Abstract
Background
Alterations in brain connectivity may underlie neuropsychiatric conditions such as schizophrenia. We here assessed the degree of convergence of frontostriatal fiber projections in 56 young adult healthy controls (HCs) and 108 matched Early Psychosis-Non-Affective patients (EP-NAs) using our novel fiber cluster analysis of whole brain diffusion magnetic resonance imaging tractography.
Methods
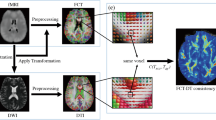
Using whole brain tractography and our fiber clustering methodology on harmonized diffusion magnetic resonance imaging data from the Human Connectome Project for Early Psychosis we identified 17 white matter fiber clusters that connect frontal cortex (FCtx) and caudate (Cd) per hemisphere in each group. To quantify the degree of convergence and, hence, topographical relationship of these fiber clusters, we measured the inter-cluster mean distances between the endpoints of the fiber clusters at the level of the FCtx and of the Cd, respectively.
Results
We found (1) in both groups, bilaterally, a non-linear relationship, yielding convex curves, between FCtx and Cd distances for FCtx-Cd connecting fiber clusters, driven by a cluster projecting from inferior frontal gyrus; however, in the right hemisphere, the convex curve was more flattened in EP-NAs; (2) that cluster pairs in the right (p = 0.03), but not left (p = 0.13), hemisphere were significantly more convergent in HCs vs EP-NAs; (3) in both groups, bilaterally, similar clusters projected significantly convergently to the Cd; and, (4) a significant group by fiber cluster pair interaction for 2 right hemisphere fiber clusters (numbers 5, 11; p = .00023; p = .00023) originating in selective PFC subregions.
Conclusions
In both groups, we found the FCtx-Cd wiring pattern deviated from a strictly topographic relationship and that similar clusters projected significantly more convergently to the Cd. Interestingly, we also found a significantly more convergent pattern of connectivity in HCs in the right hemisphere and that 2 clusters from PFC subregions in the right hemisphere significantly differed in their pattern of connectivity between groups.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Code availability
Statistical analyses were performed using R (www.r-project.org), including the “lme4” and “lmerTest” libraries for mixed model regression. The R code scripts used are available on request. Whole brain white matter tractography was computed using the unscented Kalman filter (UKF) tractography (https://github.com/pnlbwh/ukftractography). Tractography fiber clustering was performed using the whitematteranalysis (WMA) package (https://github.com/SlicerDMRI/whitematteranalysis) and the O’Donnell Research Group (ORG) atlas (http://dmri.slicer.org/atlases/), provided via the SlicerDMRI software (http://dmri.slicer.org). Code for fiber point analysis is available upon request.
References
Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36:919–34.
Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–32.
Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–19.
Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–9.
Westin CF, Maier, SE, Khidhir, B, Everett, P, Jolesz, FA, Kikinis R. Image processing for diffusion tensor magnetic resonance imaging. In: International Conference on Medical Image Computing and Computer-Assisted Intervention. Cambridge, UK: Springer; 1999. p.441–52.
Levitt JJ, Zhang F, Vangel M, Nestor PG, Rathi Y, Kubicki M, et al. The organization of frontostriatal brain wiring in healthy subjects using a novel diffusion imaging fiber cluster analysis. Cereb Cortex. 2021;31:5308–18.
Kandel E, Schwartz J, Jessell T. Principles of neural science. New York: McGraw-Hill, Health Professions Division; 2000.
Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed). 1987;295:681–2.
Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969.
Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83.
Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9.
Cronenwett WJ, Csernansky JG. Diving deep into white matter to improve our understanding of the pathophysiology of schizophrenia. Biol Psychiatry. 2013;74:396–7.
Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14.
Calhoun VD, Eichele T, Pearlson G. Functional brain networks in schizophrenia: a review. Front Hum Neurosci. 2009;3:17.
Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, et al. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69.
Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34:835–47.
Levitt JJ, Nestor PG, Levin L, Pelavin P, Lin P, Kubicki M, et al. Reduced structural connectivity in frontostriatal white matter tracts in the associative loop in schizophrenia. Am J Psychiatry. 2017;174:1102–11.
Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–71.
Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–30.
Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev. 2010;11:760–72.
Averbeck BB, Lehman J, Jacobson M, Haber SN. Estimates of projection overlap and zones of convergence within frontal-striatal circuits. J Neurosci. 2014;34:9497–505.
Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, et al. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–52.
Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–9.
Haber SN. Convergence of limbic, cognitive, and motor cortico-striatal circuits with dopamine pathways in primate brain. In: Iversen LL, Iversen SD, Dunnett SB, Bjorklund A, editors. Dopamine handbook. Oxford: Oxford University Press, Inc.; 2010. p.38–48.
Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, et al. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164:1729–36.
Liston C, Malter Cohen M, Teslovich T, Levenson D, Casey BJ. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biol Psychiatry. 2011;69:1168–77.
Haas BW, Barnea-Goraly N, Lightbody AA, Patnaik SS, Hoeft F, Hazlett H, et al. Early white-matter abnormalities of the ventral frontostriatal pathway in fragile X syndrome. Dev Med Child Neurol. 2009;51:593–9.
Heller C, Steinmann S, Levitt JJ, Makris N, Antshel KM, Fremont W, et al. Abnormalities in white matter tracts in the fronto-striatal-thalamic circuit are associated with verbal performance in 22q11.2DS. Schizophr Res. 2020;224:141–50.
Quan M, Lee SH, Kubicki M, Kikinis Z, Rathi Y, Seidman LJ, et al. White matter tract abnormalities between rostral middle frontal gyrus, inferior frontal gyrus and striatum in first-episode schizophrenia. Schizophr Res. 2013;145:1–10.
Levitt JJ, Nestor PG, Kubicki M, Lyall AE, Zhang F, Riklin-Raviv T, et al. Miswiring of frontostriatal projections in schizophrenia. Schizophr Bull. 2020;46:990–8.
Zhang F, Wu Y, Norton I, Rigolo L, Rathi Y, Makris N, et al. An anatomically curated fiber clustering white matter atlas for consistent white matter tract parcellation across the lifespan. NeuroImage. 2018;179:429–47.
Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20.
Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–9.
Levitt JJ, Rosow LK, Nestor PG, Pelavin PE, Swisher TM, McCarley RW, et al. A volumetric MRI study of limbic, associative and sensorimotor striatal subregions in schizophrenia. Schizophr Res. 2013;145:11–19.
Cetin Karayumak S, Bouix S, Ning L, James A, Crow T, Shenton M, et al. Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters. NeuroImage. 2019;184:180–200.
Cetin-Karayumak S, Di Biase MA, Chunga N, Reid B, Somes N, Lyall AE, et al. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol Psychiatry. 2020;25:3208–19.
Mirzaalian H, Ning L, Savadjiev P, Pasternak O, Bouix S, Michailovich O, et al. Inter-site and inter-scanner diffusion MRI data harmonization. NeuroImage. 2016;135:311–23.
Malcolm JG, Shenton ME, Rathi Y. Filtered multitensor tractography. IEEE Trans Med Imaging. 2010;29:1664–75.
Reddy CP, Rathi Y. Joint multi-fiber NODDI parameter estimation and tractography using the unscented information filter. Front Neurosci. 2016;10:166.
Farquharson S, Tournier JD, Calamante F, Fabinyi G, Schneider-Kolsky M, Jackson GD, et al. White matter fiber tractography: why we need to move beyond DTI. J Neurosurg. 2013;118:1367–77.
Vos SB, Viergever MA, Leemans A. Multi-fiber tractography visualizations for diffusion MRI data. PLoS ONE. 2013;8:e81453.
Fillard P, Descoteaux M, Goh A, Gouttard S, Jeurissen B, Malcolm J, et al. Quantitative evaluation of 10 tractography algorithms on a realistic diffusion MR phantom. NeuroImage. 2011;56:220–34.
Zhang F, Wu Y, Norton I, Rathi Y, Golby AJ, O’Donnell LJ. Test-retest reproducibility of white matter parcellation using diffusion MRI tractography fiber clustering. Hum Brain Mapp. 2019;40:3041–57.
Baumgartner C, Michailovich O, Levitt J, Pasternak O, Bouix S, Westin CF, et al. A unified tractography framework for comparing diffusion models on clinical scans. In: Computational Diffusion MRI Workshop of MICCAI Nice. Springer; 2012. p.27–32.
Chen Z, Tie Y, Olubiyi O, Zhang F, Mehrtash A, Rigolo L, et al. Corticospinal tract modeling for neurosurgical planning by tracking through regions of peritumoral edema and crossing fibers using two-tensor unscented Kalman filter tractography. Int J Comput Assist Radio Surg. 2016;11:1475–86.
Liao R, Ning L, Chen Z, Rigolo L, Gong S, Pasternak O, et al. Performance of unscented Kalman filter tractography in edema: Analysis of the two-tensor model. Neuroimage Clin. 2017;15:819–31.
O’Donnell LJ, Suter Y, Rigolo L, Kahali P, Zhang F, Norton I, et al. Automated white matter fiber tract identification in patients with brain tumors. Neuroimage Clin. 2017;13:138–53.
Zhang F, Norton I, Cai W, Song Y, Wells WM, O’Donnell LJ. Comparison between two white matter segmentation strategies: an investigation into white matter segmentation consistency. In: IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017); Melbourne, Australia: IEEE; 2017. p.796–9.
Descoteaux M, Angelino E, Fitzgibbons S, Deriche R. Regularized, fast, and robust analytical Q-ball imaging. Magn Reson Med. 2007;58:497–510.
Ning L, Westin CF, Rathi Y. Estimating diffusion propagator and its moments using directional radial basis functions. IEEE Trans Med Imaging. 2015;34:2058–78.
Ning L, Laun F, Gur Y, DiBella EV, Deslauriers-Gauthier S, Megherbi T, et al. Sparse Reconstruction Challenge for diffusion MRI: validation on a physical phantom to determine which acquisition scheme and analysis method to use? Med Image Anal. 2015;26:316–31.
Tournier JD, Yeh CH, Calamante F, Cho KH, Connelly A, Lin CP. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. NeuroImage. 2008;42:617–25.
Essayed WI, Zhang F, Unadkat P, Cosgrove GR, Golby AJ, O’Donnell LJ. White matter tractography for neurosurgical planning: A topography-based review of the current state of the art. Neuroimage Clin. 2017;15:659–72.
Huang H, Zhang J, van Zijl PC, Mori S. Analysis of noise effects on DTI-based tractography using the brute-force and multi-ROI approach. Magn Reson Med. 2004;52:559–65.
Radmanesh A, Zamani AA, Whalen S, Tie Y, Suarez RO, Golby AJ. Comparison of seeding methods for visualization of the corticospinal tracts using single tensor tractography. Clin Neurol Neurosurg. 2015;129:44–49.
Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36:630–44.
Guevara P, Duclap D, Poupon C, Marrakchi-Kacem L, Fillard P, Le Bihan D, et al. Automatic fiber bundle segmentation in massive tractography datasets using a multi-subject bundle atlas. NeuroImage. 2012;61:1083–99.
Jin Y, Shi Y, Zhan L, Gutman BA, de Zubicaray GI, McMahon KL, et al. Automatic clustering of white matter fibers in brain diffusion MRI with an application to genetics. NeuroImage. 2014;100:75–90.
Lefranc S, Roca P, Perrot M, Poupon C, Le Bihan D, Mangin JF, et al. Groupwise connectivity-based parcellation of the whole human cortical surface using watershed-driven dimension reduction. Med Image Anal. 2016;30:11–29.
Smith RE, Tournier JD, Calamante F, Connelly A. SIFT: spherical-deconvolution informed filtering of tractograms. NeuroImage. 2013;67:298–312.
Wu Y, Zhang F, Makris N, Ning Y, Norton I, She S, et al. Investigation into local white matter abnormality in emotional processing and sensorimotor areas using an automatically annotated fiber clustering in major depressive disorder. NeuroImage. 2018;181:16–29.
Zhang F, Savadjiev P, Cai W, Song Y, Rathi Y, Tunc B, et al. Whole brain white matter connectivity analysis using machine learning: an application to autism. NeuroImage. 2018;172:826–37.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–80.
van den Heuvel MP, Scholtens LH, de Reus MA, Kahn RS. Associated microscale spine density and macroscale connectivity disruptions in schizophrenia. Biol Psychiatry. 2016;80:293–301.
Weinberger DR. Future of days past: neurodevelopment and schizophrenia. Schizophr Bull. 2017;43:1164–8.
Goldman-Rakic PS. The corticostriatal fiber system in the rhesus monkey: organization and development. Prog Brain Res. 1983;58:405–18.
Chen SY, Huang PH, Cheng HJ. Disrupted-in-schizophrenia 1-mediated axon guidance involves TRIO-RAC-PAK small GTPase pathway signaling. Proc Natl Acad Sci USA. 2011;108:5861–6.
Mukai J, Tamura M, Fenelon K, Rosen AM, Spellman TJ, Kang R, et al. Molecular substrates of altered axonal growth and brain connectivity in a mouse model of schizophrenia. Neuron. 2015;86:680–95.
Solek CM, Farooqi NAI, Brake N, Kesner P, Schohl A, Antel JP, et al. Early inflammation dysregulates neuronal circuit formation in vivo via upregulation of IL-1β. J Neurosci. 2021;41:6353–66.
Goldman-Rakic PS. Prenatal formation of cortical input and development of cytoarchitectonic compartments in the neostriatum of the rhesus monkey. J Neurosci. 1981;1:721–35.
Haber SN. Neuroanatomy of reward: a view from the ventral striatum. In: Gottfried JA, editor. Neurobiology of sensation and reward. Boca Raton (FL): CRC Press; 2011.
Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–94.
Yeterian EH, Van Hoesen GW. Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res. 1978;139:43–63.
Barch DM, Pagliaccio D, Luking K. Mechanisms underlying motivational deficits in psychopathology: similarities and differences in depression and schizophrenia. Curr Top Behav Neurosci. 2016;27:411–49.
Haber SN, Behrens TE. The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron. 2014;83:1019–39.
Uddin LQ. Cognitive and behavioural flexibility: neural mechanisms and clinical considerations. Nat Rev. 2021;22:167–79.
Borst JP, Anderson JR. Using model-based functional MRI to locate working memory updates and declarative memory retrievals in the fronto-parietal network. Proc Natl Acad Sci USA. 2013;110:1628–33.
Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7.
Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18:177–85.
Szczepanski SM, Knight RT. Insights into human behavior from lesions to the prefrontal cortex. Neuron. 2014;83:1002–18.
Burgess PWW, H Rostral Prefrontal cortex (Brodmann Area 10) Metacognition in the Brain. In: Stuss DTKRT, editor. Principles of frontal lobe function, 2nd edition. New York: Oxford University Press; 2013. p.524–44.
Butler PD, Hoptman MJ, Smith DV, Ermel JA, Calderone DJ, Lee SH, et al. Grant report on social reward learning in schizophrenia (dagger). J Psychiatr Brain Sci. 2020;5:e200004.
Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–30.
Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170:165–72.
Oliver LD, Hawco C, Homan P, Lee J, Green MF, Gold JM, et al. Social cognitive networks and social cognitive performance across individuals with schizophrenia spectrum disorders and healthy control participants. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:1202–14.
Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–28.
Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40 Suppl 2:S107–116.
Crow TJ. Cerebral asymmetry and the lateralization of language: core deficits in schizophrenia as pointers to the gene. Curr Opin Psychiatry. 2004;17:97–106.
Levitt JJ, O’Donnell BF, McCarley RW, Nestor PG, Shenton ME. Correlations of premorbid adjustment in schizophrenia with auditory event-related potential and neuropsychological abnormalities. Am J Psychiatry. 1996;153:1347–9.
von Hohenberg CC, Pasternak O, Kubicki M, Ballinger T, Vu MA, Swisher T, et al. White matter microstructure in individuals at clinical high risk of psychosis: a whole-brain diffusion tensor imaging study. Schizophr Bull. 2014;40:895–903.
Seitz J, Zuo JX, Lyall AE, Makris N, Kikinis Z, Bouix S, et al. Tractography analysis of 5 white matter bundles and their clinical and cognitive correlates in early-course schizophrenia. Schizophr Bull. 2016;42:762–71.
Maier-Hein KH, Neher PF, Houde JC, Cote MA, Garyfallidis E, Zhong J, et al. The challenge of mapping the human connectome based on diffusion tractography. Nat Commun. 2017;8:1349.
Thomas C, Ye FQ, Irfanoglu MO, Modi P, Saleem KS, Leopold DA, et al. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci USA. 2014;111:16574–9.
Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–46.
Funding
R21MH121704 (JJL); U01MH104977 (MES, AB, DO, DH, MKes); R01MH119222 (YR, LJO); R01MH125860 (LJO, YR); P41EB015902 (LJO), R01MH074794 (Mkub, LJO), R21MH116352 (YR, Mkub, MES), NARSAD Young Investigator Award (SC-K), K24 MH110807, R01MH112748 (MKub), VA Merit Award (I01 CX000176-06; MES), R01MH117012 (KEL).
Author information
Authors and Affiliations
Contributions
JJL: conceptualization and design of the study, methodology, data analysis, writing original draft, manuscript review and editing. FZ: conceptualization and design of the study, methodology, software, data analysis, manuscript review and editing. MV: conceptualization and design of the study, methodology, software, statistical analysis, manuscript review and editing. PGN: provided input on science, review of manuscript. YR: conceptualization, methodology, manuscript review and editing. SC-K: provided input on science, methodology, review of manuscript. MK: provided input on science, manuscript review. MJC: data science, QA/QC of clinical database, review of manuscript. KEL: provided input on science, data collection, review of manuscript. DH: provided input on science, data collection, review of manuscript. MKesh: provided input on science, data collection, manuscript review and editing. SB: design and execution of MRI data processing and quality control, review of manuscript. DO: provided input on science, data collection, review of manuscript. AB: provided input on science, data collection, review of manuscript. MES: provided input on science, oversaw collection of data, manuscript review and editing. LJO: conceptualization and design of the study, methodology, software, manuscript review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Levitt, J.J., Zhang, F., Vangel, M. et al. The organization of frontostriatal brain wiring in non-affective early psychosis compared with healthy subjects using a novel diffusion imaging fiber cluster analysis. Mol Psychiatry 28, 2301–2311 (2023). https://doi.org/10.1038/s41380-023-02031-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02031-0