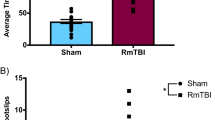
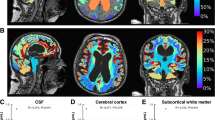
Abstract
In at least some individuals who suffer a traumatic brain injury (TBI), there exists a risk of future neurodegenerative illness. This review focuses on the association between the brain-based paravascular drainage pathway known as the “glymphatic system” and TBI-related neurodegeneration. The glymphatic system is composed of cerebrospinal fluid (CSF) flowing into the brain parenchyma along paravascular spaces surrounding penetrating arterioles where it mixes with interstitial fluid (ISF) before being cleared along paravenous drainage pathways. Aquaporin-4 (AQP4) water channels on astrocytic end-feet appear essential for the functioning of this system. The current literature linking glymphatic system disruption and TBI-related neurodegeneration is largely based on murine models with existing human research focused on the need for biomarkers of glymphatic system function (e.g., neuroimaging modalities). Key findings from the existing literature include evidence of glymphatic system flow disruption following TBI, mechanisms of this decreased flow (i.e., AQP4 depolarization), and evidence of protein accumulation and deposition (e.g., amyloid β, tau). The same studies suggest that glymphatic dysfunction leads to subsequent neurodegeneration, cognitive decline, and/or behavioral change although replication in humans is needed. Identified emerging topics from the literature are as follows: link between TBI, sleep, and glymphatic system dysfunction; influence of glymphatic system disruption on TBI biomarkers; and development of novel treatments for glymphatic system disruption following TBI. Although a burgeoning field, more research is needed to elucidate the role of glymphatic system disruption in TBI-related neurodegeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Martland HS. Punch drunk. J Am Med Assoc. 1928;91:1103–7.
Critchley M. Medical aspects of boxing, particularly from a neurological standpoint. Br Med J. 1957;1:357–62.
McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75–86.
Bieniek KF, Cairns NJ, Crary JF, Dickson DW, Folkerth RD, Keene CD, et al. The second NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2021;80:210–9.
Graham NS, Sharp DJ. Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia. J Neurol Neurosurg Psychiatry. 2019;90:1221–33.
LoBue C, Cullum CM, Didehbani N, Yeatman K, Jones B, Kraut MA, et al. Neurodegenerative dementias after traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2018;30:7–13.
McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13:287–300.
Washington PM, Villapol S, Burns MP. Polypathology and dementia after brain trauma: does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Exp Neurol. 2016;275:381–8.
Williamson MLC, Elliott TR, Bogner J, Dreer LE, Arango-Lasprilla JC, Kolakowsky-Hayner SA, et al. Trajectories of life satisfaction over the first 10 years after traumatic brain injury: race, gender, and functional ability. J Head Trauma Rehabil. 2016;31:167–79.
Juengst SB, Adams LM, Bogner JA, Arenth PM, O’Neil-Pirozzi TM, Dreer LE, et al. Trajectories of life satisfaction after traumatic brain injury: influence of life roles, age, cognitive disability, and depressive symptoms. Rehabil Psychol. 2015;60:353–64.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111.
Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180–93.
Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17:1016–24.
Bolte AC, Lukens JR. Neuroimmune cleanup crews in brain injury. Trends Immunol. 2021;42:480–94.
Simon DW, McGeachy MJ, Bayır H, Clark RSB, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:572.
Braun M, Vaibhav K, Saad NM, Fatima S, Vender JR, Baban B, et al. White matter damage after traumatic brain injury: a role for damage associated molecular patterns. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2614–26.
Tehse J, Taghibiglou C. The overlooked aspect of excitotoxicity: glutamate-independent excitotoxicity in traumatic brain injuries. Eur J Neurosci. 2019;49:1157–70.
Khatri N, Thakur M, Pareek V, Kumar S, Sharma S, Datusalia AK. Oxidative stress: major threat in traumatic brain injury. CNS Neurol Disord Drug Targets. 2018;17:689–95.
Stahel PF, Morganti-Kossmann MC, Perez D, Redaelli C, Gloor B, Trentz O, et al. Intrathecal levels of complement-derived soluble membrane attack complex (sC5b-9) correlate with blood-brain barrier dysfunction in patients with traumatic brain injury. J Neurotrauma. 2001;18:773–81.
Akeret K, Buzzi RM, Schaer CA, Thomson BR, Vallelian F, Wang S, et al. Cerebrospinal fluid hemoglobin drives subarachnoid hemorrhage-related secondary brain injury. J Cereb Blood Flow Metab. 2021;41:3000–15.
Edwards G, Zhao J, Dash PK, Soto C, Moreno-Gonzalez I. Traumatic brain injury induces tau aggregation and spreading. J Neurotrauma. 2020;37:80–92.
Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-β pathology: a link to Alzheimer’s disease? Nat Rev Neurosci. 2010;11:361–70.
Polymenidou M, Cleveland DW. Prion-like spread of protein aggregates in neurodegeneration. J Exp Med. 2012;209:889–93.
Harris JA, Devidze N, Verret L, Ho K, Halabisky B, Thwin MT, et al. Transsynaptic progression of amyloid-β-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron. 2010;68:428–41.
Woerman AL, Aoyagi A, Patel S, Kazmi SA, Lobach I, Grinberg LT, et al. Tau prions from Alzheimer’s disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proc Natl Acad Sci USA. 2016;113:E8187–96.
Hickman S, Izzy S, Sen P, Morsett L, el Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21:1359–69.
Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2009;8:16–30.
Pop V, Sorensen DW, Kamper JE, Ajao DO, Murphy MP, Head E, et al. Early brain injury alters the blood-brain barrier phenotype in parallel with β-amyloid and cognitive changes in adulthood. J Cereb Blood Flow Metab. 2013;33:205–14.
Bolte AC, Dutta AB, Hurt ME, Smirnov I, Kovacs MA, McKee CA, et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat Commun. 2020;11:4524.
Simon MJ, Iliff JJ. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta. 2016;1862:442–51.
Kaiser K, Bryja V. Choroid plexus: the orchestrator of long-range signalling within the CNS. Int J Mol Sci. 2020;21:4760.
Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11:26.
Feinberg DA, Mark AS. Human brain motion and cerebrospinal fluid circulation demonstrated with MR velocity imaging. Radiology. 1987;163:793–9.
Dreha-Kulaczewski S, Joseph AA, Merboldt K-D, Ludwig H-C, Gärtner J, Frahm J. Inspiration is the major regulator of human CSF flow. J Neurosci. 2015;35:2485–91.
Hansen EA, Romanova L, Janson C, Lam CH. The effects of blood and blood products on the arachnoid cell. Exp Brain Res. 2017;235:1749–58.
Dalkara T. Cerebral Microcirculation: an introduction | SpringerLink. 2022. https://link.springer.com/referenceworkentry/10.1007/978-3-642-37078-6_29.
Tuma RF. The cerebral microcirculation. Microcirculation. 2nd Edition. 2008:485–520.
Bacyinski A, Xu M, Wang W, Hu J. The paravascular pathway for brain waste clearance: current understanding, significance and controversy. Front Neuroanat. 2017;11:101.
Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JAR, Perry VH, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–44.
Morris AWJ, Sharp MM, Albargothy NJ, Fernandes R, Hawkes CA, Verma A, et al. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016;131:725–36.
Faghih MM, Sharp MK. Is bulk flow plausible in perivascular, paravascular and paravenous channels? Fluids Barriers CNS. 2018;15:17.
Hershenhouse KS, Shauly O, Gould DJ, Patel KM. Meningeal lymphatics: a review and future directions from a clinical perspective. Neurosci Insights. 2019;14:1179069519889027.
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–9.
Yankova G, Bogomyakova O, Tulupov A. The glymphatic system and meningeal lymphatics of the brain: new understanding of brain clearance. Rev Neurosci. 2021;32:693–705.
Nedergaard M. Neuroscience. Garbage truck of the brain. Science. 2013;340:1529–30.
Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS. 2011;8:4.
Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–103.
Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–62.
Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res. 2015;40:2583–99.
Salman MM, Kitchen P, Halsey A, Wang MX, Törnroth-Horsefield S, Conner AC, et al. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain. 2022;145:64–75.
Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–80.
Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci USA. 2001;98:14108–13.
Amiry-Moghaddam M, Frydenlund DS, Ottersen OP. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience. 2004;129:999–1010.
Szczygielski J, Kopańska M, Wysocka A, Oertel J. Cerebral microcirculation, perivascular unit, and glymphatic system: role of aquaporin-4 as the gatekeeper for water homeostasis. Front Neurol. 2021;12:767470.
Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:2.
Moshkforoush A, Ashenagar B, Harraz OF, Dabertrand F, Longden TA, Nelson MT, et al. The capillary Kir channel as sensor and amplifier of neuronal signals: modeling insights on K+-mediated neurovascular communication. Proc Natl Acad Sci USA. 2020;117:16626–37.
MacAulay N. Molecular mechanisms of K+ clearance and extracellular space shrinkage-glia cells as the stars. Glia. 2020;68:2192–211.
Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42.
Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci. 2021;24:1198–209.
Benveniste H, Heerdt PM, Fontes M, Rothman DL, Volkow ND. Glymphatic system function in relation to anesthesia and sleep states. Anesth Analg. 2019;128:747–58.
Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366:628–31.
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7.
Reddy OC, van der Werf YD. The sleeping brain: harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. 2020;10:868.
Myung J, Wu D, Simonneaux V, Lane TJ. Strong circadian rhythms in the choroid plexus: implications for sleep-independent brain metabolite clearance. J Exp Neurosci. 2018;12:1179069518783762.
Christensen J, Li C, Mychasiuk R. Choroid plexus function in neurological homeostasis and disorders: the awakening of the circadian clocks and orexins. J Cereb Blood Flow Metab. 2022;42:1163–75.
Li L, Chopp M, Ding G, Davoodi-Bojd E, Zhang L, Li Q, et al. MRI detection of impairment of glymphatic function in rat after mild traumatic brain injury. Brain Res. 2020;1747:147062.
Christensen J, Wright DK, Yamakawa GR, Shultz SR, Mychasiuk R. Repetitive mild traumatic brain injury alters glymphatic clearance rates in limbic structures of adolescent female rats. Sci Rep. 2020;10:6254.
Gama Sosa MA, de Gasperi R, Pryor D, Perez Garcia GS, Perez GM, Abutarboush R, et al. Low-level blast exposure induces chronic vascular remodeling, perivascular astrocytic degeneration and vascular-associated neuroinflammation. Acta Neuropathol Commun. 2021;9:167.
Ren Z, Iliff JJ, Yang L, Yang J, Chen X, Chen MJ, et al. ‘Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab. 2013;33:834–45.
Liu X, Xie Y, Wan X, Wu J, Fan Z, Yang L. Protective effects of aquaporin-4 deficiency on longer-term neurological outcomes in a mouse model. Neurochem Res. 2021;46:1380–9.
Katada R, Akdemir G, Asavapanumas N, Ratelade J, Zhang H, Verkman AS. Greatly improved survival and neuroprotection in aquaporin-4-knockout mice following global cerebral ischemia. FASEB J. 2014;28:705–14.
Ramirez J, Berezuk C, McNeely AA, Gao F, McLaurin J, Black SE. Imaging the perivascular space as a potential biomarker of neurovascular and neurodegenerative diseases. Cell Mol Neurobiol. 2016;36:289–99.
Doubal FN, MacLullich AMJ, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41:450–4.
Bokura H, Kobayashi S, Yamaguchi S. Distinguishing silent lacunar infarction from enlarged Virchow-Robin spaces: a magnetic resonance imaging and pathological study. J Neurol. 1998;245:116–22.
Adams HHH, Cavalieri M, Verhaaren BFJ, Bos D, van der Lugt A, Enzinger C, et al. Rating method for dilated Virchow-Robin spaces on magnetic resonance imaging. Stroke. 2013;44:1732–5.
Hilal S, Tan CS, Adams HHH, Habes M, Mok V, Venketasubramanian N, et al. Enlarged perivascular spaces and cognition: a meta-analysis of 5 population-based studies. Neurology. 2018;91:e832–42.
Vilor-Tejedor N, Ciampa I, Operto G, Falcón C, Suárez-Calvet M, Crous-Bou M, et al. Perivascular spaces are associated with tau pathophysiology and synaptic dysfunction in early Alzheimer’s continuum. Alzheimers Res Ther. 2021;13:135.
Tu Y, Zhuo W, Peng J, Huang R, Li B, Liu Y, et al. The correlation between enlarged perivascular spaces and cognitive impairment in Parkinson’s disease and vascular parkinsonism. BMC Neurol. 2022;22:282.
Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–309.
Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. 2017;140:2691–705.
Ahn SJ, Taoka T, Moon W-J, Naganawa S. Contrast-enhanced fluid-attenuated inversion recovery in neuroimaging: a narrative review on clinical applications and technical advances. J Magn Reson Imaging. 2022;56:341–53.
Wong SM, Backes WH, Drenthen GS, Zhang CE, Voorter PHM, Staals J, et al. Spectral diffusion analysis of intravoxel incoherent motion MRI in cerebral small vessel disease. J Magn Reson Imaging. 2020;51:1170–80.
Huang J, van Zijl PCM, Han X, Dong CM, Cheng GWY, Tse K-H, et al. Altered d-glucose in brain parenchyma and cerebrospinal fluid of early Alzheimer’s disease detected by dynamic glucose-enhanced MRI. Sci Adv. 2020;6:eaba3884.
Kiviniemi V, Wang X, Korhonen V, Keinänen T, Tuovinen T, Autio J, et al. Ultra-fast magnetic resonance encephalography of physiological brain activity—glymphatic pulsation mechanisms? J Cereb Blood Flow Metab. 2016;36:1033–45.
Newell DW, Nedergaard M, Aaslid R. Physiological mechanisms and significance of intracranial B waves. Front Neurol. 2022;13:872701.
Piantino JA, Iliff JJ, Lim MM. The bidirectional link between sleep disturbances and traumatic brain injury symptoms: a role for glymphatic dysfunction? Biol Psychiatry. 2022;91:478–87.
Christensen J, Yamakawa GR, Shultz SR, Mychasiuk R. Is the glymphatic system the missing link between sleep impairments and neurological disorders? Examining the implications and uncertainties. Prog Neurobiol. 2021;198:101917.
Mathias JL, Alvaro PK. Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: a meta-analysis. Sleep Med. 2012;13:898–905.
Parcell DL, Ponsford JL, Redman JR, Rajaratnam SM. Poor sleep quality and changes in objectively recorded sleep after traumatic brain injury: a preliminary study. Arch Phys Med Rehabil. 2008;89:843–50.
Chaput G, Giguère J-F, Chauny J-M, Denis R, Lavigne G. Relationship among subjective sleep complaints, headaches, and mood alterations following a mild traumatic brain injury. Sleep Med. 2009;10:713–6.
Wickwire EM, Williams SG, Roth T, Capaldi VF, Jaffe M, Moline M, et al. Sleep, sleep disorders, and mild traumatic brain injury. What we know and what we need to know: findings from a National Working Group. Neurotherapeutics. 2016;13:403–17.
Piantino J, Lim MM, Newgard CD, Iliff J. Linking traumatic brain injury, sleep disruption and post-traumatic headache: a potential role for glymphatic pathway dysfunction. Curr Pain Headache Rep. 2019;23:62.
Sandsmark DK, Elliott JE, Lim MM. Sleep-wake disturbances after traumatic brain injury: synthesis of human and animal studies. Sleep. 2017;40:zsx044.
Opel RA, Christy A, Boespflug EL, Weymann KB, Case B, Pollock JM, et al. Effects of traumatic brain injury on sleep and enlarged perivascular spaces. J Cereb Blood Flow Metab. 2019;39:2258–67.
Piantino J, Schwartz DL, Luther M, Newgard C, Silbert L, Raskind M, et al. Link between mild traumatic brain injury, poor sleep, and magnetic resonance imaging: visible perivascular spaces in veterans. J Neurotrauma. 2021;38:2391–9.
Mondello S, Muller U, Jeromin A, Streeter J, Hayes RL, Wang KKW. Blood-based diagnostics of traumatic brain injuries. Expert Rev Mol Diagn. 2011;11:65–78.
Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2:492–516.
McDonald SJ, Shultz SR, Agoston DV. The known unknowns: an overview of the state of blood-based protein biomarkers of mild traumatic brain injury. J Neurotrauma. 2021;38:2652–66.
Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35:518–26.
Plog BA, Nedergaard M. Why have we not yet developed a simple blood test for TBI? Expert Rev Neurother. 2015;15:465–8.
Lindblad C, Nelson DW, Zeiler FA, Ercole A, Ghatan PH, von Horn H, et al. Influence of blood-brain barrier integrity on brain protein biomarker clearance in severe traumatic brain injury: a longitudinal prospective study. J Neurotrauma. 2020;37:1381–91.
Murcko R, Marchi N, Bailey D, Janigro D. Diagnostic biomarker kinetics: how brain-derived biomarkers distribute through the human body, and how this affects their diagnostic significance: the case of S100B. Fluids Barriers CNS. 2022;19:32.
Xuan X, Zhou G, Chen C, Shao A, Zhou Y, Li X, et al. Glymphatic system: emerging therapeutic target for neurological diseases. Oxid Med Cell Longev. 2022;2022:6189170.
Sun B-L, Wang L-H, Yang T, Sun J-Y, Mao L-L, Yang M-F, et al. Lymphatic drainage system of the brain: a novel target for intervention of neurological diseases. Prog Neurobiol. 2018;163-4:118–43.
Cherian I, Bernardo A, Grasso G. Cisternostomy for traumatic brain injury: pathophysiologic mechanisms and surgical technical notes. World Neurosurg. 2016;89:51–7.
Cherian I, Burhan H, Dashevskiy G, Motta SJH, Parthiban J, Wang Y, et al. Cisternostomy: a timely intervention in moderate to severe traumatic brain injuries: rationale, indications, and prospects. World Neurosurg. 2019;131:385–90.
Goyal N, Kumar P. Putting ‘CSF-Shift Edema’ hypothesis to test: comparing cisternal and parenchymal pressures after basal cisternostomy for head injury. World Neurosurg. 2021;148:e252–63.
Zhou Y, Shao A, Xu W, Wu H, Deng Y. Advance of stem cell treatment for traumatic brain injury. Front Cell Neurosci. 2019;13:301.
Zhang E, Wan X, Yang L, Wang D, Chen Z, Chen Y, et al. Omega-3 polyunsaturated fatty acids alleviate traumatic brain injury by regulating the glymphatic pathway in mice. Front Neurol. 2020;11:707.
Kannan G, Kambhampati SP, Kudchadkar SR. Effect of anesthetics on microglial activation and nanoparticle uptake: implications for drug delivery in traumatic brain injury. J Control Release. 2017;263:192–9.
Sachdeva S, Persaud S, Patel M, Popard P, Colverson A, Doré S. Effects of sound interventions on the permeability of the blood-brain barrier and meningeal lymphatic clearance. Brain Sci. 2022;12:742.
Guernsey DT, Leder A, Yao S. Resolution of concussion symptoms after osteopathic manipulative treatment: a case report. J Am Osteopath Assoc. 2016;116:e13–7.
Kratz SV. Case report: Manual therapies promote resolution of persistent post-concussion symptoms in a 24-year-old athlete. SAGE Open Med Case Rep. 2021;9:2050313X20952224.
Kashyap S, Brazdzionis J, Savla P, Berry JA, Farr S, Patchana T, et al. Osteopathic manipulative treatment to optimize the glymphatic environment in severe traumatic brain injury measured with optic nerve sheath diameter, intracranial pressure monitoring, and neurological pupil index. Cureus. 2021;13:e13823.
Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–61.
Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol Neurodegener. 2015;10:58.
Zeppenfeld DM, Simon M, Haswell JD, D’Abreo D, Murchison C, Quinn JF, et al. Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 2017;74:91–9.
Zou W, Pu T, Feng W, Lu M, Zheng Y, Du R, et al. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Transl Neurodegener. 2019;8:7.
Kanaan NM, Cox K, Alvarez VE, Stein TD, Poncil S, McKee AC. Characterization of early pathological tau conformations and phosphorylation in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2016;75:19–34.
Author information
Authors and Affiliations
Contributions
MEP: conceptualization, methodology, writing—original draft preparation, writing—review and editing, visualization. CGL: conceptualization, writing—review and editing, visualization, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peters, M.E., Lyketsos, C.G. The glymphatic system’s role in traumatic brain injury-related neurodegeneration. Mol Psychiatry 28, 2707–2715 (2023). https://doi.org/10.1038/s41380-023-02070-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02070-7