increase in PTAFR expression was noted after simultaneous incubation with both TNFα and IFNγ (18.77 ± 2.96-fold; p < 0.001; Fig. 1b). Similar results were obtained using T84 IECs (Figure S1A). These results using model IECs were also confirmed in cultures of human colon epithelial cells referred to as colonoids. Consistent with the above results, PTAFR mRNA was increased after incubation of human colonoids with TNFα (3.79 ± 1.12-fold) and combined treatment with IFNγ and TNFα (7.21 ± 1.39-fold; p < 0.05; Fig. 1c) compared to untreated controls. PTAFR is expressed in a number of cells other than IECs. To determine if treatment with TNFα and/or IFNγ increased PTAFR expression in a different cell type, we isolated peripheral human blood monocytes from healthy donors and cultured them in presence or absence of the aforementioned cytokines. None of the treatment conditions lead to a significant increase in PTAFR mRNA levels (Figure S1B). To further dissect the specific roles of TNFα and IFNγ in IEC PTAFR mRNA upregulation, a functional experiment using neutralizing antibodies against TNFα and IFNγ was performed. SKCO-15 cells were treated with TNFα and IFNγ and control IgG, TNFα, and/or IFNγ neutralizing antibodies were added to the media. Interestingly, the TNFα antibody alone (0.97 ± 0.02-fold; p < 0.001) or in combination with the IFNγ antibody (1.13 ± 0.08-fold; p < 0.001) inhibited the IEC PTAFR mRNA upregulation triggered by these cytokines, while the IFNγ antibody had no effect (34.00 ± 2.94-fold) (Fig. 1d). Since TNFα activates NF-κB signaling, we determined if this signaling cascade contributes to the increase in PTAFR mRNA after TNFα/IFNγ treatment of IECs. SKCO15 cells were incubated with TNFα and IFNγ for 24 h in presence or absence of Flavopiridol (1 μM) or Bay 11–7082 (10 μM), two inhibitors that block TNFα induced NF-κB activation.14,15 As shown in Fig. 1e (left panel), both inhibitors blocked TNFα triggered NF-κB signaling as determined by a NF-κB luciferase promoter assay. Furthermore, the increase in PTAFR mRNA in response to TNFα and IFNγ incubation was abolished when cells were co-incubated with Flavopiridol (16.55 ± 2.06-fold; p < 0.001) or Bay 11-0782 (2.87 ± 0.34-fold; p < 0.001). Apoptosis of cells incubated with these inhibitors was analyzed by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, showing no significant increase in death with either (Figure S1C). To further demonstrate the role of NF-κB signaling in upregulating intestinal epithelial PTAFR mRNA expression downstream of TNFα treatment, p65 siRNA was used to downregulate this subunit in SKCO15 cells. As shown in Fig. 1f, p65 downregulation decreased TNFα and IFNγ induced upregulation of PTAFR mRNA. Finally, to clarify if other pro-inflammatory cytokines directly result in PTAFR mRNA upregulation we treated SKCO15 cells with IL-1b and IL6. As shown in Fig S1D, neither of these cytokines caused an increase in intestinal epithelial levels of PTARF.
PAFR is synthesized through NF-κB signaling in response to TNFα. a SKCO15 model IECs treated with IFNγ (100 ng/ml) and TNFα (100 ng/ml). PTAFR mRNA levels were determined as a part of a GPCR PCR array. b SKCO15 or c primary human colonoids were treated with IFNγ (100 ng/ml) and/or TNFα (100 ng/ml). PTAFR mRNA levels were determined by qPCR. d SKCO15 cells were pre-incubated 30 min with 10 μg/ml of IgG or neutralizing anti-TNFα, neutralizing anti-IFNγ and a mix of both antibodies; cells then were treated with IFNγ (100 ng/ml) and TNFα (100 ng/ml). PTAFR mRNA levels were determined by qPCR. e SKCO15 model IECs were pre-incubated 30 min with vehicle or 1 μM Flavopiridol or 10 μM Bay 11–7082 and then treated with IFNγ (100 ng/ml), TNFα (100 ng/ml). PTAFR mRNA levels were determined by qPCR. f NF-κB p65 was silenced in SKCO15 cells. After 24 h, cells were treated with IFNγ (100 ng/ml) and TNFα (100 ng/ml) and PTAFR mRNA levels were determined by qPCR. Statistical comparisons performed using one-way ANOVA with Bonferroni’s multiple comparison (***p < 0.001; mean ± SEM); NT vehicle control
These findings indicate that TNFα signaling upregulates PTAFR expression which is potentiated by IFNγ, the latter of which has been shown to upregulate TNFα receptor expression in epithelial cells.
PAFR its important for in vivo intestinal mucosal wound healing
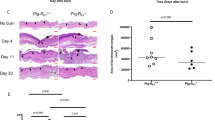
To corroborate the in vitro findings above and elucidate the relevance of PAFR in intestinal mucosal wound repair, mRNA from healing biopsy induced murine colonic mucosal wounds was isolated, and quantification of Ptafr mRNA was determined using qPCR. Ptafr was increased within 24 h (Day 1) following injury (2.83 ± 0.26-fold; p < 0.001; Fig. 2a) relative to intact intestinal mucosa. The increase in Ptafr mRNA went back to levels similar to an unwounded state on Day 2 and 3 following injury. To further characterize Ptafr expression in vivo, intestinal mucosal wounds were isolated from C57BL/6J mice and Ptafr mRNA levels were evaluated by in situ hybridization. Increased Ptafr expression was detected on Day 1 in epithelial cells adjacent to the healing intestinal mucosal wounds (Fig. 2b). To verify the contribution of Ptafr in promoting colonic mucosal wound repair, we analyzed mucosal wound healing in PAFR-deficient (Ptafr−/−) mice compared to wild-type (C57BL/6J) mice using a colonic mucosal biopsy induced injury model. While Ptafr−/− mice showed no difference in body weight, colon length, or crypt length compared to C57BL/6J mice (Figure S2), delayed mucosal wound healing was observed in Ptafr−/− mice compared to C57BL/6J mice 3 days postinjury (34.74 ± 1.1, Ptafr−/−; 47.12 ± 0.58, C57BL/6J; p < 0.001; Fig. 2c). Taken together, these results support an important role of PAFR in promoting colonic mucosal wound repair.
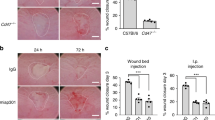
PAFR is important for in vivo intestinal mucosal wound repair. a Ptafr mRNA levels in 3-mm punch biopsies of resealing colonic wounds on different days postinjury compared to intact tissue analyzed by qPCR (n = 8–10). b In situ hybridization analysis of Ptafr expression in frozen sections from intact colon and 1 day after wounding (scale bar: 50 μm; original magnification ×20; representative images from 3 experiments). c Endoscopic images of colonic mucosal wounds in Ptafr−/− (n = 11) compared with C57BL/6J mice (n = 12) at days 1 and 3 postinjury. Graph shows quantification of wound closure. Statistical comparisons performed using one-way ANOVA with Bonferroni’s multiple comparison and unpaired two-tailed Student’s t test with Welch’s correction (***p < 0.001; mean ± SEM). IT intact tissue, NT vehicle control
PAF promotes in vitro intestinal epithelial wound repair
Intestinal mucosal wound repair is a highly regulated process orchestrated by a spatiotemporal release of pro-inflammatory and pro-repair mediators. Since the ligand for PAFR is an inflammatory lipid mediator PAF, we next analyzed PAF levels in healing colonic biopsy induced wounds by mass spectrometry. Increased PAF was detected on Day 2 after wounding (Fig. 3a, 1.58 ± 0.13-fold; p < 0.05). Functional effects of PAF on epithelial wound repair were examined in an in vitro scratch wound healing assay using SKCO15 IECs. A dose–response study revealed increasing effects of PAF in promoting wound repair at concentrations of 100 nM, 1 µM, and 10 µM (Figure S3A). Cells were then treated with 10 µM PAF for 24 h and wound repair was monitored by live time lapse imaging. Administration of PAF significantly increased wound closure within 24 h (Fig. 3b, p < 0.001). This effect was confirmed in another model IEC line, T84 (Figure S3B). To further implicate contribution of PAF/PAFR signaling in promoting wound repair, SKCO15 cells were treated with the selective PAFR inhibitor PCA 4248 and PAF. PCA 4248 dose–response studies were performed to determine its effect on epithelial wound closure. Furthermore, apoptosis was analyzed by TUNEL staining (Figure S3C, D). Treatment with 10 µM PCA 4248 and 10 µM PAF reduced wound closure compared to treatment with PAF alone (Fig. 3b). These findings suggest that the pro-repair effect of PAF was abrogated by selectively inhibiting PAFR signaling. We next evaluated the combined influence of TNFα and/or IFNγ and PAF on epithelial wound closure in vitro. Treatment of SKCO15 cells with TNFα (100 ng/ml) or IFNγ (100 ng/ml) promoted wound closure within 24 h (Fig. 3c; TNFα p < 0.001, IFNγ p < 0.05). Use of both cytokines further enhanced wound closure within 24 h (p < 0.001). In addition, we demonstrate that PAF treatment further enhanced TNFα and IFNγ mediated epithelial wound closure (Fig. 3c). To determine whether the pro-repair effect of TNFα and IFNγ is, in part, mediated by signaling downstream of the PAFR, healing wounds of SKCO15 (Fig. 3d) and primary cultured intestinal monolayers (Fig. 3e) were pre-incubated with PCA 4248 and subsequently incubated with TNFα and IFNγ alone or with PAF. PCA 4248 treatment reduced the pro-repair effects of TNFα, IFNγ, and PAF at 18 h postinjury (p < 0.001) (Fig. 3d, e). These findings suggest that the pro-repair properties of TNFα and IFNγ are, in part, mediated by PAFR signaling. Taken together, these data support an important role for PAF in promoting intestinal mucosal wound repair.
PAF is released in response to intestinal mucosal injury to promote repair. a PAF levels in 3-mm punch biopsies of resealing colonic wounds on different post-injury days compared to intact tissue analyzed by lipodomics (n = 3). b–d Wound areas of scratch wounded SKCO15 IEC monolayers, incubated with various treatments were continuously imaged. Percent wound closure was calculated by comparison of 0 and 18 or 24 h postinjury. Wounded IECs treated with b PAF (10 μM) with or without PAFR antagonist PCA 4248 (10 μM) for 24 h; c IFNγ (100 ng/ml), TNFα (100 ng/ml), and PAF alone or in combination for 24 h; d IFNγ, TNFα, and PCA 4248 alone or in combination for 18 h. e Wounded primary IECs treated with PAF, IFNγ (100 ng/ml), and TNFα (100 ng/ml), and with or without PAFR antagonist PCA 4248 (10 μM) for 12 h. Experiments were repeated three times, results of one representative experiment are shown. Statistical comparisons performed using one- or two-way ANOVA with Bonferroni’s multiple comparison (*p < 0.5; **p < 0.01; ***p < 0.001; mean ± SEM). IT intact tissue, NT vehicle control
PAF promotes cell matrix adhesion turnover by EGFR signaling via ADAM10 activation
Wound closure is mediated by cell proliferation and migration. We next analyzed the mechanisms by which PAF promotes epithelial signaling to influence cell migration. Given a report of crosstalk between PAFR and epidermal growth factor receptor (EGFR),16 we examined whether PAF influences EGFR activation leading to downstream signaling and enhanced cell motility. EGFR transactivation by GPCRs requires metalloproteases that cleave EGFR ligands. In the context of PAFR, a Disintegrin and Metalloproteinase domain-containing protein 10 (ADAM10) has been shown to cleave transmembrane heparin-binding EGF (HB-EGF) that binds to EGFR.17 To assess if ADAM10 activity increases after PAF treatment, a fluorimetric activity assay was performed using migrating SKCO15 IECs. PAF treatment increased ADAM10 activity within 30 min compared to treatment with control media (p < 0.05; Fig. 4a). We next evaluated EGFR activation by analyzing EGFR (Y845) phosphorylation in healing epithelial scratched monolayers (SKCO15, Fig. 4b) and primary cultured intestinal sparse monolayers (Fig. 4c) treated with PAF. Immunoblotting revealed increased EGFR phosphorylation at 30 min after PAF treatment (Fig. 4b, c, S4A and B). EGFR activation has been reported to initiate signaling pathways involved in regulation of cell migration. These signaling mediators include tyrosine kinase Src (Y416 phosphorylation) downstream of EGFR signaling leading to activation of cell matrix adhesion proteins including focal adhesion kinase (FAK), which regulates turnover of cell matrix adhesions and forward cell movement. In addition, Src activated by G-proteins also contributes to EGFR activation. Thus, to explore PAF induced Src activation, SKCO15 healing wounds were treated with PAF and analyzed by immunoblotting. Increased pSrc-Y416 was observed 30 min after PAF treatment compared to unstimulated cells (Fig. 4b, c, S4A and B). Increased FAK phosphorylation at the major autophosphorylation site Y397 as well as the Src-dependent pFAK-Y861 phosphorylation was also detected (Fig. 4b, c, S4A and B). In addition, increased pFAK-Y861 was identified in focal contacts of the migrating epithelial cells at the leading edge of wounds after PAF exposure for 2 h by immunofluorescence labeling (Fig. 4d). Taken together, these results support a role of EGFR activation, Src and FAK phosphorylation in response to PAF.
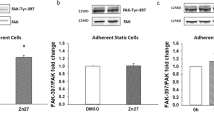
PAF promotes cell migration via ADAM10 mediated activation of EGFR signaling pathways. a Fluorimetric ADAM10 activity assay performed on spreading SKCO15 model IECs treated with PAF (10 µM) or control at 30 min (*p < 0.5; n = 3; mean ± SEM). Immunoblotting was performed on lysates from scratch-wounded SKCO15 monolayers b and sparse primary IEC cultures c treated with PAF or control for 30 min. Levels of pEGFR, pSRC, and pFAK (Y397, Y861) were compared with total EGFR, SRC, FAK, and Calnexin to assess activation (representative blots from three experiments). c Confocal micrographs of the focal contacts in migrating IECs at the leading edge of the wound after 2-h treatment with control or PAF showing staining of pFAK (Y861), phalloidin (F-actin), and nuclei. (Scale bar 50 µm; original magnification ×40; representative images from 3 experiments). Statistical comparisons performed using unpaired two-tailed Student’s t test with Welch’s correction. NT vehicle control
PAF enhances Rac1 activation, and reactive oxygen species generation, influencing cell matrix adhesion
Rac1 is a small GTPase implicated in actin cytoskeletal restructuring during epithelial cell migration and wound repair.18,19 In addition, EGFR activation enhances Rac1 activation. Thus, to further identify the mechanism by which PAF promotes epithelial cell migration and wound repair, Rac1 activation in response to PAF incubation was evaluated. Increased Rac1-GTP was observed in epithelial cells migrating to heal wounds when incubated with PAF (Fig. 5a).
Rac1 activation, ROS generation, and increased cell–matrix adhesion amplified by PAF treatment. a Treatment of scratch-wounded SKCO15 IECs with PAF (10 μM) or control for 30 min followed by analysis of Rac1 activation using a pulldown activation assay (representative blots from three experiments). b SKCO15 IECs were incubated with fMLF (100 nM), PAF (10 μM) alone and in combination with the ROS scavenger NAC (5 mM) or PCA 4248 (10 μM) for 15 min. ROS generation was detected by confocal microscopy using the fluorescent hydro-Cy3 dye in scratch-wounded monolayers adjacent to the wound edge, as indicated by asterisks (100 µm; original magnification ×20; representative images from 3 experiments). c Adhesion strength measurements of spreading SKCO15 IECs treated with PAF (1 μM) (n = 17) or control (n = 14) for 6 h. d Representative adhesion profiles for each condition. τ50 is a metric for adhesion strength representing the shear stress at which 50% adherence is observed. e Vinculin staining performed on spreading SKCO15 IECs treated with PAF (1 µM) or control for 6 h; asterisk indicating increased staining (50 µm; original magnification ×60). f Quantification of focal adhesion (FA) area (n = 2). Statistical comparisons were performed using unpaired two-tailed Student’s t test with Welch’s correction. (**p < 0.01; ***p < 0.001; mean ± SEM). NT vehicle control
Activation of Rac1 has been shown to promote reactive oxygen species (ROS) generation by associating with the intestinal epithelial oxidase NOX1, which leads to oxidative modification of phosphatases that regulate cell matrix adhesion regulatory proteins.20,21 Src has been reported to increase NOX-1 dependent ROS generation.22 ROS can enhance EGFR signaling, Src phosphorylation, and ADAM10 activation.23,24 Thus, we next analyzed the effects of PAF on ROS generation in IECs migrating to heal wounds, using an intracellular redox-sensitive dye, Hyro-Cy3. PAF exposure increased ROS generation within 15 min of treatment (2.9 ± 0.2 fold; p < 0.001; Fig. 5b, S5A). The formyl peptide receptor 1 (FPR1) agonist, fMLf was used as a positive control for ROS generation. As shown in Fig. 5b, ROS generation was most prominent in migrating IECs at the leading edge of the wound. Inhibition of PAFR signaling by incubation with PCA 4248 inhibited the increase in ROS generation. In addition, pretreatment with the ROS scavenger N-acetyl-cysteine (NAC) abrogated PAF-induced ROS generation (Fig. 5b). Since intracellular ROS signaling induces oxidative modification and inhibition of phosphatases that regulate FAK signaling in cell–matrix adhesions and forward cell movement, we next analyzed the influence of PAF incubation on the adhesion properties of epithelial cells. To assess the effects of PAF in the regulation of adhesive forces, we measured cell adhesion strength by analyzing the force required to detach the cells from the extracellular matrix. Epithelial cells were seeded on fibronectin-coated glass coverslips, allowed to adhere for 6 h after which they were exposed to hydrodynamic shear forces using a spinning disk device. The number of adherent cells were counted at differing radial positions, corresponding to known shear stress values. The fraction of adherent cells decreases nonlinearly with respect to fluid shear stress. The cell adhesion strength was defined as the shear stress that produces 50% detachment of cells. PAF treatment of SKCO15 IECs significantly increased cell adhesion strength (Fig. 5c, d; 228 vs. 271 dyn/cm2, p < 0.01), suggesting that PAF regulates cell-matrix mechanical interactions. In addition, cell spreading area was determined and FA size was analyzed by staining for vinculin, a FA protein important for FA assembly and force transmission. We did not observe a difference in cell spreading area (Figure S5B). In contrast, PAF treated SKCO15 IECs exhibited higher area of vinculin-containing FAs compared to control cells (4.3 ± 0.2 µm2 vs. 2.1 ± 0.1 µm2; p < 0.001; Fig. 5e, f). We attributed the increased adhesion strength of PAF treated cells to increased FA area, as we have previously shown cell adhesion strength increases with FA area.25 Taken together, these data indicate PAF regulates adhesion of epithelial cells to the matrix.
TNFα neutralization impairs intestinal wound repair
Epithelial cell damage and mucosal wounds are frequently seen in the mucosal lesions of individuals with IBD who have active disease. Although murine experimental colitis models consistently produce severe mucosal injury, understanding the mechanisms that control wound healing in these systems has been challenging because of the variable timing and location of wounds. We utilized a simple murine colonic mucosal biopsy-induced wound healing model to investigate the kinetics of inflammatory response and mucosal repair. Analysis of healing biopsy-induced wounds revealed increased Tnf mRNA expression with peak levels observed on day 1 after injury (4.26 ± 0.58-fold increase, p < 0.001; Fig. 6a). In contrast, significant changes in Ifng mRNA were not observed in healing wounds (Fig. 6b). Our findings suggest that the pro-inflammatory cytokines IFNγ and TNFα contribute to epithelial wound repair, in part due to PAFR upregulation. Given that Tnf mRNA expression was increased in vivo after wounding, we investigated the influence of TNFα on wound repair by intraperitoneal (ip) administration of a neutralizing TNFα antibody (MP6-XT3), which has been shown to significantly diminish TNFα levels after a single ip injection.26 Importantly, in contrast to IgG control antibody, Ptafr mRNA up-regulation was not observed in biopsy-induced wounds from mice administered TNFα neutralizing antibody (Fig. 6c).
Inhibition of TNFα signaling in vivo ablates PAFR upregulation after injury and delays intestinal mucosal wound healing. a Tnfa and b Ifng mRNA levels in 3-mm punch biopsies of resealing colonic wounds compared to intact tissue analyzed by qPCR (n = 3). c Ptafr mRNA levels in 3-mm punch biopsies of resealing colonic wounds isolated from mice treated with a TNFα neutralizing antibody or control on different days postinjury compared to intact tissue analyzed by qPCR (n = 3). d Endoscopic images of colonic mucosal wounds 1 and 3 days after biopsy injury in C57BL/6J mice treated with a TNFα neutralizing antibody or IgG control. Graph shows quantification of wound closure (n = 5). Statistical comparisons were performed using one-way ANOVA with Bonferroni’s multiple comparison and unpaired two-tailed Student’s t test with Welch’s correction (*p < 0.5; ***p < 0.001; mean ± SEM). IT intact tissue, Ctl control
There was a significant reduction in colonic mucosal wound closure in mice after anti-TNFα antibody treatment (23.56 ± 0.95%, antibody; 47.22 ± 1.34%, control; p < 0.001; Fig. 5d). Antibody neutralization efficiency was confirmed by detection of reduced mRNA levels of the TNFα target proteins Il6 and Cxcl-10 (Figure S6). Taken together these findings support an important role of TNFα in mucosal repair.
Discussion
Pro-inflammatory lipids and eicosanoids, leukotrienes, free radicals, and cytokines, including TNFα are released into injured sites during an inflammatory response. It is generally appreciated that TNFα plays an integral role in the pathogenesis of chronic inflammatory states such as inflammatory bowel disease; nevertheless evidence that this cytokine is important for the establishment of a repair phase has started to emerge.
In this work we observed that TNFα, in combination with IFNγ, has synergistic effects in upregulating PTAFR mRNA in IECs. This response seems to be specific as other pro-inflammatory cytokines as IL-1β and IL-6 do not replicate this phenomenon. Previous studies have shown that IFNγ activates STAT-1a and inhibits recruitment of this protein to the TNFα receptor 1, directing TNFα signaling toward NF-κB. In addition, IFNγ treatment increases cell surface expression of TNFα receptor in epithelial cells.13 A recent report suggested that TNFα promotes mucosal healing in colitis by activating NF-κB and Wnt/β-catenin signaling in colonic epithelial stem–progenitor cell population.10 In our study, TNFα upregulates PTAFR mRNA downstream of NF-κB signaling. In contrast to its pro-inflammatory role in myeloid cells, NF-κB has a protective role in IEC by influencing intestinal epithelial homeostasis. Mice with conditional deletion of proteins of the NF-κB in IEC display chronic colitis.27,28 These studies suggest that balanced NF-κB signaling has beneficial effects and help to restore mucosal homeostasis.
We provide evidence for crosstalk between the pro-inflammatory cytokine TNFα and PAFR in the early phase of intestinal inflammation following epithelial injury that has beneficial effects on wound repair. In these studies, we observed Ptafr upregulation during the early inflammatory phase after injury and decreased wound healing in Ptafr−/− mice. These observations are supported by previous studies in which Ptafr deletion augmented skin inflammation in a chemically induced skin injury model.29 Other studies showed a beneficial role of PAFR during pulmonary infections with Klebsiella pneumoniae.30 Ptafr−/− mice exhibited decreased anaphylactic reactions, better outcome in graft-vs.-host disease and delayed lethality following intestinal ischemia and reperfusion injury through reduced inflammatory responses.31,32,33 Increased expression of PAFR and PAF has been reported in the mucosa of individuals with IBD and in rodent models of colitis. In these conditions, increased PAFR expression are likely the result of ongoing inflammation and lack of mucosal homeostasis.
In addition to the upregulation of PAFR, we observed enhanced epithelial repair after incubation of healing wounds with the pro-inflammatory lipid mediator PAF. PAF is an endogenous PAFR ligand. PAF administration has beneficial effects on keratinocyte wound healing, and therapeutic PAF antagonists delay oral and gastric mucosal ulcer healing.11,34 However, PAF delays wound closure in corneal cells35 and PAFR antagonists have prorepair effects on corneal injury and in rodent colitis models.9,12 These studies suggest that PAF effects are likely context dependent and may differ during acute inflammatory response to injury vs. persisting chronic inflammation. Further, we demonstrate that co-incubation of TNFα and IFNγ with PAF have a synergistic effect on wound healing, most likely due to the previously described crosstalk between TNFα and PAFR. We observed increased PAF levels two days after intestinal injury, which corresponds to a transition between pro-inflammatory and pro-resolution/repair phases in the mucosal wound healing model. Elevated mucosal levels of PAF and its receptor, as observed in individuals with IBD, might be a consequence of failed resolution of inflammation with associated impaired mucosal repair.
We observed PAF-induced activation of EGFR, a receptor known to simultaneously activate multiple pathways and facilitate epithelial wound healing.36,37 Previous studies have implicated a central role for EGFR in GPCR signaling. Lemjabbar and Besbaum17 have described ADAM10-mediated EGFR transactivation by PAF-induced PAFR signaling. ADAM10 induces shedding of HB-EGF, an important EGFR ligand which is detected after injury in epithelial cells and increased after PAF treatment of model ovarian epithelial cells.16,38,39 Tokumaru et al.40 demonstrated shedding of HB-EGF after scratch wounding in keratinocytes, leading to enhanced migration and wound healing. Activation of EGFR by HB-EGF results in a long-lasting robust phosphorylation at Y845 residue. Analogous ADAM10 activation as well as pEGFR-Y845 phosphorylation consistent with its activation was observed in our study. EGFR downstream signaling pathways that promote cell migration include Src phosphorylation leading to tyrosine phosphorylation and activation of FAK, which influences cytoskeletal reorganization and focal cell matrix adhesion turnover.36,41 In addition, Src itself has been implicated in GPCR-mediated ADAM activation and pEGFR-Y845 phosphorylation.24,36 Previous studies also demonstrated G-protein mediated Src kinase involvement in PAF-induced EGFR transactivation as well as activation of EGFR, Src, FAK, and paxillin following PAF treatment in various cell systems.16,42 We show PAF treatment activates pSrc-Y416 as well as phosphorylation of FAK on Y861 (Src-dependent) and Y397 (autophosphorylation), both involved in GPCR and EGF-stimulated cell mobility.34 In addition, we observed that PAF treatment leads to activation of Rac1. Different signaling pathways downstream of PAFR can lead to such an increase of active Rac1. EGFR activation has been linked to small GTPase Rac1 activation through Src, leading to enhanced cell migration in IECs.43 Rac1 regulates cell migration and can be activated by GPCR and Src-mediated signaling downstream of NADPH oxidase NOX1, leading to ROS generation which exerts a positive influence on collective migration of the intestinal epithelium.21 In our study, we observed Rac1 activation as well as increased levels of ROS following PAF treatment. Similarly, PAF stimulation of bovine neutrophil increases ROS production.44 Additionally, we showed that PAF treatment increases FA size, thereby influencing cell–matrix adhesions.25
Biologic therapy, consisting of TNFα monoclonal antibodies (e.g., Humira), has been shown to induce and maintain remission in a number of patients with moderate-to-severe IBD refractory to conventional immunosuppressive drugs. However, such biologic therapy has had treatment failures.45,46 The possibility that TNFα plays a role in mucosal repair and that a chronic blockage of this cytokine might prevent an appropriate healing response has not been extensively explored. In our studies, inhibition of pro-inflammatory TNFα signaling by administration of a functionally inhibitory anti-TNFα antibody suppressed PAFR upregulation during wound healing. In addition, we observed impaired wound healing after anti-TNFα antibody treatment that is likely mediated by impaired upregulation of GPCRs like PAFR that signal to promote wound healing. In support of our observations showing pro-repair properties of TNFα, Bradford et al. demonstrated that TNFα plays a beneficial role in enhancing Wnt/β-catenin signaling during ulcer healing in IBD. TNFα neutralization by antibodies induces mucosal healing but blockade of soluble TNF can negatively influence colitis.10 Mucosal healing induced by anti-TNFα mAb is likely a secondary response to anti-inflammatory depletion of pathogenic effector cells. Given that the blockade of soluble TNFα (Etanercept) does not induce effector cell apoptosis it is possible that TNFα neutralization impairs IEC TNFα signaling and reduced TNFα available to IECs which could contribute to the negative clinical outcomes. Anti-TNFα mAb therapy may reduce stem IEC activation in patients with persistent mucosal inflammation. Thus, chronic inhibition of this cytokine might have detrimental effects on mucosal repair which is in part mediated by TNFα dependent upregulation of G protein coupled receptors such as PAFR.
Taken together, our findings highlight a novel pro-repair mechanism that is mediated by the pro-inflammatory cytokine TNFα, PAFR and PAF which serve to orchestrate mucosal repair and restore mucosal homeostasis (Fig. 7). The initial pro-inflammatory response sets the stage for pro-repair mechanisms that restore the epithelial barrier. Therapeutic strategies therefore need to take into consideration the time and context of chronic mucosal inflammation in order to accurately target key mediators involved in the disease process at the time of treatment.
Schematic model illustrating the molecular mechanism of PAFR signaling during epithelial wound repair. Our working hypothesis is that in response to wounding TNFα/IFNγ signaling increases expression of PAFR. Binding of PAF leads to PAFR stimulation which causes Src phosphorylation and ADAM10 activation, inducing cleavage of EGFR ligands, such as HB-EGF, and EGFR activation. Stimulation of EGFR further enhances phosphorylation of Src, which subsequently activates Rac1 via GEFs, increasing ROS accumulation and phosphorylation of FAK. In parallel, Src activation induces FAK phosphorylation to further promote turnover of focal adhesion complexes leading to modulation of cellular migration
Methods
Mice
C57BL/6 were purchased from the Jackson Laboratory. Ptafr−/− mice on a C57BL/6 background were a gift of Professor Takao Shimizu (University of Tokyo).
Human colonic enteroids (colonoids)
Human colonoids were provided by Jason Spence (University of Michigan). 2D epithelial intestinal monolayers from 3D colonoids were generated as described by Saxena et al.47
Cell lines and culture conditions
Human IECs (SKCO15, T84) were grown as previously described.21 After reaching a resistance > 700 ohms × cm2 (~5 days), they were stimulated with TNFα (100 ng/ml) and/or IFNγ (100 ng/ml) for 24 h.
IEC monolayer wounding in vitro
Wound closure was assessed using a scratch wound assay as previously published.21 See Supplementary Information for details.
In vivo wounding of colonic mucosa
A high-resolution, miniaturized colonoscope system equipped with biopsy forceps (Karl Storz; Germany) was used to injure the colonic mucosa at 5–10 sites along the dorsal artery and, healing was quantified on day 1 and 3 postinjury.
In vivo antibody administration
Mice were given i.p. injections of 250 μg of anti-TNFα monoclonal antibody (clone MP6-XT3, cat 16-7322-81, ThermoFisher Scientific) 6 h before colonic biopsy wounding. Healing was quantified, and wounds were harvested. Rat IgG1 kappa isotype control (eBRG1 cat 14-4301-82, ThermoFisher Scientific) was administered as control.
Immunoblot and immunofluorescence
For cell lysis, IEC monolayers were harvested in RIPA buffer. Immunofluorescence was performed as described previously.21
Reagents
The following antibodies were used: Calnexin (cat. C4731) Sigma (Darmstadt, Germany); FAK (cat. 610088) BD Biosciences (Franklin Lakes, NJ); pFAK (Y861) (cat. PS 1008) Calbiochem (Darmstadt, Germany); pFAK (Tyr397) (cat. 3283), Src (cat 2108), pSrc (Tyr416) (cat. 2101), EGFR (cat. 4267), pEGFR (Tyr845) (cat. 2231) Cell Signaling Technology (Danvers, MA). The hydrocyanine probe ROSstar 550, LI-COR Biosciences (cat. 926–20000). PAF (cat. 60900, Cayman Chemical, Ann Arbor, MI), PCA 4248 (cat. 0571, Tocris, Bristol, UK). PAF and PCA were used at a concentration of 10 μM. rhTNFα, rhIFNγ, rhIL-1β and rhIL-6 (cat. 300–01A, cat. 300-02, cat 200-01B and cat. 200-06; Peprotech, Rocky Hill, NJ) were used at a concentration of 100 ng/ml. fMLF (cat. F3506, Sigma, Darmstadt, Germany) was used at a concentration of 100 nM. Anti-TNFα, anti-IFNγ and Mouse IgG1 kappa Isotype Control (Mab1 cat 16-7348-81; NIB42 cat 16-7318-81 and P3.6.8.1 cat 14-4714-82, ThermoFisher Scientific) were used at a concentration of 10 μg/ml. p65 NF-κB and control siRNA were from Cell Signaling Technologies (cat 6261S and 6201) and used as suggested by the manufacturer. The NF-κB inhibitors Flavopiridol hydrochloride (cat ab 141300, abcam) and bay11–7082 (cat 196870, millipore sigma) were uses at a concentration of 1 and 10 μM, respectively.
Luciferase assay
SKCO15 cells were plated in 48-wells plates and transfected with 0.2 mg/well of pGL4.32 vector containing a promoter with 5 copies of NF-κB response elements driving expression of the luciferase reporter gene and 0.04 mg/well pRL-TK (expressing Renilla luciferase; Promega) as a control for transfection efficiency. Reporter activity was determined using the Dual Luciferase Reporter Assay System (catE1910, Promega).
Human monocyte isolation and culture
Human Peripheral blood monocytes were isolated using the EasySep™ Human Monocyte Isolation Kit (cat 19359, Stem Cell technologies). Monocytes were cultured in low adherence plates and treated with TNFα and IFNγ for 24 h.
Cell death
Cell death was evaluated using the Click-iT® TUNEL Alexa Fluor® 488 Imaging Assay (cat C10245, ThermoFisher Scientific).
Quantitative polymerase chain reaction
Total RNA was isolated from SKCO15 cells, T84 cells, human colonoids or colonic wounds using the RNeasy kit (Qiagen). Primer sequences can be found in Supplementary Information.
Lipidomic analysis of PAF levels
Totally, 25–30 punch biopsies (3 mm) of intact tissue or wounded colon from 3 animals on Day 1, 2, and 3 after wounding were analyzed for PAF levels at the Mass Spectrometry Lipidomics Core Facility, Dept. of Pharmacology, University of Colorado. The experiments were performed with 3 biological replicates.
Intracellular ROS generation
Epithelial cells were treated with PAF or control media for the indicated times and incubated with 15 μM hydro-Cy3 for 30 min at 37 °C. Epithelial cells were pretreated with NAC (20 mM) or PCA 4248 (10 μM) 30 min prior to PAF treatment. Quantification of fluorescence intensity of ROS was determined using ImageJ software.
Spinning disk assay
Cell adhesion strength was measured using the spinning disk system as previously described.25,48 For details see Supplementary Information.
FA staining
For staining of FAs, cells cultured overnight on fibronectin-coated surfaces were rinsed and permeabilized in cytoskeleton-stabilizing buffer for 10 min, fixed in 3.7% formaldehyde for 5 min, blocked in 33% goat serum in PBS, and incubated with primary antibodies against FA component Vinculin (cat. V284, Sigma) followed by AlexaFluor-labeled secondary antibody (cat. A-21422, Thermo Fisher Scientific).
Rac1 activation assay
Confluent SKCO15 cell monolayers were grown in 100 mm tissue culture plates and grid wounds were generated to enrich for migrating and spreading cells. Rac1 activity was determined by pulldown using the RhoA/Rac1/Cdc42 Activation Assay Combo Biochem Kit (cat. BK030; Cytoskeleton, Inc.) according to the manufacturer’s protocol.
In situ hybridization
Partial murine cDNA fragments for Ptfar (Genbank NM_001081211.2 corresponding to bases 565–1372) were amplified via PCR, cloned into pZERO, and sequence verified. Ptafr riboprobes were made using the Roche Sp6/T7 DIG labeling system (cat. 11175025910, Roche, Basel, Switzerland) following the manufacturer’s instructions. See Supplementary Information for details.
ADAM10 activity assay
SKCO15 cells were plated sparse (60%) in 24-well tissue culture plates and treated with PAF or control media. ADAM10 activity was measured using the fluorimetric SensoLyte 520 ADAM10 Activity Assay Kit (cat. AS-72226; AnaSpec) according to the manufacturer’s protocol.
Statistical analysis
Statistical comparisons were performed by one- or two-way ANOVA with Bonferroni’s multiple comparison or unpaired two-tailed Student’s t test, as appropriate. A p value of less than 0.05 was considered significant.
References
Leoni, G. et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J. Clin. Invest. 125, 1215–1227 (2015).
Fullerton, J. N. & Gilroy, D. W. Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug. Discov. 15, 551–567 (2016).
Buckley, C. D., Gilroy, D. W. & Serhan, C. N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327 (2014).
Serhan, C. N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 (2014).
Merendino, N., Dwinell, M. B., Varki, N., Eckmann, L. & Kagnoff, M. F. Human intestinal epithelial cells express receptors for platelet-activating factor. Am. J. Physiol. 277(4 Pt 1), G810–G818 (1999).
Ferraris, L. et al. Intestinal epithelial cells contribute to the enhanced generation of platelet activating factor in ulcerative colitis. Gut 34, 665–668 (1993).
Eliakim, R., Karmeli, F., Razin, E. & Rachmilewitz, D. Role of platelet-activating factor in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine and prednisolone. Gastroenterology 95, 1167–1172 (1988).
Kald, B., Olaison, G., Sjodahl, R. & Tagesson, C. Novel aspect of Crohn’s disease: increased content of platelet-activating factor in ileal and colonic mucosa. Digestion 46, 199–204 (1990).
Wallace, J. L. Release of platelet-activating factor (PAF) and accelerated healing induced by a PAF antagonist in an animal model of chronic colitis. Can. J. Physiol. Pharmacol. 66, 422–425 (1988).
Bradford, E. M. et al. Epithelial TNF receptor signaling promotes mucosal repair in inflammatory bowel disease. J. Immunol. 199, 1886–1897 (2017).
Feuerherm, A. J. et al. Platelet-activating factor induces proliferation in differentiated keratinocytes. Mol. Cell Biochem. 384, 83–94 (2013).
Bazan, H. & Ottino, P. The role of platelet-activating factor in the corneal response to injury. Prog. Retin. Eye Res. 21, 449–464 (2002).
Pandita, R., Pocsik, E. & Aggarwal, B. B. Interferon-gamma induces cell surface expression for both types of tumor necrosis factor receptors. FEBS Lett. 312, 87–90 (1992).
Takada, Y., Singh, S. & Aggarwal, B. B. Identification of a p65 peptide that selectively inhibits NF-kappa B activation induced by various inflammatory stimuli and its role in down-regulation of NF-kappaB-mediated gene expression and up-regulation of apoptosis. J. Biol. Chem. 279, 15096–15104 (2004).
Pierce, J. W. et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272, 21096–21103 (1997).
Yu, Y. et al. Transactivation of epidermal growth factor receptor through platelet-activating factor/receptor in ovarian cancer cells. J. Exp. Clin. Cancer Res. 33, 85 (2014).
Lemjabbar, H. & Basbaum, C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 8, 41–46 (2002).
Ridley, A. J. Rho GTPases and cell migration. J. Cell Sci. 114(Pt 15), 2713–2722 (2001).
Raftopoulou, M. & Hall, A. Cell migration: Rho GTPases lead the way. Dev. Biol. 265, 23–32 (2004).
Lambeth, J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 (2004).
Leoni, G. et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J. Clin. Invest. 123, 443–454 (2013).
Gianni, D., Taulet, N., DerMardirossian, C. & Bokoch, G. M. c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol. Biol. Cell 21, 4287–4298 (2010).
Chen, J., Chen, J. K. & Harris, R. C. Angiotensin II induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway. Mol. Cell Biol. 32, 981–991 (2012).
Ohtsu, H., Dempsey, P. J. & Eguchi, S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am. J. Physiol. Cell Physiol. 291, C1–C10 (2006).
Gallant, N. D., Michael, K. E. & Garcia, A. J. Cell adhesion strengthening: contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol. Biol. Cell 16, 4329–4340 (2005).
Herring, A. C. et al. Transient neutralization of tumor necrosis factor alpha can produce a chronic fungal infection in an immunocompetent host: potential role of immature dendritic cells. Infect. Immun. 73, 39–49 (2005).
Nenci, A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007).
Zaph, C. et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature 446, 552–556 (2007).
Sahu, R. P. et al. Loss of the platelet activating factor receptor in mice augments PMA-induced inflammation and cutaneous chemical carcinogenesis. Carcinogenesis 33, 694–701 (2012).
Soares, A. C. et al. Role of the platelet-activating factor (PAF) receptor during pulmonary infection with gram negative bacteria. Br. J. Pharmacol. 137, 621–628 (2002).
Ishii, S. et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J. Exp. Med. 187, 1779–1788 (1998).
Castor, M. G. et al. Platelet-activating factor receptor plays a role in the pathogenesis of graft-versus-host disease by regulating leukocyte recruitment, tissue injury, and lethality. J. Leukoc. Biol. 91, 629–639 (2012).
Souza, D. G. et al. Role of PAF receptors during intestinal ischemia and reperfusion injury. A comparative study between PAF receptor-deficient mice and PAF receptor antagonist treatment. Br. J. Pharmacol. 139, 733–740 (2003).
Slomiany, B. L. & Slomiany, A. Differential role of platelet-activating factor in gastric mucosal ulcer healing. Inflammopharmacology. 11, 237–248 (2003).
Ma, X., Ni, C. X., Bazan, H. & Sun, H. C. Corneal epithelial wound healing is delayed by platelet activating factor treatment. Zhonghua Yan Ke Za Zhi 40, 151–155 (2004).
Jorissen, R. N. et al. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp. Cell Res. 284, 31–53 (2003).
Boucher, I. et al. Distinct activation of epidermal growth factor receptor by UTP contributes to epithelial cell wound repair. Am. J. Pathol. 178, 1092–1105 (2011).
Boucher, I., Yang, L., Mayo, C., Klepeis, V. & Trinkaus-Randall, V. Injury and nucleotides induce phosphorylation of epidermal growth factor receptor: MMP and HB-EGF dependent pathway. Exp. Eye Res. 85, 130–141 (2007).
Sahin, U. et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 164, 769–779 (2004).
Tokumaru, S. et al. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J. Cell Biol. 151, 209–220 (2000).
Yeatman, T. J. A renaissance for SRC. Nat. Rev. Cancer 4, 470–480 (2004).
Deo, D. D., Bazan, N. G. & Hunt, J. D. Activation of platelet-activating factor receptor-coupled G alpha q leads to stimulation of Src and focal adhesion kinase via two separate pathways in human umbilical vein endothelial cells. J. Biol. Chem. 279, 3497–3508 (2004).
Dise, R. S., Frey, M. R., Whitehead, R. H. & Polk, D. B. Epidermal growth factor stimulates Rac activation through Src and phosphatidylinositol 3-kinase to promote colonic epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G276–G285 (2008).
Swain, S. D. et al. Platelet-activating factor induces a concentration-dependent spectrum of functional responses in bovine neutrophils. J. Leukoc. Biol. 64, 817–827 (1998).
Cohen, B. L. & Sachar, D. B. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. Br. Med. J. 357, j2505 (2017).
Qiu, Y. et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNFalpha dose intensification in Crohn’s disease. J. Gastroenterol. 52, 535–554 (2017).
Saxena, K. et al. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J. Virol. 90, 43–56 (2016).
Garcia, A. J., Ducheyne, P. & Boettiger, D. Quantification of cell adhesion using a spinning disc device and application to surface-reactive materials. Biomaterials 18, 1091–1098 (1997).
Acknowledgements
The authors thank Professor Takao Shimizu (University of Tokyo) for providing the Ptafr−/− mice and Nicolas Castro (Georgia Institute of Technology) for help with focal adhesion experiments. We thank Chithra K. Muraleedharan and Meenal Mhaskar for their technical support. This work was supported by NIH grants (HL127236 to A.J.G.; DK055679, DK089763, and DK059888, to A.N.; and DK61739, DK72564, and DK79392, to C.A.P.); a German Research Foundation (DFG) Research Fellowship (SI 2282/1-1, to DB); and a Crohn’s and Colitis Foundation Carreer Development Award (544599, to M.Q.).
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: D.B., M.Q., M.N.O., A.N. and C.A.P.; formal analysis: D.B., M.Q., M.N.O., D.Z., A.J.G., A.N. and C.A.P.; investigation: D.B., M.Q., M.N.O., A.N., C.A.P. V.G.H., D.Z. and J.K.; resources, A.N., C.A.P., M.B., J.C.B., R.H. and M.Y.; writing—original draft, review and editing: D.B., M.N.O., M.Q., C.A.P. and A.N.; supervision, A.N., M.Q., C.A.P. and M.N.O.; funding acquisition: A.N. and C.A.P.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Birkl, D., Quiros, M., García-Hernández, V. et al. TNFα promotes mucosal wound repair through enhanced platelet activating factor receptor signaling in the epithelium. Mucosal Immunol 12, 909–918 (2019). https://doi.org/10.1038/s41385-019-0150-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-019-0150-8
This article is cited by
-
Longitudinal DNA methylation profiling of the rectal mucosa identifies cell-specific signatures of disease status, severity and clinical outcomes in ulcerative colitis cell-specific DNA methylation signatures of UC
Clinical Epigenetics (2023)
-
Sympathetic activity regulates epithelial proliferation and wound healing via adrenergic receptor α2A
Scientific Reports (2023)
-
Large-scale sequencing identifies multiple genes and rare variants associated with Crohn’s disease susceptibility
Nature Genetics (2022)
-
Induced organoids derived from patients with ulcerative colitis recapitulate colitic reactivity
Nature Communications (2021)
-
Identifying Hub Genes, Key Pathways and Immune Cell Infiltration Characteristics in Pediatric and Adult Ulcerative Colitis by Integrated Bioinformatic Analysis
Digestive Diseases and Sciences (2021)